Abstract
The cardiac IKs current is involved in action potential repolarization, where its primary function is to limit action potential prolongation during sympathetic stimulation. The IKs channel is mainly composed of KV7.1 ion channels associated with KCNE1 auxiliary subunits. The availability of KCNE1 solution structure by nuclear magnetic resonance spectroscopy in conjunction with biochemical assays addressing KV7.1–KCNE1 residue interactions has provided new insights into the structural basis for KV7.1 modulation by KCNE1. Recent evidence further suggests that KCNE2 may associate with the KV7.1–KCNE1 channel complex and modulate its current amplitude. Here we review recent studies in this area and discuss potential roles for multiple KCNEx subunits in IKs generation and modulation as well as the clinical relevance of the new information.
Keywords: IKs, KCNE1, KCNE2, KCNQ1, KV7.1
Introduction
In human heart, the cardiac delayed rectifier current comprising IKs (cardiac slow delayed rectifier current) and IKr (cardiac rapid delayed rectifier current) is an important determinant of action potential duration. With its slow rate of activation, IKs primarily contributes to action potential repolarization during β-adrenergic stimulation, when its current amplitude is increased and rate of activation accelerated via the protein kinase A pathway. A number of studies have identified different signaling molecules, such as calmodulin and phosphatidylinositol 4,5-bisphosphate (PIP2), which contribute to regulation of IKs and IKr in the heart (for an excellent review on these topics, see Charpen-tier et al1). The α-subunit that mediates IKs is KV7.1 (also known as KCNQ1 or KvLQT1; see “The IKs Babylon” in the Online Supplemental Data). KV7.1 channels are tetramers, with each subunit containing six transmembrane segments forming peripheral voltage-sensing domains (S1–S4) and a central pore domain (S5–S6) (Figure 1A). The significance of KV7.1 in normal heart function is highlighted by more than 240 identified KCNQ1 mutations associated with arrhythmias such as long QT syndrome, short QT syndrome, and atrial fibrillation (http://www.fsm.it/cardmoc/). In the majority of cases, KV7.1 mutations associated with loss of function of the IKs current appear to result in long QT syndrome, whereas gain-of-function mutations lead to short QT syndrome or atrial fibrillation. However, KV7.1 mutations simultaneously linked to long QT syndrome and atrial fibrillation have been reported.2 As KV7.1 properties are differentially modulated by the KCNE accessory subunits (Figure 1), this complexity may be at least partially due to a heterogeneous pattern of KV7.1 association with different KCNE subunits in the heart.
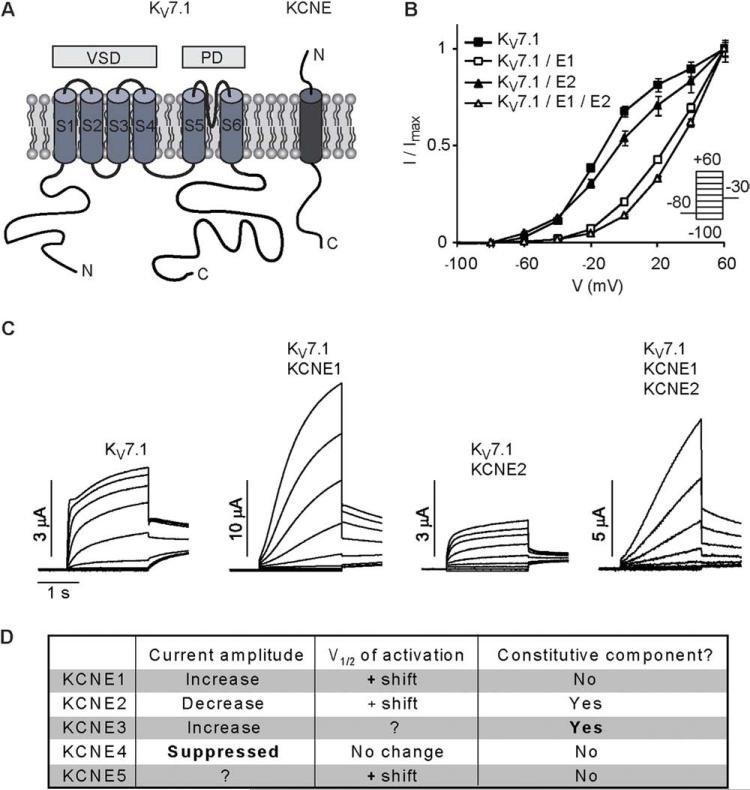
Figure 1.
KV7.1–KCNE1–KCNE2 current characteristics. A: Topology of KV7.1 and KCNE subunits. KV7.1 subunits encompass six transmembrane segments with intracellular N- and C- termini, where S1–S4 encode the voltage-sensing domain (VSD) and S5–S6 encode the pore domain (PD). The KCNE proteins contain a single transmembrane segment flanked by an extracellular N-terminus and a cytosolic C-terminus. B: Normalized amplitudes of currents elicited from Xenopus oocytes upon stimulation with the indicated voltage clamp protocol 48 hours after injection of KV7.1 and KCNE1–KCNE2 cRNA as a function of clamp potential reveals that KCNE1 shifts the voltage-dependence of KV7.1 activation in the depolarizing direction to a much greater extent than does KCNE2. C: Representative current traces from the experiments analyzed in B illustrate the diverse effects of KCNE proteins on KV7.1 channel properties. Homomeric KV7.1 channels activate relatively rapidly, exhibit sustained currents at maintained depolarization, and slowly deactivate upon repolarization. Co-expression with KCNE1 greatly increases KV7.1 current amplitude and slows activation, in addition to its effect on channel voltage-dependency. Co-expression with KCNE2 reduces current amplitudes and induces a constitutively active current component. Activation of the time-dependent current component is slower than activation in the absence of KCNE2.28 Co-expression with KCNE1 and KCNE2 generate currents with a mixture of the features, where KCNE1 dictates kinetics, whereas both KCNE1 and KCNE2 contribute to determination of current amplitude.2,29D: Effects exerted by KCNE subunits on KV7.1 currents. Strong effects are highlighted in bold.
Compelling evidence has established that KCNE1 is the major accessory subunit of the IKs channel. KCNE1 increases KV7.1 channel conductance, shifts its activation to a more positive voltage range, and, importantly, confers the unique slow activation rate of IKs. Computational work suggests that KCNE1 resides in a cleft between voltage-sensing domains in the KV7.1 channel structure. In support of this model, three KV7.1 mutations associated with cardiac arrhythmia that reveal their phenotype only upon co-expression with KCNE1 all localize to a voltage-sensing domain–pore domain interface that is part of the open-state cleft where KCNE1 resides.3 Since 1999, other members of the KCNE family have been cloned and characterized4 (note that KCNE1 is equivalent to minK, and KCNE2–KCNE5 corresponds to minK-related peptides or MiRP1-4 in previous nomenclature; see “The IKs Babylon” in the Online Supplemental Data). All KCNE genes are reportedly transcribed into mRNA in the human heart,5 and expression of KCNE1–KCNE4 proteins has been detected. Emerging evidence suggests a role for KCNE2 in regulating IKs as well. KCNE2 reduces the KV7.1 current amplitude and confers a constitutively active current component (Figures 1B–1D).6 If IKs in some cardiac myocytes is mediated by a KV7.1 channel complex encompassing KCNE2, it would have major functional consequences. Understanding the structural requirements for KCNE modulation of KV7.1 is important for delineating the role of KCNE subunits in IKs generation and regulation. Here we review current knowledge of the structural basis for KV7.1–KCNE1 interactions (with focus on the membrane-spanning regions), describe evidence for the presence of additional KCNE proteins in the channel complex (with focus on KCNE2), and discuss the clinical relevance of these recent findings.
KV7.1–KCNE1 channel stoichiometry
To resolve the structural basis for KV7.1–KCNEx interactions, it is essential to know the stoichiometry of the channel complex. The number of KCNE subunits in the IKs complex has been a matter of debate. Recently, an elegant approach was used: iterative rounds of channel blocking/modification by a chemically releasable channel inhibitor were used to bind the channel pore and simultaneously covalently modify a cysteine-bearing KCNE1 subunit. The study convincingly showed that KV7.1 and KCNE1 associate in a 4:2 stoichiometry.7 There are at least two possible mechanisms for such a stoichiometry in KV7.1–KCNE1 channel complexes. First, KV channel assembly may involve first dimerization of α-subunits, followed by dimerization of dimers to form a tetramer. For KV7.1–KCNE1 channels, KCNE1 has been speculated to be involved in such a dimeric assembly process.7 Recent reports support this mechanism. The carboxy end of KCNE1 (aa 109–129) binds to a double-helix bundle (helices C) in the cytoplasmic carboxy-terminus of KV7.1,8 and these helix C bundles may be involved in dimerization of KV7.1 dimers during channel biogenesis.9 Second, the KCNE1 solution nuclear magnetic resonance (NMR) structure implies that binding of additional KCNE1 subunits in the KV7.1–KCNE1 channel complex may face steric hindrance due to helical secondary structures present in the KCNE1 extracellular and intracellular domains, which form an interface large enough to span multiple KV7.1 subunits.3
KCNE1 location in KV7.1 channels
Experimental observations suggest that KCNE1 is located in close proximity to the KV7.1 pore domain so that the transmembrane segment of KCNE1 interacts directly with S6 of KV7.1.10–12 For example, it has been shown that KCNE1 position 42 can come close to KV7.1 position 324 in S6 in the open state so that cysteine side chains engineered into these two positions can form a disulfide bond and lock the channel in the open state.13 In addition to interacting with the pore domain, KCNE1 interacts with the KV7.1 voltage-sensing domain. For example, KV7.1 position 226 in S4 can come close to KCNE1 position 44 in the open state so that cysteine side chains engineered into these positions can form a disulfide bond and stabilize S4 in its activated state.14 These interactions may be related to the ability of KCNE1 to right-shift the voltage-dependency of KV7.1 channel activation. Although the association of KCNE1 with the KV7.1 channel is mediated mainly by the transmembrane regions, secondary association between the carboxyl end of KCNE1 and bundles of helices C of KV7.1 also contribute.8
Based on the KV1.2 crystal structure and the KCNE1 solution NMR structure, models for the KV7.1–KCNE1 complex in the open and the closed states have been proposed (Figure 2).3 Computational analysis of the channel complex localizes KCNE1 to a cleft between pore and voltage-sensing domains but with different interaction interfaces in the two states.3 In the closed state, the extracellular end of KCNE1 transmembrane segment forms contacts with S3 of one subunit and with S5 and S6 of another subunit, whereas in the open state, it forms an interface with S1, S5, and S6 from three different subunits.3 In general, these predictions are in agreement with functional studies using cysteine scanning mutagenesis and disulfide trapping approaches.12,13 Hence, although still not completely resolved, a picture emerges that KCNE proteins are in contact with several helices from distinct subunits of the KV7.1 channel.
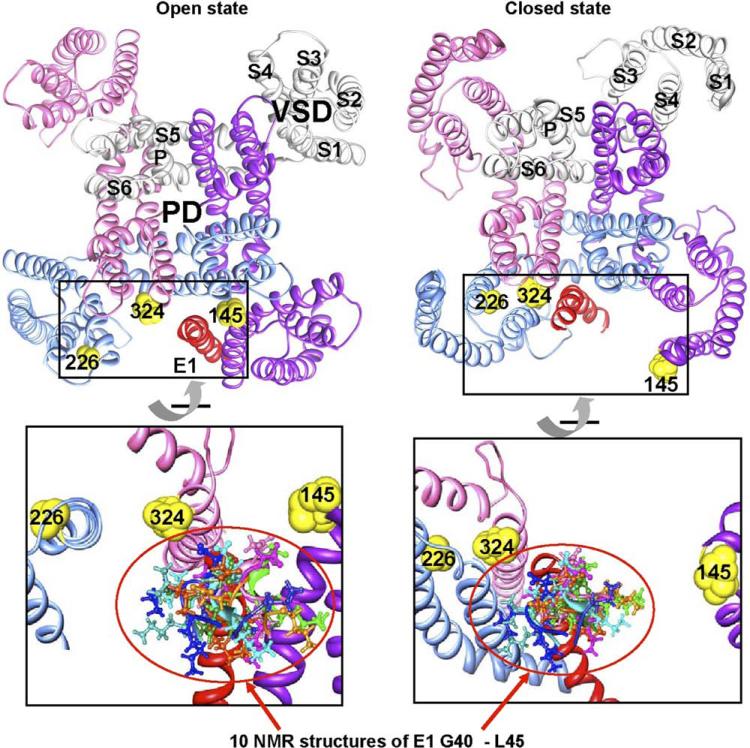
Figure 2.
Model of KV7.1–KCNE1 channel structure. KV7.1–KCNE1 closed- and open-state models as generated by Kang et al3 and graphed using UCSF-Chimera. Top: Docking of the nuclear magnetic resonance (NMR) structure of one KCNE1 transmembrane segment (red) onto a homology model of KV7.1 in the open (left) and closed (right) state, respectively, shows that KCNE1 resides in different clefts in the two states. The channel complex is visualized from the extracellular side. KV7.1 subunits are colored gray, pink, blue, and purple, with S1–S6 and P helices labeled for one subunit. The voltage-sensing domain (VSD) and pore domain (PD) are also marked. Yellow spheres indicate the extracellular ends of S1 (I145), S4 (A226), and S6 (V324). In the open state, the extracellular end of the KCNE1 transmembrane segment forms an interface with S1, S5, and S6 from three different subunits; in the closed state, it forms contact with S3 of one subunit and S5 and S6 of another subunit. Bottom: Enlarged and rotated view of the boxed regions in the top panels, with addition of 10 NMR structures of KCNE1 residues 40–45 (color coded differently for individual NMR structures). This was achieved by aligning the 10 NMR KCNE1 structures (residues 40–70) to the KCNE1 transmembrane segment of the KV7.1–KCNE1 model6 using the MatchMaker function of UCSF-Chimera (for clarity, only residues 40–45 are shown). Assuming (1) a wide swing of S1–S4 upon transition between closed and open states and (2) changing KCNE1 position and orientation relative to KV7.1 in these states, the model suggests a wide range of motions in the extracellular end of the KCNE1 transmembrane segment (highlighted by the red circle) and possibilities of contacts with KV7.1. This model is partially supported by the proximity of –S–S– partners as delineated in cysteine mutagenesis, although further experimental confirmation is needed.
KCNE1 interaction with KV7.1 voltage sensor
Computational model simulations of KV7.1–KCNE1 interactions suggest that the intracellular end of the KCNE1 transmembrane segment is close to the S5 end of the S4–S5 linker in KV7.1. This may be the mechanism by which KCNE1 opposes S4–S5 linker movement and accordingly slows KV7.1 channel opening.3,15 This scenario is supported by functional studies reporting differential effects of S4–S5 linker mutations dependent on channel assembly with KCNE1.16,17 Furthermore, KCNE1 is proposed to impede salt bridge formation between charged residues in S2 and S4 of the KV7.1 voltage sensor.18 The pattern of salt bridge formation appears to differ between the channel's open and closed states, which correlates with axial rotation of KCNE1 transmembrane segments during IKs gating transitions between the two states.13 Compelling evidence thus supports a strategic location of KCNE1 between the KV7.1 voltage sensor and pore domains, which allows KCNE1 to effectively modulate the KV7.1 gating transition between the closed and open states.
How does KCNE1 slow KV7.1 activation?
KCNE1 can transform KV7.1-mediated currents into the characteristic slowly activating IKs by two possible mechanisms. KCNE1 either slows voltage sensor movement or delays coupling of voltage sensor movement to the activation gate. A careful study combining cysteine scanning mutagenesis and varying voltage-clamp pulse durations suggests that KV7.1–KCNE1 voltage sensors reach their equilibrium position in less than 100 ms.19 Together with KCNE1's restriction of S4–S5 linker movement, it seems likely that the rate-limiting step in KV7.1–KCNE1 channel activation is in the opening of the activation gate. In K+ channels, conformational changes allowing gating are attributed to a so-called gating hinge located deep within the membrane. In most K+ channels, the hinge is a conserved glycine residue, but KV7.1 channels carry an alanine at the homologous position (336). KV7.1 comprises a Pro-Ala-Gly sequence further downstream on S6, which corresponds to the KV channel Pro-Val-Pro motif that adds to the flexibility of the region. In the absence of KCNE1, the ability of KV7.1 to kink at Ala 336 facilitates gating, whereas the integrity of the Pro-Ala-Gly motif is required for channel activation.20 Thus, it can be speculated that an additional mechanism by which KCNE1 slows KV7.1 activation is by restraining the tendency of S6 to bend at either of these regions.
Structural determinants for diverse KCNE effects on KV7.1 channels
Additional members of the KCNE family may contribute to IKs generation. The five KCNE proteins all have diverse effects on KV7.1 conduction properties in heterologous systems, and their importance for cardiac function is evidenced by co-segregation of mutations in each of them and cardiac disorders (for reviews on these subjects, see Charpentier et al1 and McCrossan and Abbott4). A cysteine scanning mutagenesis study suggested a similar position and orientation of the KCNE2 transmembrane segment with respect to KV7.1 as found for KCNE1, with one face of KCNE2 transmembrane segment making intimate contact with the KV7.1 pore domain and another face making more dynamic contacts with the voltage-sensing domain.6 However, it appears that minor structural differences determine the specific modulatory effects of KCNEs on KV7.1. For instance, the drastic effect of KCNE3 in converting KV7.1 into a constitutively active channel by shifting the equilibrium position of its voltage sensors to the activated state at all potentials19,21 can be altered into that of KCNE1 by swapping just one amino acid within the transmembrane segment.22 Likewise, exchange of a single amino acid within the KCNE2 transmembrane segment converts the KV7.1 modulatory properties of KCNE2 to those of KCNE1.6 It seems that discrete contact points between KV7.1 and the KCNEs are determinants of the distinct modulatory effects of the different KCNE subunits on the KV7.1 channel.
KCNE2 as a dynamic IKs regulator
As KCNE2–KCNE4 protein expression has been confirmed in human heart,23–25 IKs may not be a simple channel complex encompassing four KV7.1 subunits and two KCNE1 subunits. Experiments using heterologous expression systems have shown that different members of the KCNE family can associate with the same KV7.1 channel simultaneously,26–29 and exchange of KCNE1–KCNE2 subunits can occur even during the lifespan of KV7.1–KCNEx channel complexes in cells.29 Because only KCNE1 can confer the unique slow activation rate of IKs, it is an obligate part of the IKs channel complex. Given the constraint that KV7.1 and KCNE proteins associate in a 4:2 stoichiometry, it is conceivable that a portion of IKs channels in human myocytes may be mediated by a channel complex consisting of four KV7.1 subunits, one KCNE1 subunit, and one KCNE subunit other than KCNE1. Thus, spatial heterogeneity in the KCNE expression profile is a potential mechanism for cellular regulation of IKs density.
Experiments performed on rat cardiomyocytes suggest a role for KCNE2 in IKs.27,28 As KCNE2 decreases the amplitude of KV7.1–KCNE1 currents (Figure 1C),2,28 it is tempting to speculate that KCNE2 functions as a down-regulator of KV7.1–KCNE1-mediated IKs (Figure 3).27–29 The feasibility of this proposition is supported by experiments on adult guinea pig ventricular myocytes, where expression of KCNE2 protein by adenovirus-mediated gene transfer reduces IKs current density but not its gating kinetics or KV7.1 protein level.29
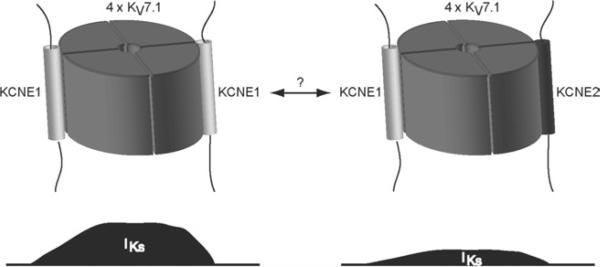
Figure 3.
Possible heterogeneity in IKs channel components. IKs density may be dynamically regulated by the composition of KCNE proteins in the channel complex. Expression of KV7.1–KCNE1 channels gives rise to greater current amplitude than do KV7.1–KCNE1–KCNE2 channels. As KCNE2 can modulate current mediated by KV7.1–KCNE1 channels in the plasma membrane, it can be speculated that KCNE2 may function as a dynamical down-regulator of KV7.1–KCNE1 currents, where KCNE2 may be able to exchange position with KCNE1 in the channel complex.29 Such regulation of IKs density by KCNE2 could have a temporal and/or a spatial component, but this remains speculative based on the current evidence.
Clinical relevance
The clinical aspects of the information presented in this review are twofold. First, recent studies suggest that the subunit composition of IKs channels in cardiac myocytes likely is more complex and dynamic than previously envisioned. Not only KCNE1 but also KCNE2–KCNE4 may be involved in IKs generation. Because these other KCNE subunits can confer distinct phenotypes to KV7.1 channels, changes in the subunit composition can have profound impact on IKs current amplitude and/or gating kinetics and thus action potential shape and duration. Concurrent atrial fibrillation and QT prolongation have been reported for individuals carrying mutations in KV7.1. How KV7.1 can form the molecular substrate for both conditions is perplexing, as QT prolongation is caused by a reduced repolarization reserve, whereas atrial fibrillation can be caused by increased K+ currents that shorten the repolarization phase and increase the risk of multiple reentrant wavelets. The complexity conveyed by the KCNE subunits may help explain some of the paradoxical phenotypes of KV7.1 mutations. As a case in point, a carrier of the KV7.1 mutation Q147R presented with concomitant atrial fibrillation and prolonged QT interval.2 Functional studies revealed that the mutation causes a gain of function for channels encompassing KCNE2 and a loss of function for channels encompassing KCNE1. That functional consequences of the mutation are manifested only in presence of KCNE subunits underscores the importance of KCNE subunits for IKs generation. However, perhaps more importantly, it offers a possible molecular explanation for the patient's phenotype. Assuming a heterogenous distribution of KCNE1 and KCNE2 subunits in the heart, where KCNE1 plays a dominant role in ventricular IKs generation and KCNE2 is important for atrial IKs generation, the Q147R mutation may simultaneously lead to loss of function for IKs in the ventricles (QT prolongation) and gain of function for IKs in the atria (atrial fibrillation).
The second clinical implication is that the dynamic nature of IKs channel subunit composition can influence the channel's sensitivity to antiarrhythmic agents. It has long been recognized that KCNE1 association with KV7.1 can dramatically increase its sensitivity to experimental IKs channel blocker (azimilide) or activator (mefenamic acid). On the other hand, KCNE2 association with KV7.1 does not affect its sensitivity to either azimilide (unpublished observation) or mefenamic acid.27
Conclusion
Reasoning that specific current densities may be regulated by dynamically altering the composition of accessory subunits in a particular ion channel complex rather than by regulating plasma membrane expression of the entire complex correlates with the finding that most protein complexes contain entities that are both periodically and constitutively expressed.30 The task of delineating in detail how changes in IKs channel subunit composition influence its sensitivity to clinically used antiarrhythmic agents is important and, just like resolving the heterogeneity in cardiac KCNE protein expression profiles, requires future investigations.
Supplementary Material
Acknowledgments
This work was supported by the Danish National Research Foundation to Drs. Lundby and Schmitt and by the National Heart, Lung, and Blood Institute of the National Institutes of Health Grant RO1-HL67840 to Dr. Tseng.
ABBREVIATIONS
- IKr
cardiac rapid delayed rectifier current
- IKs
cardiac slow delayed rectifier current
- KV
voltage-gated potassium channel
- NMR
nuclear magnetic resonance
- PIP2
phosphatidylinositol 4,5-bisphosphate
Footnotes
Appendix Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.hrthm.2009.12.017.
References
- 1.Charpentier F, Merot J, Loussouarn G, Baro I. Delayed rectifier K(+) currents and cardiac repolarization. J Mol Cell Cardiol. 2010;48:37–44. doi: 10.1016/j.yjmcc.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Lundby A, Ravn LS, Svendsen JH, Olesen SP, Schmitt N. KCNQ1 mutation Q147R is associated with atrial fibrillation and prolonged QT interval. Heart Rhythm. 2007;4:1532–1541. doi: 10.1016/j.hrthm.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 3.Kang C, Tian C, Sonnichsen FD, et al. Structure of KCNE1 and implications for how it modulates the KCNQ1 potassium channel. Biochemistry. 2008;47:7999–8006. doi: 10.1021/bi800875q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCrossan ZA, Abbott GW. The MinK-related peptides. Neuropharmacology. 2004;47:787–821. doi: 10.1016/j.neuropharm.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 5.Gaborit N, Le BS, Szuts V, et al. Regional and tissue specific transcript signatures of ion channel genes in the non-diseased human heart. J Physiol. 2007;582:675–693. doi: 10.1113/jphysiol.2006.126714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu XS, Zhang M, Jiang M, Wu DM, Tseng GN. Probing the interaction between KCNE2 and KCNQ1 in their transmembrane regions. J Membr Biol. 2007;216:117–127. doi: 10.1007/s00232-007-9047-7. [DOI] [PubMed] [Google Scholar]
- 7.Morin TJ, Kobertz WR. Counting membrane-embedded KCNE beta-subunits in functioning K+ channel complexes. Proc Natl Acad Sci U S A. 2008;105:1478–1482. doi: 10.1073/pnas.0710366105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haitin Y, Wiener R, Shaham D, et al. Intracellular domains interactions and gated motions of I(KS) potassium channel subunits. EMBO J. 2009;28:1994–2005. doi: 10.1038/emboj.2009.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiener R, Haitin Y, Shamgar L, et al. The KCNQ1 (Kv7.1) COOH terminus, a multitiered scaffold for subunit assembly and protein interaction. J Biol Chem. 2008;283:5815–5830. doi: 10.1074/jbc.M707541200. [DOI] [PubMed] [Google Scholar]
- 10.Melman YF, Um SY, Krumerman A, Kagan A, McDonald TV. KCNE1 binds to the KCNQ1 pore to regulate potassium channel activity. Neuron. 2004;42:927–937. doi: 10.1016/j.neuron.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Panaghie G, Tai KK, Abbott GW. Interaction of KCNE subunits with the KCNQ1 K+ channel pore. J Physiol. 2006;570:455–467. doi: 10.1113/jphysiol.2005.100644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu X, Jiang M, Hsu KL, Zhang M, Tseng GN. KCNQ1 and KCNE1 in the IKs channel complex make state-dependent contacts in their extracellular domains. J Gen Physiol. 2008;131:589–603. doi: 10.1085/jgp.200809976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung DY, Chan PJ, Bankston JR, et al. Location of KCNE1 relative to KCNQ1 in the I(KS) potassium channel by disulfide cross-linking of substituted cysteines. Proc Natl Acad Sci U S A. 2009;106:743–748. doi: 10.1073/pnas.0811897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakajo K, Kubo Y. KCNE1 and KCNE3 stabilize and/or slow voltage sensing S4 segment of KCNQ1 channel. J Gen Physiol. 2007;130:269–281. doi: 10.1085/jgp.200709805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shamgar L, Haitin Y, Yisharel I, et al. KCNE1 constrains the voltage sensor of Kv7.1 K+ channels. PLoS ONE. 2008;3:e1943. doi: 10.1371/journal.pone.0001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chouabe C, Neyroud N, Richard P, et al. Novel mutations in KvLQT1 that affect Iks activation through interactions with Isk. Cardiovasc Res. 2000;45:971–980. doi: 10.1016/s0008-6363(99)00411-3. [DOI] [PubMed] [Google Scholar]
- 17.Franqueza L, Lin M, Splawski I, Keating MT, Sanguinetti MC. Long QT syndrome-associated mutations in the S4-S5 linker of KvLQT1 potassium chan nels modify gating and interaction with minK subunits. J Biol Chem. 1999;274:21063–21070. doi: 10.1074/jbc.274.30.21063. [DOI] [PubMed] [Google Scholar]
- 18.Restier L, Cheng L, Sanguinetti MC. Mechanisms by which atrial fibrillation-associated mutations in the S1 domain of KCNQ1 slow deactivation of IKs channels. J Physiol. 2008;586:4179–4191. doi: 10.1113/jphysiol.2008.157511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rocheleau JM, Kobertz WR. KCNE peptides differently affect voltage sensor equilibrium and equilibration rates in KCNQ1 K+ channels. J Gen Physiol. 2008;131:59–68. doi: 10.1085/jgp.200709816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seebohm G, Strutz-Seebohm N, Ureche ON, et al. Differential roles of S6 domain hinges in the gating of KCNQ potassium channels. Biophys J. 2006;90:2235–2244. doi: 10.1529/biophysj.105.067165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panaghie G, Abbott GW. The role of S4 charges in voltage-dependent and voltage-independent KCNQ1 potassium channel complexes. J Gen Physiol. 2007;129:121–133. doi: 10.1085/jgp.200609612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melman YF, Domenech A, de la LS, McDonald TV. Structural determinants of KvLQT1 control by the KCNE family of proteins. J Biol Chem. 2001;276:6439–6444. doi: 10.1074/jbc.M010713200. [DOI] [PubMed] [Google Scholar]
- 23.Delpon E, Cordeiro JM, Nunez L, et al. Functional effects of KCNE3 mutation and its role in the development of Brugada syndrome. Circ Arrhythm Electro-physiol. 2008;1:209–218. doi: 10.1161/CIRCEP.107.748103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang M, Zhang M, Tang DG, et al. KCNE2 protein is expressed in ventricles of different species, and changes in its expression contribute to electrical remodeling in diseased hearts. Circulation. 2004;109:1783–1788. doi: 10.1161/01.CIR.0000124225.43852.50. [DOI] [PubMed] [Google Scholar]
- 25.Manderfield LJ, George AL., Jr KCNE4 can co-associate with the I(Ks) (KCNQ1-KCNE1) channel complex. FEBS J. 2008;275:1336–1349. doi: 10.1111/j.1742-4658.2008.06294.x. [DOI] [PubMed] [Google Scholar]
- 26.Morin TJ, Kobertz WR. A derivatized scorpion toxin reveals the functional output of heteromeric KCNQ1-KCNE K+ channel complexes. ACS Chem Biol. 2007;2:469–473. doi: 10.1021/cb700089s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toyoda F, Ueyama H, Ding WG, Matsuura H. Modulation of functional properties of KCNQ1 channel by association of KCNE1 and KCNE2. Biochem Biophys Res Commun. 2006;344:814–820. doi: 10.1016/j.bbrc.2006.03.213. [DOI] [PubMed] [Google Scholar]
- 28.Wu DM, Jiang M, Zhang M, et al. KCNE2 is colocalized with KCNQ1 and KCNE1 in cardiac myocytes and may function as a negative modulator of I(Ks) current amplitude in the heart. Heart Rhythm. 2006;3:1469–1480. doi: 10.1016/j.hrthm.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 29.Jiang M, Xu X, Wang Y, et al. Dynamic partnership between KCNQ1 and KCNE1 and influence on cardiac IKs current amplitude by KCNE2. J Biol Chem. 2009;284:16452–16462. doi: 10.1074/jbc.M808262200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Lichtenberg U, Jensen LJ, Brunak S, Bork P. Dynamic complex formation during the yeast cell cycle. Science. 2005;307:724–727. doi: 10.1126/science.1105103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.