Abstract
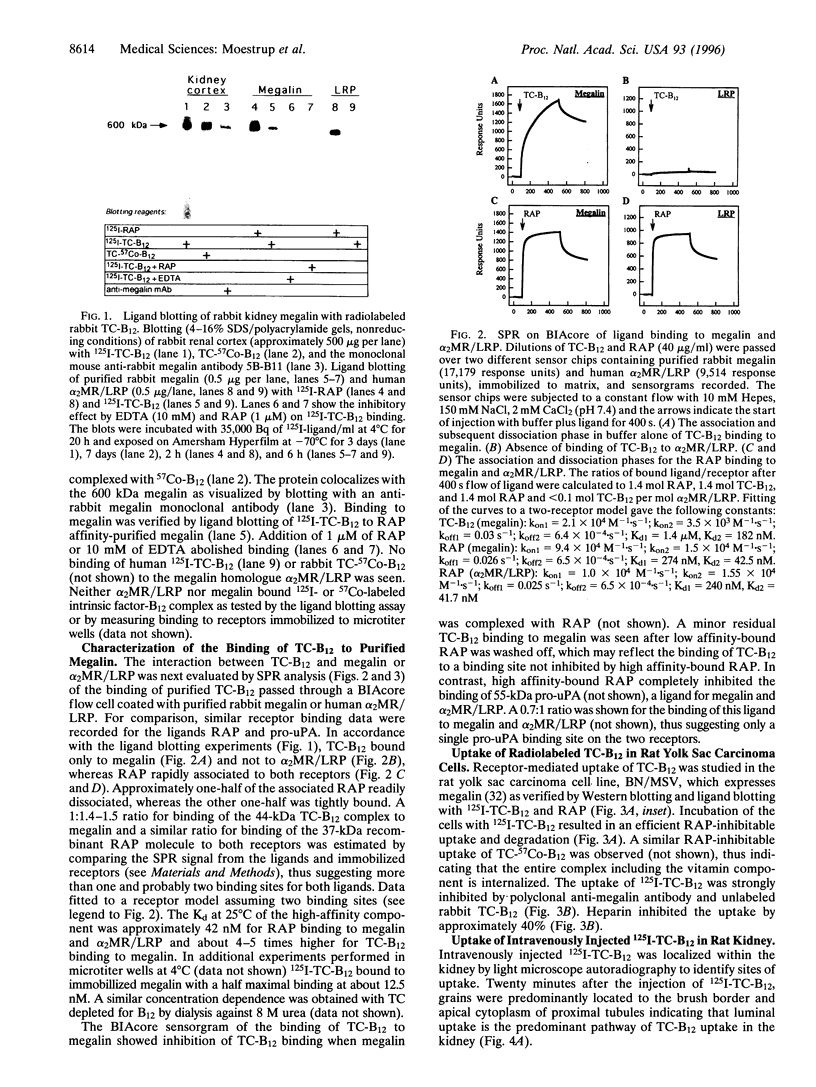
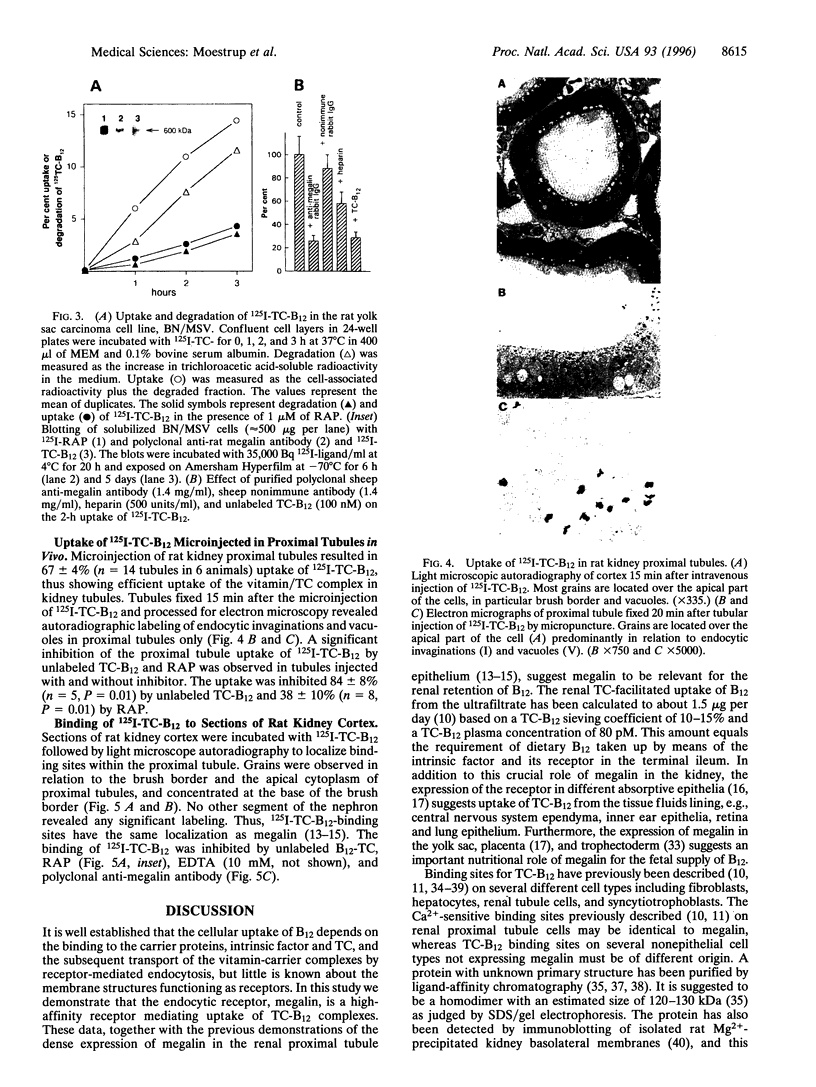
Kidney cortex is a main target for circulating vitamin B12 (cobalamin) in complex with transcobalamin (TC). Ligand blotting of rabbit kidney cortex with rabbit 125I-TC-B12 and human TC-57Co-B12 revealed an exclusive binding to megalin, a 600-kDa endocytic receptor present in renal proximal tubule epithelium and other absorptive epithelia. The binding was Ca2+ dependent and inhibited by receptor-associated protein (RAP). Surface plasmon resonance analysis demonstrated a high-affinity interaction between purified rabbit megalin and rabbit TC-B12 but no measurable affinity of the vitamin complex for the homologous alpha 2-macroglobulin receptor (alpha 2MR)/low density lipoprotein receptor related protein (LRP). 125I-TC-B12 was efficiently endocytosed in a RAP-inhibitable manner in megalin-expressing rat yolk sac carcinoma cells and in vivo microperfused rat proximal tubules. The radioactivity in the tubules localized to the endocytic compartments and a similar apical distribution in the proximal tubules was demonstrated after intravenous injection of 125I-TC-B12. The TC-B12 binding sites in the proximal tubule epithelium colocalized with megalin as shown by ligand binding to cryosections of rat kidney cortex, and the binding was inhibited by anti-megalin polyclonal antibody, EDTA, and RAP. These data show a novel nutritional dimension of megalin as a receptor involved in the cellular uptake of vitamin B12. The expression of megalin in absorptive epithelia in the kidney and other tissues including yolk sac and placenta suggests a role of the receptor in vitamin B12 homeostasis and fetal vitamin B12 supply.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bose S., Seetharam S., Hammond T. G., Seetharam B. Regulation of expression of transcobalamin II receptor in the rat. Biochem J. 1995 Sep 15;310(Pt 3):923–929. doi: 10.1042/bj3100923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose S., Seetharam S., Seetharam B. Membrane expression and interactions of human transcobalamin II receptor. J Biol Chem. 1995 Apr 7;270(14):8152–8157. doi: 10.1074/jbc.270.14.8152. [DOI] [PubMed] [Google Scholar]
- Boukhzer E., Ennya A., Felden F., Gérard A., Nexo E., Nicolas J. P., Gérard H., Guéant J. L. Transcobalamin II--cobalamin binding sites are present on rabbit germ cells. Biochim Biophys Acta. 1992 Dec 15;1175(1):128–131. doi: 10.1016/0167-4889(92)90019-8. [DOI] [PubMed] [Google Scholar]
- Bu G., Geuze H. J., Strous G. J., Schwartz A. L. 39 kDa receptor-associated protein is an ER resident protein and molecular chaperone for LDL receptor-related protein. EMBO J. 1995 May 15;14(10):2269–2280. doi: 10.1002/j.1460-2075.1995.tb07221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin A. D., Weintraub H. J. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis. 1989 Jan-Feb;9(1):21–32. doi: 10.1161/01.atv.9.1.21. [DOI] [PubMed] [Google Scholar]
- Chatelet F., Brianti E., Ronco P., Roland J., Verroust P. Ultrastructural localization by monoclonal antibodies of brush border antigens expressed by glomeruli. I. Renal distribution. Am J Pathol. 1986 Mar;122(3):500–511. [PMC free article] [PubMed] [Google Scholar]
- Christensen E. I., Gliemann J., Moestrup S. K. Renal tubule gp330 is a calcium binding receptor for endocytic uptake of protein. J Histochem Cytochem. 1992 Oct;40(10):1481–1490. doi: 10.1177/40.10.1382088. [DOI] [PubMed] [Google Scholar]
- Dryden L. P., Hartman A. M. Relative concentration of vitamin B12 in the organs of the male rat as affected by its intake of the vitamin. J Nutr. 1966 Dec;90(4):382–386. doi: 10.1093/jn/90.4.382. [DOI] [PubMed] [Google Scholar]
- Farquhar M. G., Saito A., Kerjaschki D., Orlando R. A. The Heymann nephritis antigenic complex: megalin (gp330) and RAP. J Am Soc Nephrol. 1995 Jul;6(1):35–47. doi: 10.1681/ASN.V6135. [DOI] [PubMed] [Google Scholar]
- GRASBECK R., GORDIN R., KANTERO I., KUHLBACK B. Selective vitamin B12 malabsorption and proteinuria in young people. A syndrome. Acta Med Scand. 1960 Jul 15;167:289–296. doi: 10.1111/j.0954-6820.1960.tb03549.x. [DOI] [PubMed] [Google Scholar]
- Gueth-Hallonet C., Santa-Maria A., Verroust P., Maro B. Gp330 is specifically expressed in outer cells during epithelial differentiation in the preimplantation mouse embryo. Development. 1994 Nov;120(11):3289–3299. doi: 10.1242/dev.120.11.3289. [DOI] [PubMed] [Google Scholar]
- Hakami N., Neiman P. E., Canellos G. P., Lazerson J. Neonatal megaloblastic anemia due to inherited transcobalamin II deficiency in two siblings. N Engl J Med. 1971 Nov 18;285(21):1163–1170. doi: 10.1056/NEJM197111182852103. [DOI] [PubMed] [Google Scholar]
- Hansen M., Nexø E. Cobalamin binding proteins in human seminal plasma. Scand J Clin Lab Invest. 1992 Nov;52(7):647–652. doi: 10.3109/00365519209115508. [DOI] [PubMed] [Google Scholar]
- IMERSLUND O. Idiopathic chronic megaloblastic anemia in children. Acta Paediatr Suppl. 1960 Jan;49(Suppl 119):1–115. [PubMed] [Google Scholar]
- Katz M., Mehlman C. S., Allen R. H. Isolation and characterization of an abnormal human intrinsic factor. J Clin Invest. 1974 May;53(5):1274–1283. doi: 10.1172/JCI107674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Farquhar M. G. The pathogenic antigen of Heymann nephritis is a membrane glycoprotein of the renal proximal tubule brush border. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5557–5561. doi: 10.1073/pnas.79.18.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kounnas M. Z., Haudenschild C. C., Strickland D. K., Argraves W. S. Immunological localization of glycoprotein 330, low density lipoprotein receptor related protein and 39 kDa receptor associated protein in embryonic mouse tissues. In Vivo. 1994 May-Jun;8(3):343–351. [PubMed] [Google Scholar]
- Kounnas M. Z., Loukinova E. B., Stefansson S., Harmony J. A., Brewer B. H., Strickland D. K., Argraves W. S. Identification of glycoprotein 330 as an endocytic receptor for apolipoprotein J/clusterin. J Biol Chem. 1995 Jun 2;270(22):13070–13075. doi: 10.1074/jbc.270.22.13070. [DOI] [PubMed] [Google Scholar]
- Krieger M., Herz J. Structures and functions of multiligand lipoprotein receptors: macrophage scavenger receptors and LDL receptor-related protein (LRP). Annu Rev Biochem. 1994;63:601–637. doi: 10.1146/annurev.bi.63.070194.003125. [DOI] [PubMed] [Google Scholar]
- Le Panse S., Galceran M., Pontillon F., Lelongt B., van de Putte M., Ronco P. M., Verroust P. J. Immunofunctional properties of a yolk sac epithelial cell line expressing two proteins gp280 and gp330 of the intermicrovillar area of proximal tubule cells: inhibition of endocytosis by the specific antibodies. Eur J Cell Biol. 1995 Jun;67(2):120–129. [PubMed] [Google Scholar]
- Levine J. S., Allen R. H. Intrinsic factor within parietal cells of patients with juvenile pernicious anemia. A retrospective immunohistochemical study. Gastroenterology. 1985 May;88(5 Pt 1):1132–1136. doi: 10.1016/s0016-5085(85)80071-8. [DOI] [PubMed] [Google Scholar]
- Li N., Seetharam S., Rosenblatt D. S., Seetharam B. Expression of transcobalamin II mRNA in human tissues and cultured fibroblasts from normal and transcobalamin II-deficient patients. Biochem J. 1994 Jul 15;301(Pt 2):585–590. doi: 10.1042/bj3010585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemans J., Kroes A. C., van Geel J., van Kapel J., Schoester M., Abels J. Uptake of transcobalamin II-bound cobalamin by HL-60 cells: effects of differentiation induction. Exp Cell Res. 1989 Oct;184(2):449–460. doi: 10.1016/0014-4827(89)90343-1. [DOI] [PubMed] [Google Scholar]
- Lindemans J., van Kapel J., Abels J. Uptake of transcobalamin II-bound cobalamin by isolated rat kidney tubule cells. Scand J Clin Lab Invest. 1986 May;46(3):223–232. doi: 10.3109/00365518609083663. [DOI] [PubMed] [Google Scholar]
- Linnell J. C., Collings L., Down M. C., England J. M. Distribution of endogenous cobalamin between the transcobalamins in various mammals. Clin Sci (Lond) 1979 Aug;57(2):139–144. doi: 10.1042/cs0570139. [DOI] [PubMed] [Google Scholar]
- Moestrup S. K., Cui S., Vorum H., Bregengård C., Bjørn S. E., Norris K., Gliemann J., Christensen E. I. Evidence that epithelial glycoprotein 330/megalin mediates uptake of polybasic drugs. J Clin Invest. 1995 Sep;96(3):1404–1413. doi: 10.1172/JCI118176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moestrup S. K., Kaltoft K., Sottrup-Jensen L., Gliemann J. The human alpha 2-macroglobulin receptor contains high affinity calcium binding sites important for receptor conformation and ligand recognition. J Biol Chem. 1990 Jul 25;265(21):12623–12628. [PubMed] [Google Scholar]
- Moestrup S. K., Nielsen S., Andreasen P., Jørgensen K. E., Nykjaer A., Røigaard H., Gliemann J., Christensen E. I. Epithelial glycoprotein-330 mediates endocytosis of plasminogen activator-plasminogen activator inhibitor type-1 complexes. J Biol Chem. 1993 Aug 5;268(22):16564–16570. [PubMed] [Google Scholar]
- Moestrup S. K. The alpha 2-macroglobulin receptor and epithelial glycoprotein-330: two giant receptors mediating endocytosis of multiple ligands. Biochim Biophys Acta. 1994 Jun 29;1197(2):197–213. doi: 10.1016/0304-4157(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Nexo E. A new principle in biospecific affinity chromatography used for purification of cobalamin-binding proteins. Biochim Biophys Acta. 1975 Jan 30;379(1):189–192. [PubMed] [Google Scholar]
- Nexø E., Hollenberg M. D. Characterization of the particulate and soluble acceptor for transcobalamin II from human placenta and rabbit liver. Biochim Biophys Acta. 1980 Mar 3;628(2):190–200. doi: 10.1016/0304-4165(80)90366-9. [DOI] [PubMed] [Google Scholar]
- OKUDA K. Starvation and renal uptake of vitamin B12. Nature. 1962 Jul 21;195:292–293. doi: 10.1038/195292a0. [DOI] [PubMed] [Google Scholar]
- Orlando R. A., Farquhar M. G. Functional domains of the receptor-associated protein (RAP). Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3161–3165. doi: 10.1073/pnas.91.8.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A., Pietromonaco S., Loo A. K., Farquhar M. G. Complete cloning and sequencing of rat gp330/"megalin," a distinctive member of the low density lipoprotein receptor gene family. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9725–9729. doi: 10.1073/pnas.91.21.9725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R. J., Burger R. L., Mehlman C. S., Allen R. H. The role and fate of rabbit and human transcobalamin II in the plasma transport of vitamin B12 in the rabbit. J Clin Invest. 1976 Jan;57(1):27–38. doi: 10.1172/JCI108265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. S., Treston A. M., Bowman E. P., Owens J. A., Cooksley W. G. The regulatory roles of liver and kidney in cobalamin (vitamin B12) metabolism in the rat: the uptake and intracellular binding of cobalamin and the activity of the cobalamin-dependent enzymes in response to varying cobalamin supply. Clin Sci (Lond) 1984 Sep;67(3):299–306. doi: 10.1042/cs0670299. [DOI] [PubMed] [Google Scholar]
- Seligman P. A., Allen R. H. Characterization of the receptor for transcobalamin II isolated from human placenta. J Biol Chem. 1978 Mar 25;253(6):1766–1772. [PubMed] [Google Scholar]
- Strickland D. K., Kounnas M. Z., Argraves W. S. LDL receptor-related protein: a multiligand receptor for lipoprotein and proteinase catabolism. FASEB J. 1995 Jul;9(10):890–898. doi: 10.1096/fasebj.9.10.7615159. [DOI] [PubMed] [Google Scholar]
- Warshawsky I., Bu G., Schwartz A. L. Sites within the 39-kDa protein important for regulating ligand binding to the low-density lipoprotein receptor-related protein. Biochemistry. 1995 Mar 14;34(10):3404–3415. doi: 10.1021/bi00010a032. [DOI] [PubMed] [Google Scholar]
- Willnow T. E., Armstrong S. A., Hammer R. E., Herz J. Functional expression of low density lipoprotein receptor-related protein is controlled by receptor-associated protein in vivo. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4537–4541. doi: 10.1073/pnas.92.10.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S., Riittinen L., Majuri R., Fukuda M., Gräsbeck R. Studies on the transcobalamin receptor in hog kidney. Kidney Int. 1991 Feb;39(2):289–294. doi: 10.1038/ki.1991.35. [DOI] [PubMed] [Google Scholar]
- Yang Y. M., Ducos R., Rosenberg A. J., Catrou P. G., Levine J. S., Podell E. R., Allen R. H. Cobalamin malabsorption in three siblings due to an abnormal intrinsic factor that is markedly susceptible to acid and proteolysis. J Clin Invest. 1985 Dec;76(6):2057–2065. doi: 10.1172/JCI112208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngdahl-Turner P., Rosenberg L. E., Allen R. H. Binding and uptake of transcobalamin II by human fibroblasts. J Clin Invest. 1978 Jan;61(1):133–141. doi: 10.1172/JCI108911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G., Bachinsky D. R., Stamenkovic I., Strickland D. K., Brown D., Andres G., McCluskey R. T. Organ distribution in rats of two members of the low-density lipoprotein receptor gene family, gp330 and LRP/alpha 2MR, and the receptor-associated protein (RAP). J Histochem Cytochem. 1994 Apr;42(4):531–542. doi: 10.1177/42.4.7510321. [DOI] [PubMed] [Google Scholar]
- van Kapel J., Wouters N. M., Lindemans J. Application of heparin-conjugated Sepharose for the measurement of cobalamin-saturated and unsaturated transcobalamin II. Clin Chim Acta. 1988 Mar 15;172(2-3):297–310. doi: 10.1016/0009-8981(88)90336-1. [DOI] [PubMed] [Google Scholar]