Abstract
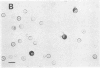
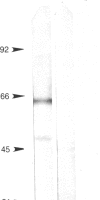
A molecular recognition code has been hypothesized to exist in which ligands and their binding sites are encoded on complementary segments of genomic DNA. We have tested this hypothesis by generating a rabbit antibody to a synthetic decapeptide (complementary peptide) encoded by an RNA complementary to the mRNA for luteinizing hormone-releasing hormone (LHRH) and determining whether this antibody recognizes the LHRH receptor. When the antibody was used for immunoperoxidase staining of enzymatically dispersed rat anterior pituitary cells, only those that contained and secreted luteinizing hormone (i.e., the gonadotropes) were recognized. This staining could be abolished by preincubation with the complementary peptide or with an LHRH agonist, suggesting that the antibody is specific to the complementary peptide and is directed at the binding site of the receptor. Further evidence that the antibody recognizes the LHRH receptor was obtained in immunoblot experiments on solubilized receptors from pituitary glands. Immunoperoxidase staining with the antibody revealed two bands at 60 kDa and 51 kDa, which are values similar to those previously obtained for the LHRH receptor in photoaffinity-labeling experiments. The staining of these bands was inhibited by preincubation with the complementary peptide or an LHRH agonist. The antibody as well as the complementary peptide to LHRH also suppressed LHRH-stimulated luteinizing hormone release in a quantitative reverse hemolytic plaque assay, presumably by binding to the LHRH receptor and by binding LHRH, respectively. These findings suggest that the synthetic decapeptide whose sequence is specified by the complementary RNA to LHRH mRNA is sufficiently similar to an LHRH binding site that the peptide not only binds LHRH but was also recognized by the immune system as such a site. These findings provide strong support for the hypothesis that recognition molecules are encoded by complementary segments of genomic DNA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blalock J. E., Smith E. M. Hydropathic anti-complementarity of amino acids based on the genetic code. Biochem Biophys Res Commun. 1984 May 31;121(1):203–207. doi: 10.1016/0006-291x(84)90707-1. [DOI] [PubMed] [Google Scholar]
- Bost K. L., Blalock J. E. Molecular characterization of a corticotropin (ACTH) receptor. Mol Cell Endocrinol. 1986 Jan;44(1):1–9. doi: 10.1016/0303-7207(86)90099-7. [DOI] [PubMed] [Google Scholar]
- Bost K. L., Smith E. M., Blalock J. E. Regions of complementarity between the messenger RNAs for epidermal growth factor, transferrin, interleukin-2 and their respective receptors. Biochem Biophys Res Commun. 1985 May 16;128(3):1373–1380. doi: 10.1016/0006-291x(85)91092-7. [DOI] [PubMed] [Google Scholar]
- Bost K. L., Smith E. M., Blalock J. E. Similarity between the corticotropin (ACTH) receptor and a peptide encoded by an RNA that is complementary to ACTH mRNA. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1372–1375. doi: 10.1073/pnas.82.5.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton R. N., Catt K. J. Gonadotropin-releasing hormone receptors: characterization, physiological regulation, and relationship to reproductive function. Endocr Rev. 1981 Spring;2(2):186–209. doi: 10.1210/edrv-2-2-186. [DOI] [PubMed] [Google Scholar]
- Clayton R. N., Shakespear R. A., Duncan J. A., Marshall J. C., Munson P. J., Rodbard D. Radioiodinated nondegradable gonadotropin-releasing hormone analogs: new probes for the investigation of pituitary gonadotropin-releasing hormone receptors. Endocrinology. 1979 Dec;105(6):1369–1376. doi: 10.1210/endo-105-6-1369. [DOI] [PubMed] [Google Scholar]
- Eidne K. A., Hendricks D. T., Millar R. P. Demonstration of a 60K molecular weight luteinizing hormone-releasing hormone receptor in solubilized adrenal membranes by a ligand-immunoblotting technique. Endocrinology. 1985 May;116(5):1792–1795. doi: 10.1210/endo-116-5-1792. [DOI] [PubMed] [Google Scholar]
- Hazum E. Photoaffinity labeling of luteinizing hormone releasing hormone receptor of rat pituitary membrane preparations. Endocrinology. 1981 Oct;109(4):1281–1283. doi: 10.1210/endo-109-4-1281. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Iwashita M., Catt K. J. Photoaffinity labeling of pituitary and gonadal receptors for gonadotropin-releasing hormone. Endocrinology. 1985 Aug;117(2):738–746. doi: 10.1210/endo-117-2-738. [DOI] [PubMed] [Google Scholar]
- LEVY H. B., SOBER H. A. A simple chromatographic method for preparation of gamma globulin. Proc Soc Exp Biol Med. 1960 Jan;103:250–252. doi: 10.3181/00379727-103-25476. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McCann S. M. Physiology and pharmacology of LHRH and somatostatin. Annu Rev Pharmacol Toxicol. 1982;22:491–515. doi: 10.1146/annurev.pa.22.040182.002423. [DOI] [PubMed] [Google Scholar]
- Seeburg P. H., Adelman J. P. Characterization of cDNA for precursor of human luteinizing hormone releasing hormone. Nature. 1984 Oct 18;311(5987):666–668. doi: 10.1038/311666a0. [DOI] [PubMed] [Google Scholar]
- Smith P. F., Frawley L. S., Neill J. D. Detection of LH release from individual pituitary cells by the reverse hemolytic plaque assay: estrogen increases the fraction of gonadotropes responding to GnRH. Endocrinology. 1984 Dec;115(6):2484–2486. doi: 10.1210/endo-115-6-2484. [DOI] [PubMed] [Google Scholar]
- Smith P. F., Luque E. H., Neill J. D. Detection and measurement of secretion from individual neuroendocrine cells using a reverse hemolytic plaque assay. Methods Enzymol. 1986;124:443–465. doi: 10.1016/0076-6879(86)24034-3. [DOI] [PubMed] [Google Scholar]
- Thorpe D. H. Opiate structure and activity--a guide to understanding the receptor. Anesth Analg. 1984 Feb;63(2):143–151. [PubMed] [Google Scholar]
- Tsernoglou D., Petsko G. A., Hudson R. A. Structure and function of snake venom curarimimetic neurotoxins. Mol Pharmacol. 1978 Jul;14(4):710–716. [PubMed] [Google Scholar]