Abstract
Background
Associations between blood pressure (BP) and ambient air pollution have been inconsistent. No studies have used ambulatory BP monitoring and outdoor home air-pollutant measurements with time-activity-location data. We address these gaps in a study of 64 elderly subjects with coronary artery disease, living in retirement communities in the Los Angeles basin.
Methods
Subjects were followed up for 10 days with hourly waking ambulatory BP monitoring (n = 6539 total measurements), hourly electronic diaries for perceived exertion and location, and real-time activity monitors (actigraphs). We measured hourly outdoor home pollutant gases, particle number, PM2.5, organic carbon, and black carbon. Data were analyzed with mixed models controlling for temperature, posture, actigraph activity, hour, community, and season.
Results
We found positive associations of systolic and diastolic BP with air pollutants. The strongest associations were with organic carbon (especially its estimated fossil-fuel- combustion fraction), multiday average exposures, and time periods when subjects were at home. An interquartile increase in 5-day average organic carbon (5.2 μg/m3) was associated with 8.2 mm Hg higher mean systolic BP (95% confidence interval = 3.0–13.4) and 5.8 mm Hg higher mean diastolic BP (3.0–8.6). Associations of BP with 1–8 hour average air pollution were stronger with reports of moderate to strenuous physical exertion but not with higher actigraph motion. Associations were also stronger among 12 obese subjects.
Conclusions
Exposure to primary organic components of fossil fuel combustion near the home were strongly associated with increased ambulatory BP in a population at potential risk of heart attack. Low fitness or obesity may increase the effects of pollutants.
Ambient particulate matter (PM) air pollution has been associated with morbidity and mortality from cardiovascular diseases.1 Possible mechanisms behind these associations include autonomic dysfunction, oxidative stress and inflammation, and vasomotor dysfunction.2 Increased blood pressure (BP) or less vasodilatation was found in humans experimentally exposed to concentrated ambient PM2.5.3 One experimental study found the effects on BP were attributable to the organic carbon fraction of urban PM2.5, which suggests that fossil fuel combustion products may be causal.4 Organic components of particles, especially ultrafine particles (>0.1 μm in diameter), induce oxidative stress responses,5 and oxidative stress is a major mechanism underlying hypertension.6
Despite this experimental evidence, air pollutants have been inconsistently associated with BP in epidemiologic studies—some show positive associations7–15 and others inverse16–18 or no associations.19,20 In most studies, air-pollutant data came from regional air monitors situated far from subject locations, possibly leading to exposure error. Five of these studies collected home or personal air pollution data, but the number of BP measurements overall was small (n > 180) or was averaged.9,14,15,17,20 Only 2 studies have used ambulatory BP monitoring.8,14 One of these studies8 found positive associations of BP with ambient carbon monoxide (CO) in 46 young healthy nonsmoking vehicular traffic controllers. However, analyses were based on only three 24-hour averaged BP measurements across 3 seasons rather than real-time exposure-response relationships, which are among the potential strengths of ambulatory BP monitoring. The other study14 showed that systolic BP was higher by 7 mm Hg when 15 young healthy adults wore particle filter face-masks while walking for 2 hours on busy Beijing streets. None of these studies examined organic carbon, and none statistically evaluated confounding or effect modification by emotional stress or ambulatory physical activity.
We addressed these gaps in methods by using ambulatory BP monitoring to assess acute effects of real-time changes in air pollution exposures, including organic carbon, and by simultaneously collecting ambulatory data on other important determinants of BP. Numerous studies have shown that BP assessment from ambulatory monitoring is more closely associated with end-organ damage than the isolated BP readings in clinics.21 Nevertheless, ambulatory BP needs to be clinically interpreted in reference to concurrent physical activity levels.22 Positive associations between ambulatory BP and activity are small, largely attributable to major changes in the level of activity,23 and show considerable between-individual variation.22
We tested the hypothesis that ambulatory BP would be positively associated with exposure to outdoor home air pollutants, especially combustion-derived pollutants in addition to PM2.5. This hypothesis is supported by the evidence that redox-active pollutant components impair vasomotor responses by inducing oxidative stress, systemic inflammation, and endothelial dysfunction.2 We recently reported associations of outdoor home PM with increased blood biomarkers of inflammation.24,25
We followed a cohort of 64 elderly individuals with a history of coronary artery disease, who might be expected to have increased susceptibility to the adverse cardiovascular effects of air pollutants.26 The design allowed us to test for the first time the responses of ambulatory BP to variations in acute (hourly) and long-term air-pollutant exposures (up to 9 days). Electronic diary data on time-location were used to assess the representativeness of outdoor home exposures and the effect of perceived exertion on the relation of BP to air pollution exposure.
METHODS
Population and Design
This repeated measures study was designed to assess exposure-response relationships within subjects. Between-subject fixed characteristics were primarily of interest as effect modifiers.
Subjects were recruited from 4 retirement communities in the Los Angeles air basin. Eligibility criteria included age of at least 65 years, history of coronary artery disease, being a nonsmoker, and no exposure to environmental tobacco smoke. We confirmed coronary artery disease diagnoses with a medical records review. Study cardiologists and nurses clinically evaluated 105 potentially eligible subjects in our mobile medicine clinic. Twenty-one subjects were not eligible, 18 dropped out, and 2 had an insufficient number of ambulatory BP monitoring hours (>28 of 140 maximum expected), leaving 64 subjects with 6539 total hours (subject average, 103 ± 44 hours). The research protocol was approved by the Institutional Review Board of the University of California, Irvine. We obtained informed written consent from the subjects.
We followed up the subjects from 2 communities in 2005–2006 (32 subjects) and 2 communities in 2006–2007 (32 subjects). Subjects were studied with ambulatory BP monitoring in 2 sessions of 5 consecutive days during a warm period with higher photochemical activity (July to mid October) and 5 consecutive days during a cooler period of higher air stagnation (mid October to February). This provided contrasts in air-pollutant exposures.25 Each subject received a 1-day run-in trial for training. Ambulatory monitoring started Monday morning and ended Friday afternoon. A research assistant made daily home visits to download electronic data, including ambulatory BP monitoring, actigraphs, and personal digital assistant (PDA) diaries.
Ambulatory Data Collection
Ambulatory BP monitoring was carried out using the Burdick Ultralite ambulatory BP monitoring model 90217 (Burdick Inc, Deerfield, WI). This equipment fulfills standardized criteria for accuracy and performance protocols.27 A microprocessor program discriminates between pressure signals, patient movement, and respiratory artifact and initiates up to 2 additional measurements if a reading fails to satisfy program criteria. The device was programmed to measure and record BP at the top of every waking hour (up to 14 hr/d).
We electronically monitored physical activity continuously with an actigraph (Mini-motionlogger; Ambulatory Monitoring, Inc, Ardsley, NY) placed near the waist. This device records all 3 axes of movement with high test-retest reliability and validity.28 We measured the movement intensity by summing the absolute value of deviations from 0 volts each 0.1 seconds. Periods when the device was not worn were identified and categorized as missing. There were 6071 actigraph observations in the 60 minutes preceding 6539 ambulatory BP measurements (93%). For analyses, we first normalized the 1-minute data by subject-specific z-scores and then determined average movement intensity for 5-minute periods across 120 minutes before each BP measurement.
Subjects answered an hourly PDA diary after being prompted by a preprogrammed alarm that signaled the end of BP measurements. Diary responses included emotional states, moderate-to-strenuous physical exertion in the previous hour, and quarter-hour time-location (eTable 1, http://links.lww.com/EDE/A383). Paper diaries (11%) were used when the PDA was not working, or for subjects unable to use it. Subjects were instructed to sit whenever possible during their BP measurements; they also recorded their posture in the diary. To assess psychological states that could affect BP,29 the subjects were asked 3 questions (with ordinal responses) for stress or anxiety, anger, and quality of interpersonal interactions (positive to confrontational). We also asked questions about caffeine and alcohol consumption. To assess time-location, we asked 4 questions pertaining to each quarter hour in the preceding hour (indoor or outdoor home, in transit, or elsewhere). There were 6421 diary observations for the hour preceding 6539 ambulatory BP measurements (98%).
Air Pollution Exposures
We chose subjects in retirement communities to enhance the accuracy of exposure assessments using outdoor home air monitoring. In addition, retirees living in these communities are less likely to leave the monitoring location, which we confirmed with PDA data. We measured hourly outdoor home air-pollutant concentrations 9 days before each BP measurement. We used only hourly measurements rather than 24-hour filter-based gravimetric PM mass measurements, which did not match the hourly time frame of the ambulatory BP monitoring. Exposures included total particle number (Condensation Particle Counter model 3785; TSI Inc, Shoreview, MN), PM2.5 black carbon (Aethalometer; Magee Scientific, Berkeley, CA), PM2.5 organic carbon (OC_EC Analyzer model 3F; Sunset Laboratory Inc, Tigard, OR), PM2.5 mass (Beta-Attenuation Mass Monitor model 1020; Met One instruments Inc, Grants Pass, OR), and pollutant gases (ozone [O3], CO, and nitrogen oxides [NOx]), measured using federal reference methods. We detail the measurement methods elsewhere.25,30,31
Total organic carbon was further refined into 2 fractions, secondary and primary, using estimation methods that we describe elsewhere.30 Secondary organic carbon derives from products of photochemical reactions involving reactive volatile and semivolatile organics from anthropogenic and biogenic sources. Primary organic carbon is representative of particulate organics from fossil fuel combustion sources (in Los Angeles, primarily traffic).
Analysis
We used linear mixed effects models to analyze within-subject relations of systolic and diastolic BP to air pollution exposures. A model with random intercepts and slopes did not fit significantly better than a model with random intercepts only. The covariance structure was fitted best as an autoregressive-1 correlation. As proposed by Janes et al,32 to interpret regression estimates at the subject level, we adjusted for between-subject and between-session exposure effects on BP by mean-centering exposures as shown in the following mixed model:
where ai,j,k is the random intercept for subject i nested in session j and community k, X̄i is the between-subject (bs) component or the average exposure of subject i, X̄ij – X̄i is the within-subject, between-session (wsbs) component for session j or the average exposure in session j minus the overall subject average exposure, Xijt – X̄ij is the within-subject, within-session (wsws) component of primary interest or the exposure at BP measurement time t for subject i minus the average exposure for session j, Zi,j,t is a vector of additional adjustment covariates, and εi,j,t is the random within-person error in the BP measurement.
We assessed acute associations from exposures in the last 1, 4, and 8 hours before the BP measurement. We also assessed longer-term associations using the last 24-hour (1-day) average and cumulative exposures up to 9 days before each BP measurement. We present a selected span of averaging times to simplify the presentation (1-day, 3-day, 5-day, 7-day, and 9-day moving averages). Additional findings for a representative distributed lag model are presented in eFigure 1 (http://links.lww.com/EDE/A383). Averages were calculated if data for at least 75% of hours were available.
We used residual diagnostics in the SAS 9.2 Mixed procedure (SAS Institute Inc, Cary, NC) to investigate deviations from standard linear mixed-model assumptions and the presence of influential observations and subject clusters. Residuals were sufficiently normally distributed. We found no influential observations and only one potentially influential subject cluster with high leverage for modeling systolic BP. This subject was retained in the analysis because data values were plausible, and estimated effects did not qualitatively change when the subject was removed.
We decided a priori to control for temperature at the same averaging time as the air pollutant, hour of the day to control for circadian variation in BP, diary-reported posture during the BP measurement, and actigraph activity. Ambulatory BP was positively associated with actigraph activity in the 25 minutes before both systolic and diastolic BP measurements, turning toward null and then negative during the earlier times (26–120 minutes) (eFigure 2, http://links.lww.com/EDE/A383). Therefore, we adjusted associations using 25-minute average actigraph motion. In secondary analyses, we also considered residual confounding and potential effect modification from diary-derived variables, including stress or anxiety, anger, interpersonal interactions, intake of alcohol, and intake of caffeinated beverages.
To assess the potential effect of exposure error, sensitivity analyses were conducted, restricting analyses to the 91% of ambulatory BP monitoring hours when the subjects reported they were home for all quarter-hour blocks. These results were compared with analyses using all observed hours.
We tested effect modification by physical activity of the associations between air pollution and BP in product-term models of the pollutant by diary-reported exertion or last 1-hour average actigraph motion. We also tested effect modification by antihypertensive medication, statins, nonsteroidal anti-inflammatory drugs, sex, body mass index (BMI), season, and current hypertension status (mean ambulatory BP; Table 1) using JNC-7 report guidelines.33 We did not collect data on sodium intake. In these secondary analyses, we considered a product-term significance level of P > 0.1 to suggest interaction.
TABLE 1.
Characteristics of Subjectsa (n = 64)
| Characteristics | |
|---|---|
| Sex | |
| Males | 38 (59) |
| Females | 26 (41) |
| BMI (kg/m2); mean (SD) | 27.0 (4) |
| <30 | 52 (81) |
| ≥30 | 12 (19) |
| History of hypertension | 45 (70) |
| Current hypertension status (systolic BP/diastolic BP)b | |
| Normal (< 120/80 mm Hg) | 42 (66) |
| Prehypertension (120–139/80–89 mm Hg) | 11 (17) |
| Hypertension (≥ 140/90 mm Hg) | 11 (17) |
| Antihypertensive medicationsc | 55 (86) |
| β-receptor blocking medications | 37 (58) |
| Antiadrenergic agentsd | 6 (9) |
| Calcium channel blockers | 21 (33) |
| Angiotensin-converting enzyme inhibitors and angiotensin II receptor antagonists | 32 (50) |
| Nonsteroidal anti-inflammatory drugs (person-d)e | 77 (13) |
| 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins)c | 41 (64) |
Number (%) except where indicated.
From ambulatory average.
Daily use of each prescription and over-the-counter medication was reported by subjects in paper diaries daily. The subjects counted here took the medication on a regular daily schedule.
Includes α2 receptor agonists and α1-adrenergic receptor blocking agents.
Includes the total person-days of reported use of aspirin, naproxen, ibuprofen, and celecoxib (% of total person-days of diary reports).
SD indicates standard deviation.
RESULTS
Descriptive data are presented in Table 1. All subjects were white except 1 black and 1 Hispanic, and the mean (SD) age was 84 (5.6) years. Table 2 shows descriptive statistics for exposures. Exposure correlations were strong among the various markers of traffic-related air pollution (black carbon, primary organic carbon, NOx, and CO) (Table 3). These pollutants were more weakly correlated with particle number and PM2.5, not correlated with secondary organic carbon, and inversely correlated with O3.
TABLE 2.
Descriptive Statistics of Outdoor Home Air Pollutant Exposures
| Exposure (24-hr Averages) | No. Days (No. Missing)a | Mean (SD) | Interquartile Range | Min/Max |
|---|---|---|---|---|
| Outdoor hourly particle measurements | ||||
| Black carbon (μg/m3) | 235 (0) | 1.67 (0.79) | 1.02 | 0.29/4.51 |
| Organic carbon (μg/m3) | 188 (47) | 7.78 (3.68) | 5.20 | 2.46/18.7 |
| Primary organic carbon (μg/m3) | 157 (78) | 5.34 (2.92) | 4.37 | 1.41/12.5 |
| Secondary organic carbon (μg/m3) | 157 (78) | 2.90 (1.54) | 2.14 | 0.27/7.65 |
| Particle number (no./cm3) | 184 (51) | 12,817 (5889) | 6351 | 2019/30180 |
| PM2.5 (μg/m3) | 235 (0) | 21.1 (11.4) | 16.0 | 2.3/77.4 |
| Outdoor hourly gases | ||||
| NOx (ppb) | 235 (0) | 46.63 (31.4) | 42.3 | 3.2/184 |
| CO (ppm) | 224 (11) | 0.53 (0.30) | 0.42 | 0.01/1.68 |
| O3 (ppb) | 232 (3) | 27.1 (11.5) | 17.4 | 3.8/60.7 |
There were fewer missing observations for black carbon, PM2.5, and the gases because 2 samplers were operated in parallel at all times.
SD indicates standard deviation.
TABLE 3.
Spearman Correlation Matrix for Outdoor Home Air-Pollutant Exposuresa
| Black Carbon | Primary Organic Carbon | Secondary Organic Carbon | Particle Number | PM2.5 | Nitrogen Oxides | Carbon Monoxide | Ozone | |
|---|---|---|---|---|---|---|---|---|
| Organic carbon | 0.63 | 0.65 | 0.72 | 0.27 | 0.44 | 0.46 | 0.59 | –0.05 |
| Black carbon | 1.00 | 0.88 | 0.07 | 0.40 | 0.58 | 0.83 | 0.79 | –0.38 |
| Primary organic carbon | 1.00 | 0.01 | 0.47 | 0.43 | 0.79 | 0.75 | –0.36 | |
| Secondary organic carbon | 1.00 | –0.08 | 0.22 | –0.09 | 0.11 | 0.26 | ||
| Particle number | 1.00 | –0.13 | 0.63 | 0.45 | –0.38 | |||
| PM2.5 | 1.00 | 0.14 | 0.31 | 0.04 | ||||
| Nitrogen oxides | 1.00 | 0.82 | –0.53 | |||||
| Carbon monoxide | 1.00 | –0.29 |
All exposures are mean-centered by retirement community and seasonal phase.
Diary-reported moderate to strenuous exertion (6% of reports) was associated with 1.45 mm Hg increase in systolic BP (95% confidence interval [CI] = 0.02–2.88) and a 1.28 mm Hg increase in diastolic BP (0.41–1.70). Similarly, compared with the bottom 95%, the top fifth percentile of 1-hour average actigraph data were associated with a 1.64 mm Hg increase in systolic BP (0.09–3.19) and a 1.78 mm Hg increase in diastolic BP (0.84–2.73). In a mixed model, actigraph activity was predicted by diary-reported exertion (z-score = 0.47 [95% CI = 0.42–0.51]).
An indicator variable for intense, strong, or moderate stress or anxiety, adjusted for posture and hour of day, was positively associated with systolic BP (2.21 mm Hg [95% CI = 0.01–4.41]) and diastolic BP (2.12 mm Hg [0.78–3.45]) compared with little to no stress or anxiety. This is consistent with our previous research.29 Anger and interpersonal interactions were not associated with BP. These variables were not observed to confound or interact with associations of air pollutants and BP, and were therefore not included in the models.
A report of intake of 2 or more cups of caffeinated beverages in the last hour was associated with an increase in systolic BP of 4.74 mm Hg (1.14–8.33), but not diastolic BP. Alcohol intake was not associated with BP. Caffeine and alcohol were not included in the models because they did not confound or interact with associations of air pollutants and BP.
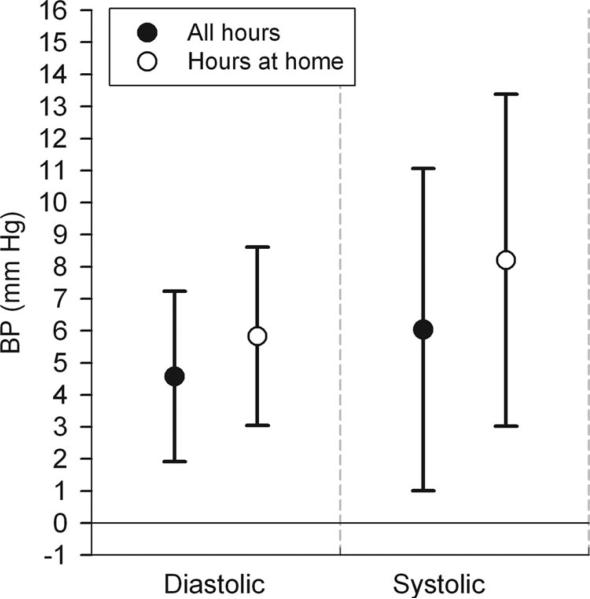
In multiple regression analyses adjusted for temperature, hour, posture, and actigraph activity, we found positive associations of air pollution with both diastolic and systolic BP (eTable 2, http://links.lww.com/EDE/A383). Positive associations were found for all particle measurements except particle numbers. NOx and CO were positively associated with diastolic BP, but 95% CIs were wider than those for the PM variables. O3 was not associated with BP. After excluding the 9% of hours when subjects reported not being at home (near air monitors), associations were generally stronger (eTable 2, http://links.lww.com/EDE/A383). This is exemplified with 5-day average organic carbon in Figure 1.
FIGURE 1.
Associations of blood pressure with 5-day average outdoor home organic carbon: influence on models by hours the subjects were at home. Estimates and 95% CIs are for interquartile increases in the air pollutant, adjusted for temperature of the same averaging time, hour of day, posture during the BP measurement, and last 25-minute average of z-scored actigraph activity.
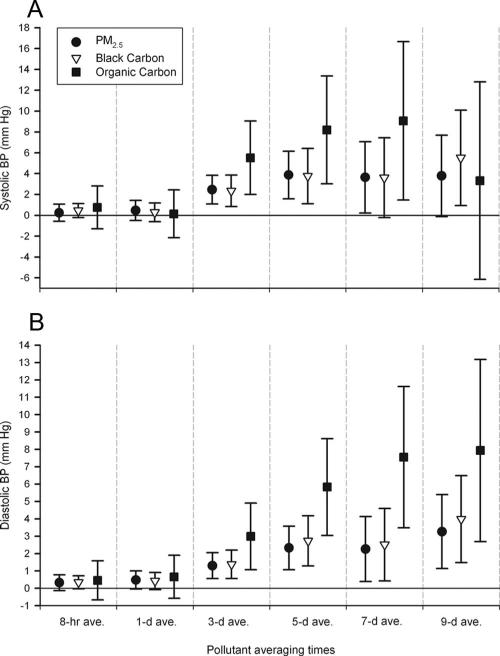
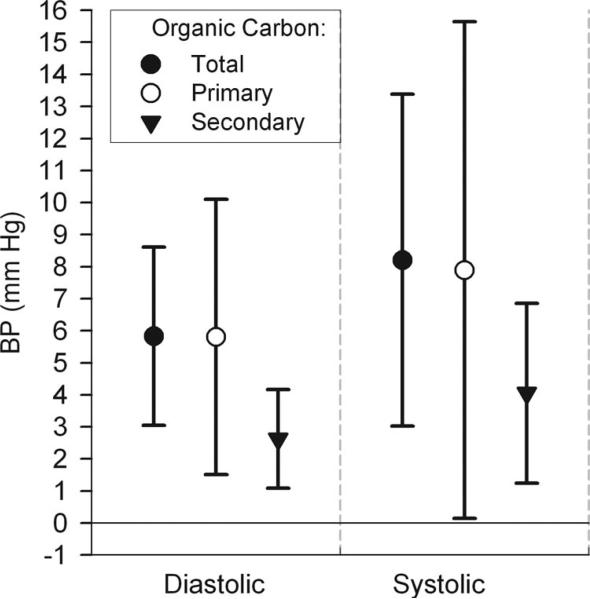
Selected models in Figure 2 illustrate differences in exposure averaging times and in particle composition (black carbon and organic carbon) compared with total mass (PM2.5). Longer multiday averaging times showed stronger magnitudes of association. For shorter averaging times, only 8- and 24-hour black carbon and PM2.5 showed small associations with diastolic BP. There was little difference in magnitude of associations with BP between black carbon and PM2.5; however, associations were generally stronger for multiday averages of organic carbon. This finding for total organic carbon can be attributed to its combustion-related primary fraction, which was more strongly associated with BP than its secondary photochemical fraction (Fig. 3).
FIGURE 2.
Associations of blood pressure with outdoor home air pollutants: differences by averaging time and particle characteristics. A, Systolic BP. B, Diastolic BP. Estimates and 95% CIs are for interquartile increases in the air pollutant, adjusted for the same variables as in Figure 1, and restricted to hours the subjects were at home.
FIGURE 3.
Associations of blood pressure with 5-day average outdoor home organic carbon: differences by primary and secondary organic carbon fractions. Estimates and 95% CIs are for interquartile increases in the air pollutant, adjusted for the same variables as in Figure 1, and restricted to hours the subjects were at home.
A few differences by seasonal phase of study showed that organic carbon, secondary organic carbon, and PM2.5 were stronger for shorter-term averages through 3 days in the warmer phase whereas black carbon, primary organic carbon, and PM2.5 were stronger in the colder phase for 3-day through 9-day averages (data not shown).
To assess whether associations with PM2.5 were attributable to organic carbon or black carbon we compared 1- to 2-pollutant models of 5-day average PM2.5 with organic carbon or black carbon. We found that regression coefficients for both PM2.5 and organic carbon decreased to a similar magnitude in 2-pollutant models. This was also the case for PM2.5 and black carbon in relation to diastolic BP, but PM2.5 confounded the association between systolic BP and black carbon (eFigure 3, http://links.lww.com/EDE/A383).
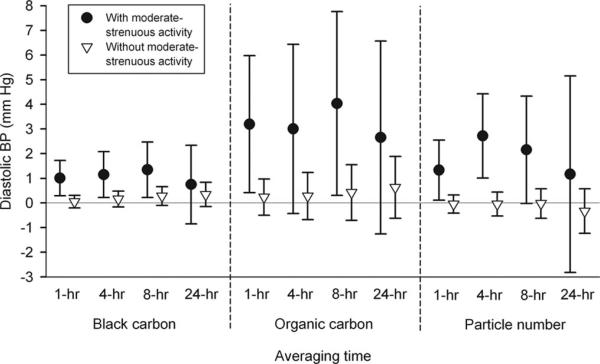
Positive associations of BP with 1-, 4-, and 8-hour average black carbon and total and primary organic carbon were stronger for the 6% of hours for which subjects reported moderate to strenuous exertion in the hour preceding the BP measurement (eTable 3, http://links.lww.com/EDE/A383). In contrast to unstratified models, there were also positive associations between BP and hourly particle numbers with reports of high exertion. These associations are illustrated for diastolic BP in Figure 4. However, there was inconsistent evidence of stronger associations of systolic BP with 3- and 5-day average black carbon and organic carbon exposures when subjects reported no exertion. Findings were also not consistent in product-term models for air pollution and average actigraph-derived activity, with little to no evidence of effect modification (data not shown).
FIGURE 4.
Associations of diastolic blood pressure with black carbon, organic carbon, and particle number: effect modification by diary-reported moderate to strenuous physical activity in the hour preceding the BP measurement. Because there was little evidence of interaction for longer averaging times, only 24 hour averages are shown for comparison. Estimates and 95% CIs are for interquartile increases in the air pollutant, adjusted for temperature of the same averaging time, hour of day, and posture during the BP measurement, and restricted to hours the subjects were at home.
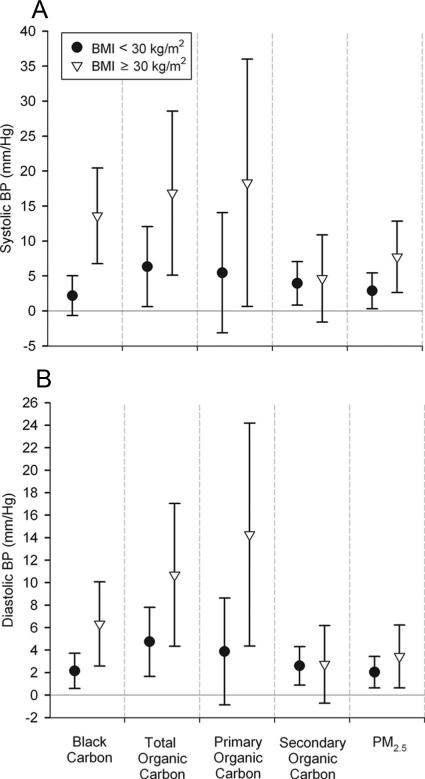
Several pollutants (black carbon, organic carbon, primary organic carbon, PM2.5, NOx, and CO), from hourly to multiday averages, were more strongly associated with systolic and diastolic BP among 12 obese subjects (BMI ≥30 kg/m2) than among the remaining 52 subjects (Fig. 5; eTable 4, http://links.lww.com/EDE/A383). The effect modification of pollutant associations by high exertion was consistent in both the BMI groups (data not shown).
FIGURE 5.
Associations of blood pressure with 5-day average outdoor home air pollution: Effect modification by body mass index. (A) systolic BP; (B) diastolic BP. Estimates and 95% CI are for interquartile increases in the air pollutant, adjusted for the same variables as in Figure 4, and restricted to hours the subjects were at home.
There was little convincing evidence for effect modification by hypertension status (data not shown) or use of antihypertensive medications, and most product terms did not reach a P > 0.1 (eTables 5 and 6, http://links.lww.com/EDE/A383). Although we found stronger positive associations for black carbon and organic carbon among subjects not using β-blockers, the reverse was evident for antiadrenergics. Positive associations of diastolic BP with air pollutants were stronger among subjects not taking statins, which is consistent with expectations—although most product terms were nonsignificant (eTables 7 and 8, http://links.lww.com/EDE/A383). There were generally stronger positive associations of BP with air pollutants when subjects used nonsteroidal anti-inflammatory drugs or just aspirin (eTables 7 and 8), which is counterintuitive, given the cardioprotective effects of aspirin. There were inconsistent differences in associations by sex (eTable 9, http://links.lww.com/EDE/A383).
DISCUSSION
Exposures to outdoor home-pollutant particles were associated with increased ambulatory BP in a panel cohort of elderly subjects with a history of coronary artery disease. This physiologic response may contribute to associations of cardiovascular morbidity and mortality with regional ambient air pollution.1 Associations were stronger when analyses were restricted to times when subjects were at home, suggesting that this reduced exposure error. Alternatively, other unmeasured factors while outside of the community may have influenced BP. NOx and CO were more imprecisely associated with BP than particles, whereas O3 was not associated with BP.
This study provides clues to possible causal pollutant components in epidemiologic associations not otherwise explained by the Environmental Protection Agency-regulated PM2.5 mass concentrations.34 Our strongest overall associations were for organic carbon. The magnitude of associations with 5-day and 7-day average organic carbon (8.2 and 9.1 mm Hg for systolic BP and 5.8 and 7.6 mm Hg for diastolic BP, respectively, per interquartile range of 5.2 μg/m3) is generally greater than those of previous studies for other exposures, including PM2.5,7–20 possibly due to differences in design. These large population-average BP changes are likely clinically relevant to long-term risk and may pose a risk to acute cardiovascular events induced by higher BP, such as atherosclerotic plaque disruption. The stronger organic carbon associations seem to be attributable to the fraction that represents primary products of fossil fuel combustion (Fig. 3). This result is coherent with our findings that blood biomarkers of inflammation were more strongly associated with primary organic carbon than secondary organic carbon.25 Particles linked to traffic sources (especially ultrafine PM)35 are implicated in inducing systemic oxidative stress because of high concentrations of redox-active organic components.5,35
We used ambulatory BP monitoring over multiple days to test the time course of pollutant exposure-response relationships. BP was associated with 1- to 8-hour exposure averages with reported exertion, suggesting an acute effect. However, the strongest associations were for multiday moving averages of air pollutants. Zanobetti et al11 reported on a panel study of 62 subjects with preexisting cardiac disease attending a cardiac rehabilitation program with exercise. The authors found that 5-day average ambient PM2.5 was more strongly associated with in-clinic BP than shorter averaging times. Our results are internally consistent with previous findings in the present panel, showing associations of multiday average air pollutants with increased biomarkers of inflammation, such as interleukin-6.24,25
We speculate that elevations of carbonaceous aerosols over several days have cumulative effects on systemic oxidative stress and inflammation, which induces increased BP, perhaps related to a sensitization of the vasculature to endogenous vasoconstrictors, leading to microvascular constriction. Exposure of rodents to concentrated outdoor PM2.5 over many weeks potentiates the hypertensive response to angiotensin II, and is accompanied by an upregulation of RhoA/Rho kinase,36,37 NAD(P)H oxidase, and nitric oxide synthase-dependent generation of superoxide.36 An increase in endothelin-1, which upregulates BP, could play a role,15 but there is evidence to the contrary.3 The multiday effect on BP may depend on the location of exposure, and thus on particle composition—as has been shown experimentally using traffic-related PM2.5 from downtown Toronto.3
We found effect modification of the relation between recent hourly exposures to air pollutants and BP by perceived high exertion. The pollutants included black carbon, organic carbon, primary organic carbon, and particle number, but not PM2.5. Experimental data suggest that acute (hourly) effects are likely mediated by autonomic nervous system responses via airway receptors.3 However, there were inconsistent interactions with 3- and 5-day black carbon and organic carbon for systolic BP, and no interactions with actigraph motion data. Our findings can be roughly compared with the in-clinic panel study of Zanobetti et al11 in which 2-day average ambient PM2.5 (13.9 μg/m3) was associated with a 7.0 mm Hg increase in diastolic BP (95% CI = 2.3–12.1) during exercise in persons with a resting heart rate ≥70 bpm. This association was larger than associations at rest, but the authors found no associations for ≤24 hour averages of PM2.5.
We speculate that the lack of pollutant interactions with actigraph motion data is because physiologic determinants of stress represented by diary responses are somewhat different from actigraph measurements. Diary reports serve as a subjective assessment of exertion not captured by the actigraph. For example, the actigraph is less sensitive for activities that do not incorporate increased skeletal movement, such as walking uphill. More importantly, poorly conditioned subjects may experience a higher level of perceived exertion because relatively low levels of physical activity measured by the actigraph in this study are closer to their maximal level of performance than for subjects who are physically fit. This may be a clue to potentially important mechanisms of air pollutant effects in susceptible populations, given the well-known link between low physical fitness and increased cardiac risk. Increased pollutant dose is also expected during exertion.
We also found stronger associations of BP with air pollution among 12 obese subjects, for both hourly and multiday pollutant averages. This did not explain the effect modification by exertion. This finding is coherent with findings of other panel studies showing stronger inverse associations of heart rate variability with PM2.5 among obese persons with coronary artery disease38 and stronger positive associations of C-reactive protein with PM2.5 among obese elderly people.39 Potential mechanisms of obesity-pollutant interactions may include an enhancement of the proinflammatory and autonomic effects of air pollution by factors thought to be involved in obesity-hypertension linkages (endothelial dysfunction, renin-angiotensin system activation, insulin resistance, adipokines, sympathetic activation, and oxidative stress).40 Similar to our results for BP, the coronary artery disease panel study also found stronger heart-rate variability associations among subjects not using β-blockers.38
A limitation of our study was the lack of data on hourly personal exposures, although analyses of community exposures were improved when restricted to the hours when subjects were at home. We also did not assess hourly particle size distribution, which could have provided information on relative associations with ultrafine versus larger particle size fractions.
Our findings suggest that organic components in traffic-related air pollution exposures near the home are strongly associated with increased BP. We also found evidence indicating the importance of fitness as a susceptibility factor in these associations. First, obesity strengthened the positive associations between BP and air pollutants. Second, we found limited evidence that acute exposure to air pollutants may enhance the deleterious interrelation between perceived physical exertion and BP. In both cases, the risk of acute myocardial ischemia could increase among those with underlying coronary artery disease. Strengths of this study include the use of ambulatory BP monitoring, real-time monitoring of exposure and physical activity, measurements of air pollution near subject residences, and detailed exposure measurements that include global markers of pollutant aerosol components from fossil fuel combustion and photochemistry.
Supplementary Material
ACKNOWLEDGMENTS
We thank the staff from the Department of Epidemiology, University of California Irvine; Department of Civil and Environmental Engineering, University of Southern California; the General Clinical Research Center, University of California Irvine; the California Air Resources Board; and the South Coast Air Quality Management District.
Supported by the National Institute of Environmental Health Sciences grant ES12243, the National Center for Research Resources, NIH, grant MO1 RR00827, the United States Environmental Protection Agency grant RD83241301, the California Air Resources Board contract number 03-329, and by the Larry K. Dodge and Susan Samueli endowed chairs (to J.L.).
Footnotes
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com).
REFERENCES
- 1.Pope CA, 3rd, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc. 2006;56:709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- 2.Mills NL, Törnqvist H, Robinson SD, et al. Air pollution and atherothrombosis. Inhal Toxicol. 2007;19(suppl 1):81–89. doi: 10.1080/08958370701495170. [DOI] [PubMed] [Google Scholar]
- 3.Brook RD, Urch B, Dvonch JT, et al. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension. 2009;54:659–667. doi: 10.1161/HYPERTENSIONAHA.109.130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Urch B, Silverman F, Corey P, et al. Acute blood pressure responses in healthy adults during controlled air pollution exposures. Environ Health Perspect. 2005;113:1052–1055. doi: 10.1289/ehp.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li N, Sioutas C, Cho A, et al. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect. 2003;111:455–460. doi: 10.1289/ehp.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paravicini TM, Touyz RM. Redox signaling in hypertension. Cardiovasc Res. 2006;71:247–258. doi: 10.1016/j.cardiores.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Auchincloss AH, Roux AV, Dvonch JT, et al. Associations between recent exposure to ambient fine particulate matter and blood pressure in the Multi-Ethnic Study of Atherosclerosis (MESA). Environ Health Perspect. 2008;116:486–491. doi: 10.1289/ehp.10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Paula Santos U, Braga AL, Giorgi DM, et al. Effects of air pollution on blood pressure and heart rate variability: a panel study of vehicular traffic controllers in the city of São Paulo, Brazil. Eur Heart J. 2005;26:193–200. doi: 10.1093/eurheartj/ehi035. [DOI] [PubMed] [Google Scholar]
- 9.Linn WS, Gong H, Jr, Clark KW, Anderson KR. Day-to-day particulate exposures and health changes in Los Angeles area residents with severe lung disease. J Air Waste Manag Assoc. 1999;49(9 Spec No.):108–115. doi: 10.1080/10473289.1999.10463890. [DOI] [PubMed] [Google Scholar]
- 10.Ibald-Mulli A, Stieber J, Wichmann HE, Koenig W, Peters A. Effects of air pollution on blood pressure: a population-based approach. Am J Public Health. 2001;91:571–577. doi: 10.2105/ajph.91.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zanobetti A, Canner MJ, Stone PH, et al. Ambient pollution and blood pressure in cardiac rehabilitation patients. Circulation. 2004;110:2184– 2189. doi: 10.1161/01.CIR.0000143831.33243.D8. [DOI] [PubMed] [Google Scholar]
- 12.Choi JH, Xu QS, Park SY, et al. Seasonal variation of effect of air pollution on blood pressure. J Epidemiol Community Health. 2007;61:314–318. doi: 10.1136/jech.2006.049205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dvonch JT, Kannan S, Schulz AJ, et al. Acute effects of ambient particulate matter on blood pressure: differential effects across urban communities. Hypertension. 2009;53:853–859. doi: 10.1161/HYPERTENSIONAHA.108.123877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langrish JP, Mills NL, Chan JK, et al. Beneficial cardiovascular effects of reducing exposure to particulate air pollution with a simple facemask. Part Fibre Toxicol. 2009;6:8. doi: 10.1186/1743-8977-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu L, Ruddy T, Dalipaj M, et al. Effects of indoor, outdoor, and personal exposure to particulate air pollution on cardiovascular physiology and systemic mediators in seniors. J Occup Environ Med. 2009;51:1088–1098. doi: 10.1097/JOM.0b013e3181b35144. [DOI] [PubMed] [Google Scholar]
- 16.Harrabi I, Rondeau V, Dartigues JF, Tessier JF, Filleul L. Effects of particulate air pollution on systolic blood pressure: a population-based approach. Environ Res. 2006;101:89–93. doi: 10.1016/j.envres.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 17.Ebelt ST, Wilson WE, Brauer M. Exposure to ambient and nonambient components of particulate matter: a comparison of health effects. Epidemiology. 2005;16:396–405. doi: 10.1097/01.ede.0000158918.57071.3e. [DOI] [PubMed] [Google Scholar]
- 18.Ibald-Mulli A, Timonen KL, Peters A, et al. Effects of particulate air pollution on blood pressure and heart rate in subjects with cardiovascular disease: a multicenter approach. Environ Health Perspect. 2004;112:369–377. doi: 10.1289/ehp.6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madsen C, Nafstad P. Associations between environmental exposure and blood pressure among participants in the Oslo Health Study (HUBRO). Eur J Epidemiol. 2006;21:485–491. doi: 10.1007/s10654-006-9025-x. [DOI] [PubMed] [Google Scholar]
- 20.Jansen KL, Larson TV, Koenig JQ, et al. Associations between health effects and particulate matter and black carbon in subjects with respiratory disease. Environ Health Perspect. 2005;113:1741–1746. doi: 10.1289/ehp.8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mancia G, Parati G. Ambulatory blood pressure monitoring and organ damage. Hypertension. 2000;36:894–900. doi: 10.1161/01.hyp.36.5.894. [DOI] [PubMed] [Google Scholar]
- 22.Leary AC, Donnan PT, MacDonald TM, Murphy MB. The influence of physical activity on the variability of ambulatory blood pressure. Am J Hypertens. 2000;13:1067–1073. doi: 10.1016/s0895-7061(00)01186-9. [DOI] [PubMed] [Google Scholar]
- 23.Fahrenberg J. Concurrent assessment of blood pressure, physical activity, and emotional state in natural settings. In: Fahrenberg J, Myrtek M, editors. Ambulatory Assessment: Computer-Assisted Psychological and Psychophysiological Methods in Monitoring and Field Studies. Hogrefe & Huber Publishers; Seattle, WA: 1996. [Google Scholar]
- 24.Delfino RJ, Staimer N, Tjoa T, et al. Circulating biomarkers of inflammation, antioxidant activity, and platelet activation are associated with urban air pollution in elderly subjects with a history of coronary artery disease. Environ Health Perspect. 2008;116:898–906. doi: 10.1289/ehp.11189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delfino RJ, Staimer N, Tjoa T. Air pollution exposures and circulating biomarkers of effect in a susceptible population: clues to potential causal component mixtures and mechanisms. Environ Health Perspect. 2009;117:1232–1238. doi: 10.1289/ehp.0800194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Klot S, Peters A, Aalto P, et al. Ambient air pollution is associated with increased risk of hospital cardiac readmissions of myocardial infarction survivors in five European cities. Circulation. 2005;112:3073–3079. doi: 10.1161/CIRCULATIONAHA.105.548743. [DOI] [PubMed] [Google Scholar]
- 27.Baumgart P, Kamp J. Accuracy of the Labs Medical 90217 ambulatory blood pressure monitor. Blood Press Monit. 1998;3:303–307. [PubMed] [Google Scholar]
- 28.Patterson SM, Krantz DS, Montgomery LC, Deuster PA, Hedges SM, Nebel E. Automated physical activity monitoring: validation and com parison with physiological and self-report measures. Psychophysiology. 1993;30:296–305. doi: 10.1111/j.1469-8986.1993.tb03356.x. [DOI] [PubMed] [Google Scholar]
- 29.Shapiro D, Jamner LD, Goldstein IB, Delfino RJ. Striking a chord: moods, blood pressure, and heart rate in everyday life. Psychophysiology. 2001;2:197–204. [PubMed] [Google Scholar]
- 30.Polidori A, Arhami M, Delfino RJ, Allen R, Sioutas C. Indoor-outdoor relationships, trends and carbonaceous content of fine particulate matter in retirement communities of the Los Angeles basin. J Air Waste Manag Assoc. 2007;57:366–379. doi: 10.1080/10473289.2007.10465339. [DOI] [PubMed] [Google Scholar]
- 31.Arhami M, Kuhn T, Fine PM, Delfino RJ, Sioutas C. Effects of sampling artifacts and operating parameters on the performance of a semi-continuous particulate EC/OC monitor. Environ Sci Technol. 2006;40:945–953. doi: 10.1021/es0510313. [DOI] [PubMed] [Google Scholar]
- 32.Janes H, Sheppard L, Shepherd K. Statistical analysis of air pollution panel studies: an illustration. Ann Epidemiol. 2008;18:792–802. doi: 10.1016/j.annepidem.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 33.National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee . The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. US Department of Health and Human Services; Washington, DC: 2004. [NIH Publication No. 04-5230. [DOI] [PubMed] [Google Scholar]
- 34.Delfino RJ, Sioutas C, Malik S. Potential role of ultrafine particles in associations between airborne particle mass and cardiovascular health. Environ Health Perspect. 2005;113:934–946. doi: 10.1289/ehp.7938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sioutas C, Delfino RJ, Singh M. Exposure assessment for atmospheric ultrafine particles (UFP) and implications in epidemiological research. Environ Health Perspect. 2005;113:947–955. doi: 10.1289/ehp.7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun Q, Yue P, Ying Z, et al. Air pollution exposure potentiates hypertension through reactive oxygen species-mediated activation of Rho/ROCK. Arterioscler Thromb Vasc Biol. 2008;28:1760–1766. doi: 10.1161/ATVBAHA.108.166967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ying Z, Yue P, Xu X, et al. Air pollution and cardiac remodeling: a role for RhoA/Rho-kinase. Am J Physiol Heart Circ Physiol. 2009;296:H1540–H1550. doi: 10.1152/ajpheart.01270.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Hartog JJ, Lanki T, Timonen KL, et al. Associations between PM2.5 and heart rate variability are modified by particle composition and beta-blocker use in patients with coronary heart disease. Environ Health Perspect. 2009;117:105–111. doi: 10.1289/ehp.11062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dubowsky SD, Suh H, Schwartz J, Coull BA, Gold DR. Diabetes, obesity, and hypertension may enhance associations between air pollution and markers of systemic inflammation. Environ Health Perspect. 2006;114:992–998. doi: 10.1289/ehp.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singer GM, Setaro JF. Secondary hypertension: obesity and the metabolic syndrome. J Clin Hypertens (Greenwich) 2008;10:567–574. doi: 10.1111/j.1751-7176.2008.08178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.