Abstract
An understanding of the anatomic distributions of major neurodegenerative disease lesions is important to appreciate the differential clinical profiles of these disorders and to serve as neuropathological standards for emerging molecular neuroimaging methods. To address these issues, here we present a comparative survey of the topographical distribution of the defining molecular neuropathological lesions among ten neurodegenerative diseases from a large and uniformly assessed brain collection. Ratings of pathological severity in sixteen brain regions from 671 cases with diverse neurodegenerative diseases were summarized and analyzed. These included: a) amyloid-β and tau lesions in Alzheimer’s disease, b) tau lesions in three other tauopathies including Pick’s disease, progressive supranuclear palsy and corticobasal degeneration, c) α-synuclein inclusion ratings in four synucleinopathies including Parkinson’s disease, Parkinson’s disease with dementia, dementia with Lewy bodies and multiple system atrophy, and d) TDP-43 lesions in two TDP-43 proteinopathies, including frontotemporal lobar degeneration associated with TDP-43 and amyotrophic lateral sclerosis. The data presented graphically and topographically confirm and extend previous pathological anatomic descriptions and statistical comparisons highlight the lesion distributions that either overlap or distinguish the diseases in each molecular disease category.
Keywords: Alzheimer’s disease, Pick’s disease, corticobasal degeneration, progressive supranuclear palsy, Parkinson’s disease, Parkinson’s disease dementia, dementia with Lewy bodies, multiple system atrophy, frontotemporal lobar degeneration - TDP, amyotrophic lateral sclerosis, amyloid-β, Tau α-synuclein, TDP-43
INTRODUCTION
The neuropathological diagnosis of major neurodegenerative diseases is based on the presence of microscopic lesions of distinctive morphologies and molecular composition (Dickson et al., 2002; Forman et al., 2002; Gelb et al., 1999; Hughes et al., 2001; Litvan et al., 1996; Mackenzie et al., 2011a; Mackenzie et al., 2011b; Mackenzie et al., 2010; Montine et al., 2012; Neumann et al., 2009; Zhukareva et al., 2002). Clinical diagnosis of these diseases on the other hand is still mostly dependent on the presentation and course of symptoms and signs. These clinical features correlate approximately with the degree to which particular neurodegenerative disease lesions affect the neural systems that underlie the affected brain functions. Differential diagnosis of molecularly distinct diseases in clinical practice is often difficult due to similar symptomatology that is likely due to the overlap in the distribution of the different neuropathologies in the same neural systems among the different neurodegenerative diseases. It is also recognized that distinct clinical phenotypes may arise from molecularly identical disease lesions that occur differentially among different neural systems. In addition, mixed pathologies are common and it can be difficult to attribute the relative contributions of a given neuropathology to the disease’s clinical profile during life (Jellinger and Attems, 2008; Nelson et al., 2012; Schneider et al., 2007; Toledo et al., 2012).
Much has already been described about the topographical distribution of neuropathological lesions within major neurodegenerative diseases. For instance, in 1991 Gary Van Hoesen (to whom this article is dedicated) and colleagues (Arnold et al., 1991) and Heiko and Eva Braak (Braak and Braak, 1991) provided detailed maps of the distribution of tangle and plaque lesions in Alzheimer’s disease (AD), highlighting the widespread, but selective and hierarchical involvement of different regions of cerebral cortex. Later, Thal proposed a staging scheme for the distribution of amyloid-β plaques in AD (Thal et al., 2002). These studies have been instrumental in our understanding of AD and its clinical features and have informed many subsequent neuropathological, neuroimaging and diagnostic studies. Analogous topographical studies have been performed in Lewy body diseases (Braak et al., 2003), frontotemporal degeneration (FTD) variants (Armstrong et al., 2010; Hof et al., 1994b), and amyotrophic lateral sclerosis (ALS)(Geser et al., 2008). However, to our knowledge, there have been no studies that have presented the topographical profiles of neurodegenerative diseases together along with less common though still important neurodegenerative diseases.
With the ongoing development of amyloid-β, tau and α-synuclein targeted ligands as neuroimaging biomarkers that will be used for differential diagnosis, it is important to deepen and broaden understanding of the comparative patterns of molecular lesion distribution among different neurodegenerative diseases characterized by plaques, tangles, Lewy bodies and other inclusions such as those formed by TDP-43 (TAR DNA-binding protein 43). The work presented in this article contributes to a neuropathological lesion distribution “standard” against which neuroimaging distributions might be evaluated. We present a comparative survey of the topographical distribution of the defining molecular neuropathological lesions among major neurodegenerative diseases from a single, large and diverse brain collection in the Perelman School of Medicine at the University of Pennsylvania. The ten diseases we consider include a) the amyloid-β and tau proteinopathy AD; b) the most common tauopathies: Pick’s disease, progressive supranuclear palsy (PSP), and corticobasal degeneration (CBD); c) the major α-synucleinopathies: Parkinson’s disease (PD), Parkinson’s disease with dementia (PDD), dementia with Lewy bodies (DLB), and multiple system atrophy (MSA); and d) the TAR DNA-binding protein 43 (TDP-43) proteinopathies: frontotemporal lobar degeneration (FTLD) associated with TDP-43 (FTLD-TDP) and ALS.
MATERIALS AND METHODS
Study Sample
Demographic, clinical diagnostic, and neuropathological data were obtained from the Perelman School of Medicine’s Integrated Neurodegenerative Disease Database (INDD) (Xie et al., 2011) for all brain autopsy cases conducted by the Center for Neurodegenerative Disease Research (CNDR) from 1998 (when the NIA-Reagan criteria for AD (Hyman and Trojanowski, 1997) were implemented and when a-synuclein was discovered to be the main constituent of Lewy pathology (Spillantini et al., 1997)) through February, 2013. Criteria for inclusion into the topographical analyses included: 1) Neuropathological diagnosis of a single neurodegenerative disease; 2) Standardized ratings of thioflavin-S (ThioS) labeled neuritic plaques and/or amyloid-β immunohistochemical plaque ratings, pathological tau immunoreactive lesions, α-synuclein immunoreactive lesions and TDP-43 immunoreactive lesions; and 3) No secondary neuropathological diagnoses (e.g., second neurodegenerative disease, major strokes, multiple sclerosis, tumor, etc.). Cases with incidental, small lesions such as small or microscopic infarcts or small meningiomas were allowed. Cases with incidental, rare Lewy bodies, ventromedial temporal lobe tangles or senile plaques (which are almost universal in older adults) that did not rise to a threshold of a secondary neuropathological diagnosis according to established criteria were also allowed.
Most cases had been clinically evaluated, followed and referred for autopsy from specialty clinical practices at the University of Pennsylvania Health System, including the Alzheimer’s Disease Center, Parkinson’s Disease and Movement Disorders Center, the Frontotemporal Degeneration Center and the Amyotrophic Lateral Sclerosis Clinic. In addition, 74 cases with suspected FTD variants had been evaluated and referred from the University of California San Francisco Alzheimer’s Disease Center. Informed consent for autopsy had been obtained in all cases from the patients or legal representative in accordance with Pennsylvania and California state laws as well as protocols approved by the Institutional Review Board and Hospital of the University of Pennsylvania.
Neuropathological Assessment
General Tissue Processing
Brain extractions, gross inspections, tissue processing and diagnostic microscopic examinations were conducted by neuropathology fellows and staff in the CNDR as previously described (Arnold et al., 1995; Neumann et al., 2006; M. L. Schmidt et al., 1988). Briefly, after removal and weighing, visual inspection documented any gross abnormalities of the whole brain, meninges, and extracerebral blood vessels, including circle of Willis atherosclerosis. The hindbrain and cerebral hemispheres were separated and coronally sectioned into 1-2 cm slabs for further inspection of any infarctions, tumors or other lesions. Fresh tissues from multiple central nervous system areas were fixed in 10% neutral buffered formalin or 70% ethanol with 150 mM sodium chloride, paraffin embedded, and cut into 6-μm sections. Brains from the University of California San Francisco Alzheimer’s Disease Center had been extracted, immersion fixed in neutral buffered formalin and shipped to the CNDR for inspection and processing as above. Sections were stained with hematoxylin-eosin, thioflavin S and special stains as indicated for establishing diagnosis AD and other neurodegenerative diseases topical to this study according to established criteria and standards of practice (Montine et al., 2012; Neumann et al., 2006; Schmidt et al., 1988; Uryu et al., 2008) as well as to recognize other neuropathological conditions (e.g., neoplasms, infections, Creutzfeldt-Jakob) that were not topical and ineligible for inclusion in this study..
Antibodies and Immunohistochemistry
Antibodies are summarized in Immunohistochemistry was conducted with antibodies directed at amyloid-β, hyperphosphorylated or paired helical filament tau, α-synuclein and TDP-43 and developed using the avidin-biotin complex detection method (VECTASTAIN ABC kit; Vector Laboratories, Burlingame, California) or the BioGenex Super Sensitive MultiLink IHC Detection System Kit (BioGenex Laboratories, San Ramon, California) with 3,3-diaminobenzidine as the chromogen (Table 1) The antibody used for amyloid-β was NAB228, a mouse monoclonal antibody generated in the CNDR against synthetic Aβ1-40 peptide mouse and applied at a dilution of 1:15,000 (Lee et al., 2006). PHFTau was labeled with PHF1, a mouse monoclonal antibody (gift of Peter Davies) raised against detergent-extracted PHF preparations that has been epitope mapped to the region around phosphoserine 396/404 (Greenberg et al., 1992; Otvos et al., 1994) and used at a concentration of 1:1000. α-Synuclein pathology was labeled with a mouse monoclonal antibody Syn303, used at a concentration of 1:4000. Syn303 was generated in the CNDR against oxidized α-synuclein and preferentially recognizes oxidized and/or nitrated aggregated and oligomeric α-synuclein (Giasson et al., 2002). Pathological TDP-43 was stained with a rat antibody to phosphorylated TDP-43 at a concentration of 1:1000 (gift of Dr Manuela Neumann). This was generated against phosphopeptide pS409/410 (SMDSKS(p)S(p)GWG), corresponding to amino acid residues 404-413 of human TDP-43 and phosphorylation of serine residues 409/410 {Neumann, 2009 #9533}. In some cases, alternative but equally sensitive and specific antibodies were used at earlier time periods in the existence of the CNDR brain collection.
Table 1. Antibodies.
| Antibody Name | Immunoqen | Source | Species | Type |
|---|---|---|---|---|
| NAB228 | Synthetic Aβ1-40 peptide | CNDR | Mouse | Monoclonal |
| PHF1 | Paired helical filament extracts, alter characterized as tau phosphorylated around serine residues 396/404 |
Gift of Peter Davies | Mouse | Monoclonal |
| Syn303 | Oxidized α-Synuclein | CNDR | Mouse | Monoclonal |
| pTDP-43 | TDP-43 phosphopeptide (SMDSKS(p)S(p)GWG), corresponding TDP-43 amino acid residues 404-413 phosphorylated at serine residues 409/410 |
Gift of Manuella to Neumann |
Rat | Monoclonal |
Regions of Interest
Sixteen regions were routinely investigated for the molecular disease lesions of interest. These included four limbic cortices: 1) amygdaloid complex (a “corticoid” structure), 2) CA1/subiculum of the body of the hippocampus (allocortex) at the level of the lateral geniculate nucleus, 3) entorhinal cortex (paralimbic periallocortex), and 4) anterior cingulate (paralimbic proisocortex) above the genu of the corpus callosum; three isocortical association areas: 5) superior and mid-temporal gyrus including superior temporal sulcus approximately mid-way along their rostral-caudal axis, 6) mid-frontal gyrus and 7) angular gyrus; three cerebral subcortical nuclear regions: 8) putamen within the dorsal striatum, 9) globus pallidus, and 10) thalamus (principally pulvinar); four brainstem regions: 11) midbrain tectum at the level of the superior colliculus, 12) midbrain substantia nigra, 13) pons (excluding locus ceruleus), 14) locus ceruleus and 15) medulla; and finally, 16) cerebellum including dentate nucleus. In ALS cases only, sufficient data was also available for analysis of primary motor cortex as well.
Assessment and Neuropathology Ratings
Analyses used the data from all stained sections within each disease that were graded with a global score for the neuropathological lesions of interest. In AD, this included plaque pathology (as identified with thioflavin S staining and amyloid-β immunohistochemistry), and neurofibrillary tangle and thread/neuritic pathology (as identified with tau immunohistochemistry). In tauopathies, this included tau neuronal and/or glial intracytoplasmic inclusions of all types (tangles, Pick bodies, etc.), neuropil threads and dystrophic neuritic and glial processes. In α-synucleinopathies, this included α-synuclein-immunoreactive Lewy pathology (Lewy bodies and neurites), dots, axonal spheroids and/or oligodendroglial cytoplasmic inclusions. In the TDP-43 proteinopathies, TDP-43 neuropathological lesions included intracytoplasmic and nuclear inclusions of various types, granular or diffuse intracytoplasmic immunoreactivity, and neuritic immunoreactivity. The global burden of pathological lesions was semi-quantitatively graded on a four-point scale in each of the sixteen regions by experienced neuropathologists and neuropathology fellows under the supervision of a single senior neuropathologist (JQT) until 2011 when a second neuropathology faculty (EBL) joined this effort (0 = none, 1 = rare/mild, 2 = occasional/moderate, 3 = numerous/severe). We confirmed the reliability and accuracy of such a grading paradigm for plaque and tangle lesions in the CNDR collection in a recent study in which we re-assessed a representative sample of sections from 150 brains using a computer-assisted quantitative method (Yarchoan et al., 2012). This found high associations between ordinal ratings and % area measures of amyloid-β plaques (r=0.83, p<0.0001) and density counts of neurofibrillary tangles (r=0.91, p<0.0001).
Descriptive and Statistical Analysis and Topographical Heat Mapping
For graphical display of regional lesion severity among specific disease groups within a molecular pathological category neuropathology scores for each section were averaged and standard deviations were calculated. For topographical display, these average grades were entered into a custom-designed brain heat map template that plotted the values in measured regions of interest and extrapolated them into proximal and functionally similar cortical and subcortical regions that were not sampled. The heat map is composed of two sagittal templates (showing a lateral view of the brain convexity and medial section at the longitudinal cerebral fissure), two coronal sections (representing sections at the anterior commissure and the mammillary bodies), a coronal section of the medial temporal lobe at the level of the lateral geniculate body and axial sections of the midbrain (at the level of the SN and red nucleus), pons (at the level of the locus coeruleus) and the medulla oblongata (at the level of the inferior olive). The sampled sections and their corresponding staining procedures are detailed above and elsewhere (Toledo et al., 2013). The scores that were mapped to the template were the mean values of the semi-quantitative scores described above for each area in each disease group and each stain, excluding cases with missing values.
For statistical analyses comparing different diseases (e.g., PSP, CBD) within a molecular disease group (e.g., tauopathies), the 16 brain regions were collapsed into 5 categories according to their functional neuroanatomic system, as follows: a. Limbic Cortices = amygdala, CA1/subiculum of hippocampus, entorhinal cortex and anterior cingulate; b. Isocortical Association Cortices = superior and mid-temporal gyri including superior temporal sulcus, mid-frontal gyrus and angular gyrus; c. Subcortical Nuclei = caudate/putamen, globus pallidus and thalamus/subthalmus; d. Brainstem = midbrain, substantia nigra, pons, locus ceruleus and medulla; e. Cerebellum = cerebellar cortex and dentate nucleus. For each case, an average pathology score for each neuroanatomic category was calculated by taking the mean of the raw scores (0 to 3) of each of the regions that comprise each category.
A repeated measure ANOVA was used to model the mean pathology scores, using disease group, neuroanatomic category, and their interaction as predictors. An additional covariate adjusted for in the model was patient age at death. Repeated measure ANOVA accounts for the correlations of the pathology scores from different brain regions within the same individual. Each molecular pathology type (ThioS and amyloid-β, tau, α-synuclein and TDP-43) was modeled separately.
Estimated mean differences between disease groups for each brain area category were compared via 2-sample t-tests on the predicted scores obtained from the models. All statistical tests were two-sided. Because we conducted many statistical tests, we used significance level of 0.01 to reduce the likelihood of false positive discovery findings.
All analyses were conducted using SAS version 9.3 (SAS Institute, Inc). Illustrative photomicrographs in Figures 1-4 were captured on an Olympus BX61 microscope, DP71 camera and DP software (Olympus America, Inc). Photomicrographs were captured as TIFF files and cropped and finished with auto contrast/brightness adjustment in Adobe Photoshop CS4 v11.0.2 (Adobe Systems, Inc.) prior to figure assembly with graphs and heatmap templates in Microsoft Powerpoint 2008 for Mac v12.2.6 (Microsoft Corporation).
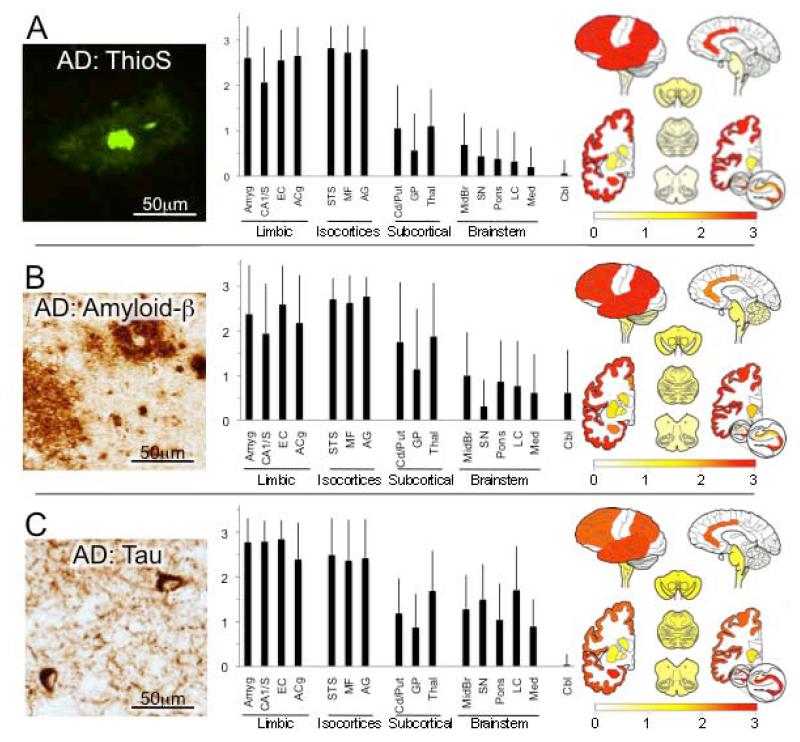
Figure 1.
Alzheimer’s disease (AD). (A) Thioflavin S (ThioS)-stained amyloid plaques in mid-frontal gyrus. Photomicrograph shows classical “dense core” plaque with intensely fluorescent amyloid core surrounded by more diffuse amyloid halo. Bar graph depicts average (with standard deviations) ThioS plaque ratings in each of the 16 regions of interest, grouped into neuroanatomic categories (limbic cortices, association isocortices, subcortical nuclei, brainstem and cerebellum). At right, these ratings were projected onto brain mapping template with the rating values indicated in the color bar gradient. (B) Amyloid-β immunohistochemistry. Photomicrograph from mid-frontal gyrus cortex shows major types of plaques - dense cored (upper right) and diffuse (lower left) as well as other scattered amyloid-β deposits. Overall, amyloid-β immunohistochemistry identifies plaque pathology more sensitively than ThioS, as evident in the relatively higher ratings in subcortical nuclei, brainstem and cerebellum. (C) Tau immunohistochemistry. Photomicrograph of angular gyrus shows two neurofibrillary tangles as well as reticular and thread-like neuritic pathology. In graph and map, note very high and consistent tau ratings in limbic cortices but also widespread but more variable involvement throughout brain, including rarely the cerebellum. Abbreviations in graph from left to right: Amyg = amygdala, CA1/S = CA1 and subiculum subfields of hippocampal formation, EC = entorhinal cortex, ACg = anterior cingulate gyrus, STS = superior temporal sulcus including portions of superior and middle temporal gyri, MF = mid-frontal gyrus, AG = angular gyrus, Cd/Put = caudate and putamen, GP = globus pallidus, Thal = thalamus and subthalamus, Midbr = midbrain, SN = substantia nigra, Pons = pons, LC = locus ceruleus, Med = medulla, Cbl = cerebellum.
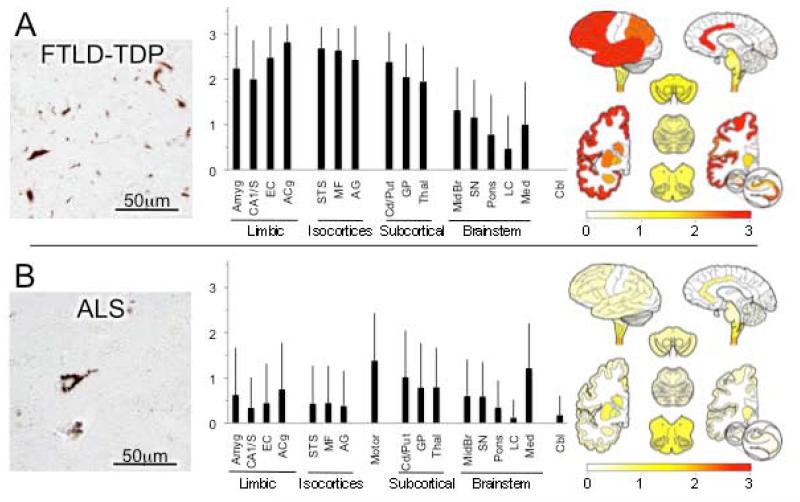
Figure 4.
TDP-43 proteinopathies.* (A) Frontotemporal lobar degeneration associated with TDP-43 (FTDL-TDP). Neuronal and neuritic pathological TDP-43 inclusions of various morphologies (photomicrograph) severely affect limbic and isocortical association cortices as well as subcortical nuclei, more so than brain stem. TDP-43 lesions were not observed in cerebellum in any case. (B) Amyotrophic lateral sclerosis (ALS). Photomicrograph shows neuron with diffuse perikaryal TDP-43 immunoreactivity. Graph and map depict widespread but generally mild lesion ratings throughout the brain in ALS with exception of higher ratings motor cortex and lower brainstem. In addition, cervical spinal cord was commonly examined in ALS cases and showed high ratings (data not shown). *Both photomicrographs are from mid-frontal gyrus.
RESULTS
Data from 671 cases were included in this study from the 1032 completed autopsies accrued by the CNDR from January 1998 through February 2013. 260 “mixed” cases were excluded because they had more than one neurodegenerative disease diagnosis, 71 cases were unremarkable (“normal”) without abnormalities qualifying for a neuropathological diagnosis, 23 tauopathy cases were excluded because they were rare or could not be classified into a specific disease diagnosis and 21 cases were excluded because of non-neurodegenerative or very rare disease diagnoses (e.g., neoplasms, Creutzfeldt-Jakob disease). Of the mixed cases, most exhibited co-morbid AD. This was especially so for Lewy body diseases for which we excluded 81 cases with DLB + AD, 42 cases with PDD + AD, and 5 cases with PD (no dementia) + AD, and 3 cases of MSA + AD. The excluded tauopathy cases consisted of 10 unclassifiable, 5 FTD with parkinsonism associated with chromosome-17, 4 argyrophilic grain disease, and 4 tangle-predominant senile dementia. For statistical analyses and topographical mapping, only cases with complete data from the lesions of interest were used.
Demographic and diagnostic data of the cases with single neurodegenerative disease diagnoses are presented in Table 1. Statistical comparisons among diagnostic categories were significant for age (ANOVA F= 34.0, p<0.0001) with post hoc t-test comparisons finding AD significantly older than normal, tauopathy and TDP-43 groups (all p<0.0001) but not synucleinopathies, and synucleinopathies significantly older than the TDP-43 group (P<0.0001) but not normal or tauopathy groups (p>0.01). There were no significant differences among categories for postmortem interval (F= 2.1, p=0.08). Brain weights varied among categories for both females (F=12.1, p<0.0001) and males (F=12.0, p<0.0001). In individual category posthoc t-test comparisons for females, brain weights in AD were significantly less than in normal, synucleinopathy and TDP43 categories (all p<0.0001) but not tauopathies (p=0.02). Differences between tauopathies, synucleinopathies and TDP-43 proteinopathies in females were not significant (all p>0.01). In males, AD brain weights were less than in normals, synucleinopathies and TDP-43 proteinopathies (all p<0.0001), but not tauopathies (p=0.90). Tauopathy brain weights in males were significantly less than normals (p=0.0002), synucleinopathies (p<0.0001) and TDP-43 proteinopathies (p=0.002). There were no significant differences in male brain weights between normals, synucleinopathies and TDP-43 proteinopathies (all p>0.01).
Alzheimer’s disease
Plaques of all morphologies were globally graded in sections labeled with thioflavin S in all cases (n = 270 with complete data, Figure 1A) and with amyloid-β immunohistochemistry in a subset of cases (n = 16, since this labeling was routinely implemented in 2011, Figure 1B). Highest and most consistent scores were present in the isocortical association cortices, followed more variably by limbic cortical regions in the ventromedial temporal lobe and cingulate gyrus. Modest plaque scores were also observed in the striatum and thalamus, and lower levels and less frequent plaques were present in brainstem and cerebellum.
For tau immunoreactive neurofibrillary tangles and neurites, scores in AD cases (n =270) were uniformly highest in the limbic cortices, followed by isocortical association regions (Figure 1C). Moderate densities of tau pathology were recorded in cerebral subcortical and brain stem regions with minimal involvement of the cervical spinal cord and cerebellum.
Tauopathies
Data from the three major tauopathies in the CNDR collection (other than AD) are presented in Figure 2.
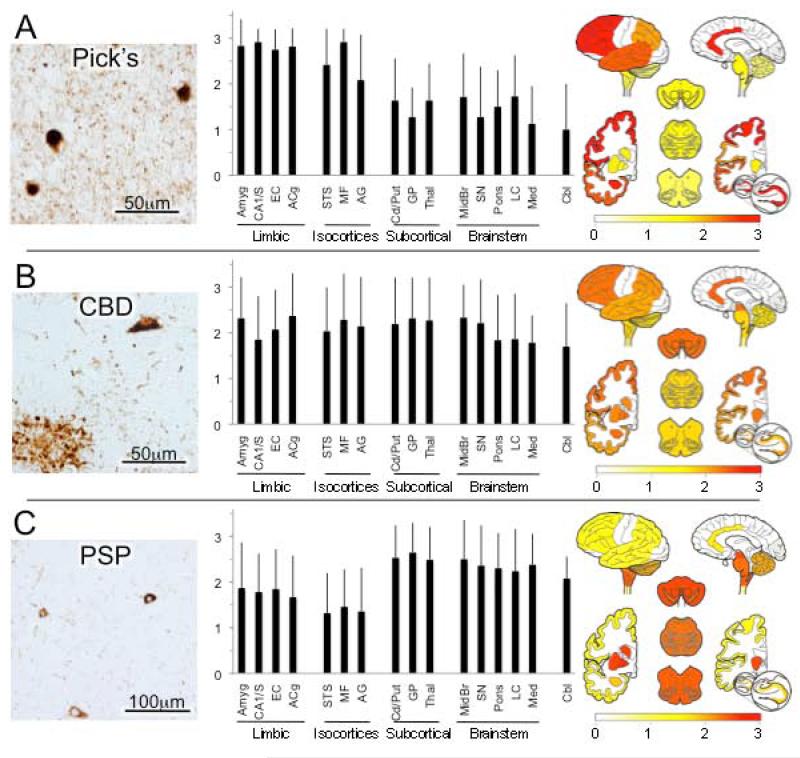
Figure 2.
Tauopathies.* (A) Pick’s disease. Photomicrograph of shows 3 Pick bodies as well as diffuse neuritic pathology in background. As shown in the graph and map, tau pathology is greatest in limbic and isocortical association areas, but can also be found throughout the brain. (B) Corticobasal degeneration (CBD). Photomicrograph shows tau-immunoreactive tangle-line neuronal inclusion (upper right) and astrocytic “plaque” (lower left) as well as scattered immunoreactive neuritic/glial processes. As depicted in the graph and map, tau pathology is widely distributed. (C) Progressive supranuclear palsy (PSP). Global ratings were assigned based on abnormal tau inclusions in glia and neurons as well as glial and neuronal processes. In contrast to Pick’s disease and AD, graphs portray the predominant subcortical nuclei and hindbrain pathology of PSP. *All photomicrographs are from mid-frontal gyrus.
Pick’s disease cases (n = 12) had very high and consistent scores for cellular and neuritic tau lesions in the limbic cortices and frontal association cortex, and to a slightly lesser and more variable degree in the temporal and parietal association cortices (Figure 2A). Modest scores of tau pathology were also commonly noted in subcortical and brainstem regions and in cerebellum too.
In CBD (n = 28), we found that average ratings of tau pathology were generally moderate, variable across cases and very widespread, with little differentiation in scores among cortical, subcortical, brain stem and cerebellar regions (Figure 2B).
For PSP (n = 50), highest ratings of tau pathologies were recorded in the basal ganglia and thalamus and all regions of the brain stem, and the cerebellum (Figure 2C).
There were moderate scores in the limbic and isocortical association areas, largely due to the abundant white matter tau pathology and especially neuritic tau pathology.
To compare the four major tauopathies (including AD), we collapsed the scores within individual regions into functionally relevant neuroanatomic categories as described above. Neuroanatomic category differences for tau pathology scores among the four tauopathies were statistically assessed using repeated measures ANOVA. Results are presented in Table 2. In the limbic cortices, the mean tau pathology scores of all four diseases were significantly different from each other, with the exception of AD and Pick’s disease, where the scores were highest, but showed no real difference. In the isocortical association cortices, scores for patients with PSP were significantly lower than for all the other diseases, but there were no statistically significant differences between scores among AD, CBD, and Pick’s disease patients. In contrast to the first two categories, in the brainstem and the area including the basal ganglia and thalamus regions, scores among PSP patients were significantly higher than those of the other 3 diseases. CBD scores were also higher than those among Pick’s disease patients, but these differences did not reach the level of statistical significance once we adjusted for multiple comparisons. Within the cerebellum, scores from all four diseases were significantly different from each other; PSP scores were the highest on average, followed by scores for CBD, then Pick’s disease, while AD scores were lowest, with very few AD patients having non-zero cerebellum scores.
Table 2. Case Information.
| Female Brain | Male Brain | |||||
|---|---|---|---|---|---|---|
| n | Age at Death (SD) | % Female | PMI hrs (SD) | Weight, grams (SD) | Weight, grams (SD) | |
| Alzheimer’s Disease | 271 | 76.9 (11.5) | 57.4 | 12.6 (7.6) | 1045 (133) | 1210 (155) |
| Tauopathies | ||||||
| Pick’s | 12 | 65.1 (13.9) | 41.7 | 19.2 (16.3) | 974 (110) | 1056 (211) |
| PSP | 53 | 75.2 (7.4) | 35.9 | 15.9 (13.3) | 1126 (125) | 1255 (262) |
| CBD | 28 | 66.0 (9.3) | 60.7 | 11.4 (7.0) | 1121 (131) | 1172 (121) |
| Synucleinopathies | ||||||
| PD | 60 | 77.2 (9.1) | 23.3 | 15 (12.7) | 1193 (106) | 1338 (113) |
| PDD | 41 | 77.1 (8.2) | 12.2 | 10.8 (6.6) | 1080 (99) | 1357 (141) |
| DLB | 8 | 77.1 (6.0) | 25.0 | 10.8 (6.2) | 1177 (173) | 1148 (574) |
| MSA | 36 | 65.7 (8.8) | 33.3 | 16.4 (9.0) | 1216 (58) | 1397 (167) |
| TDP-43 Proteinopathies | ||||||
| FTLD-TDP | 63 | 67.6 (10.5) | 49.2 | 12.4 (8.2) | 1020 (180) | 1181 (228) |
| ALS | 99 | 61.8 (10.2) | 41.4 | 12.7 (7.8) | 1258 (125) | 1393 (126) |
α-Synucleinopathies
Data from the four major synucleinopathies in the CNDR collection are presented in Figure 3.
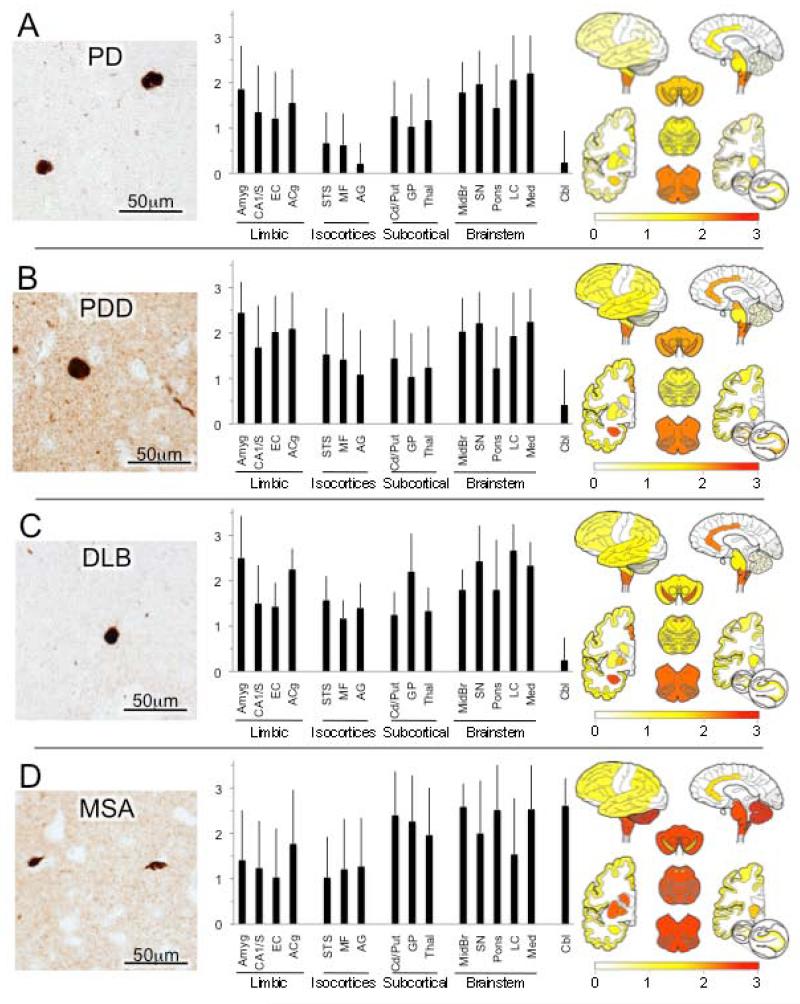
Figure 3.
α-Synucleinopathies.* (A) Parkinson’s disease (PD). While predominant in brainstem, Lewy pathology is often found in subcortical nuclei and cortex, especially limbic cortex. Photomicrograph shows Lewy bodies in mid-frontal gyrus. (B) Parkinson’s Disease Dementia (PDD). α-Synuclein pathology in the form of intraneuronal Lewy bodies and dystrophic Lewy neurites (photomicrograph shows mid-frontal cortex) is greater and more widely disseminated, especially in cerebral cortex in PDD. (C) Dementia with Lewy bodies (DLB) shows virtually identical pathology and distribution pattern to PDD. (D) Multiple system atrophy (MSA). The pathological α-synuclein lesions that predominate in MSA are oligodendrocyte glial cell inclusions (GCIs, photomicrograph) that distinguish MSA from PD, PDD and DLB. GCIs as well as some Lewy-like neuronal pathology are widespread in MSA but predominate in hindbrain and subcortical nuclei. *All photomicrographs are from mid-frontal gyrus.
In our series of PD cases without known dementia, Lewy pathology (n = 50) was scored as moderate-high in the substantia nigra, midbrain, locus ceruleus and medulla as expected, but moderate pathology was also evident in amygdala and limbic cortices as well as the caudate/putamen, globus pallidus and thalamus (Figure 3A). Mild and occasional Lewy pathology was scored in the association isocortices. Compared to PD without dementia, PDD cases (n = 32) had generally greater ratings of Lewy pathology in all brain regions, but particularly in limbic and isocortical association cortices, where the differences were statistically significant (Figure 3B, Table 3). As expected, regional scores for Lewy pathology were very similar in DLB (n = 8) and PDD (Figure 3C). In contrast, MSA (n = 33) showed moderate and high scores consistently in the brain stem and cerebellum as well as in the cerebral subcortical nuclei with modestly lower scores in limbic and isocortical association areas (Figure 3D).
Table 3. Mean Differences and Significance in Tau Pathology Ratings Among Tauopathies.
| a. Limbic Cortices | |||
| Comparison | CBD | AD | Pick’s |
| PSP vs. | Mean Diff: −0.30 Pval: 0.0149 | Mean Diff: −0.91 Pval: <0.0001 | Mean Diff: −1.01 Pval: <0.0001 |
| Pick’s vs. | Mean Diff: 0.71 Pval: 0.0001 | Mean Diff: 0.10 Pval: 0.5069 | |
| AD vs. | Mean Diff: 0.61 Pval: <0.0001 | ||
| b. Isocortical Association Cortices | |||
| Comparison | CBD | AD | Pick’s |
| PSP vs. | Mean Diff: −1.13 Pval: <0.0001 | Mean Diff: −0.83 Pval: <0.0001 | Mean Diff: −1.14 Pval: <0.0001 |
| Pick’s vs. | Mean Diff: 0.31 Pval: 0.2625 | Mean Diff: 0.02 Pval: 0.9442 | |
| AD vs. | Mean Diff: 0.30 Pval: 0.0674 | ||
| c. Subcortical Nuclei | |||
| Comparison | CBD | AD | Pick’s |
| PSP vs. | Mean Diff: 0.43 Pval: 0.0138 | Mean Diff: 1.25 Pval: <0.0001 | Mean Diff: 1.00 Pval: <0.0001 |
| Pick’s vs. | Mean Diff: −0.57 Pval : 0.0255 | Mean Diff: 0.25 Pval: 0.2597 | |
| AD vs. | Mean Diff: −0.82 Pval: <0.0001 | ||
| d. Brainstem | |||
| Comparison | CBD | AD | Pick’s |
| PSP vs. | Mean Diff: 0.47 Pval: 0.0008 | Mean Diff: 1.06 Pval: <0.0001 | Mean Diff: 0.95 Pval: <0.0001 |
| Pick’s vs. | Mean Diff: −0.48 Pval: 0.0182 | Mean Diff: 0.11 Pval: 0.5311 | |
| AD vs. | Mean Diff: −0.59 Pval: <0.0001 | ||
| e. Cerebellum | |||
| Comparison | CBD | AD | Pick’s |
| PSP vs. | Mean Diff: 0.44 Pval: 0.0029 | Mean Diff: 2.05 Pval: <0.0001 | Mean Diff: 1.12 Pval: <0.0001 |
| Pick’s vs. | Mean Diff: −0.69 Pval: 0.0014 | Mean Diff: 0.93 Pval: <0.0001 | |
| AD vs. | Mean Diff: −1.62 Pval: <0.0001 |
Note: Individual regions scores were grouped and averaged according functional neuroantomic system categories: a. Limbic Cortices = amygdala, CA1/subiculum of hippocampus, entorhinal cortex and anterior cingulate; b. Isocortical Association Cortices = superior and mid-temporal gyri including superior temporal sulcus, mid-frontal gyrus and angular gyrus; c. Subcortical Nuclei = caudate/putamen, globus pallidus and thalamus/subthalmus; d. Brainstem = midbrain, substantia nigra, pons, locus ceruleus and medulla; e. Cerebellum = cerebellar hemisphere cortex and dentate nucleus.
As above, synuclein pathology within the five neuroanatomic groupings above was compared among the four major synucleinopathies (Table 3). Multiple system atrophy was especially distinguished by its significantly greater basal ganglia and cerebellar pathology as compared to that in all three Lewy body diseases; all of these comparisons, other than MSA vs. DLB in basal ganglia, were highly statistically significant. PD was especially distinguished from both PDD and DLB by its much lower limbic and association isocortical scores, although the difference versus DLB was not statistically significant in the limbic cortices. Scores among PDD patients were significantly higher than those in MSA patients in these regions, however. In the brainstem area, none of the scores differed from the others enough to warrant statistical significance.
TDP-43 Proteinopathies
Data from the two principal TDP-43 diseases are presented in Figure 4.
TDP-43 scores in FTLD-TDP (n = 22 data, Figure 4A) were highest in isocortical association and limbic cortices areas, but also striatum, globus pallidus and thalamus while brainstem scores were generally low. No TDP-43 lesions were detected in cerebellum in any FTLD-TDP case.
In ALS (n = 56, Figure 4B), TDP-43 lesion scores were highest in medulla, and also primary motor cortex (which was only evaluated in ALS cases), to a lesser degree in basal ganglia and thalamus and while they were frequently detected in limbic and association isocortical regions, the density scores were low.
Statistical comparison of FTLD-TDP’s and ALS’s TDP-43 lesion scores found significantly higher average scores in limbic and isocortical association cortices, basal ganglia and thalamus among FTLD-TDP patients as compared to ALS patients, but this relationship did not hold in the brainstem or cerebellum (Table 4).
Table 4. Mean Differences and Significance Values in α-Synuclein Pathology Ratings Among Synucleinopathies.
| a. Limbic Cortices | |||
| Comparison | MSA | DLB | PDD |
| PD vs. | Mean Diff: 0.15 Pval: 0.3894 | Mean Diff: −0.52 Pval: 0.0779 | Mean Diff: −0.59 Pval: 0.0003 |
| PDD vs. | Mean Diff: 0.75 Pval: 0.0002 | Mean Diff: 0.07 Pval: 0.8294 | |
| DLB vs. | Mean Diff: 0.68 Pval: 0.0324 | ||
| b. Isocortical Association Cortices | |||
| Comparison | MSA | DLB | PDD |
| PD vs. | Mean Diff: −0.66 Pval: 0.0003 | Mean Diff: −0.85 Pval: 0.0039 | Mean Diff: −0.75 Pval: <0.0001 |
| PDD vs. | Mean Diff: 0.09 Pval: 0.6570 | Mean Diff: −0.1 Pval: 0.7348 | |
| DLB vs. | Mean Diff: 0.19 Pval: 0.5464 | ||
| c. Subcortical Nuclei | |||
| Comparison | MSA | DLB | PDD |
| PD vs. | Mean Diff: −1.07 Pval: <0.0001 | Mean Diff: −0.65 Pval: 0.0501 | Mean Diff: −0.23 Pval: 0.1933 |
| PDD vs. | Mean Diff: −0.83 Pval: 0.0001 | Mean Diff: −0.42 Pval: 0.2172 | |
| DLB vs. | Mean Diff: −0.42 Pval: 0.2325 | ||
| d. Brainstem | |||
| Comparison | MSA | DLB | PDD |
| PD vs. | Mean Diff: −0.27 Pval: 0.1196 | Mean Diff: −0.38 Pval: 0.1935 | Mean Diff: −0.15 Pval: 0.3490 |
| PDD vs. | Mean Diff: −0.13 Pval: 0.4936 | Mean Diff: −0.23 Pval: 0.4369 | |
| DLB vs. | Mean Diff: 0.10 Pval: 0.7405 | ||
| e. Cerebllum | |||
| Comparison | MSA | DLB | PDD |
| PD vs. | Mean Diff: −2.27 Pval: <0.0001 | Mean Diff: −0.27 Pval: 0.4487 | Mean Diff: −0.26 Pval: 0.1822 |
| PDD vs. | Mean Diff: −2.01 Pval: <0.0001 | Mean Diff: −0.01 Pval: 0.9773 | |
| DLB vs. | Mean Diff: −1.99 Pval: <0.0001 |
Note: per legend of Table 2.
DISCUSSION
Appreciation of the similarities and differences in the topographical distribution of neurodegenerative disease lesions is important for clinicopathological correlation, radiological-pathological correlation in the emerging era of molecular neuroimaging, and for our pathophysiological understanding of the major neurodegenerative diseases.
Additionally, as our understanding of brain-behavior relationships grows in specificity with regard to local and network level interactions, careful description of the distinctions (and overlap) in the topography of neurodegeneration across diseases will further elucidate the cognitive and functional consequences of these conditions. Here, we described and compared the ten principal neurodegenerative diseases using data from a large and uniformly processed neurodegenerative disease brain collection.
Alzheimer’s disease
AD is the most common neurodegenerative dementia, accounting for 60-80% of all dementias (Alzheimer’s Association, 2013). Early in its course, episodic memory impairment is usually the sole or predominant symptom, indicative of ventromedial temporal lobe dysfunction. This is followed by progressive amnesia and deterioration in other cognitive domains, reflecting pathological involvement of more widespread neural systems.
AD is definitively diagnosed with neuropathological examination that finds abundant senile plaques and neurofibrillary tangles. Molecularly, AD is both an amyloidopathy and a tauopathy. Senile plaques (Figure 1A) are extracellular deposits of amyloid-β peptides of various lengths, polymeric fibrillizations, C- or N- terminal truncations, and post-translational modifications. These variations along with the neural milieu presumably give rise to the diversity of plaque morphologies observed (Dickson, 1997; Thal et al., 2002; Walsh and Teplow, 2012). When deposits occur in association with tau containing dystrophic axons and/or dendrites, they are called neuritic plaques and these are the types of plaques most associated with neural injury in AD. Neurofibrillary tangles (Figure 1B) are intraneuronal cytoplasmic (or extra-neuronal after cell death) aggregates chiefly composed of abnormally phosphorylated tau protein assembled into paired helical filaments (PHFtau). PHFtau also accumulates in axons and dendrites to form neuropil threads, dystrophic neurites and neuritic plaques just noted (Alafuzoff et al., 2008).
Our data are consistent with other studies that have mapped the distribution of tangle and plaque pathology in AD (Arnold et al., 1991; Braak and Braak, 1991; Braak et al., 1998; Hyman et al., 1990; Thal et al., 2002). For tau immunoreactive neurofibrillary tangles and neurites, scores were uniformly highest in the limbic cortices, followed by isocortical association regions and then subcortical regions. The distribution of tau pathology has been considered to be particularly distinctive and predictable, giving rise to the well-known staging system of Braak and Braak (Braak and Braak, 1991) in which tau pathology is first apparent in the perirhinal and entorhinal cortices and subsequently “spreads” into select subfields of the hippocampus, limbic subcortical structures (basal ganglia, thalamus) and then out to isocortical association areas (but much less so, primary motor or sensory areas) with more advanced disease. The distribution and severity of tau pathology in AD also correlates with the severity of dementia in AD (Arriagada et al., 1992; Bennett et al., 2004; Giannakopoulos et al., 2003)
Amyloid-β plaques (of all types) were uniformly abundant in all isocortical association areas measured and somewhat less so in limbic regions. This pattern stands in contrast to that of tau lesions, which are greatest in limbic cortices. These observations are also consistent with previous topographical staging studies indicating earliest and most abundant amyloid-β plaque deposition in isocortices followed by limbic allocortical regions, cerebral subcortical nuclei and then hindbrain (Arnold et al., 1991; Thal et al., 2002).
Tauopathies
Pick’s disease typically presents clinically as a frontotemporal dementia with impairment of social and executive behaviors and/or a primary progressive non-fluent aphasia, although AD-like amnestic presentations also occur. It is considered the second most common cause of FTLD, after FTLD-TDP (Wider and Wszolek, 2008). At autopsy, the brain is classically noted to exhibit circumscribed lobar atrophy, neuronal loss and gliosis that particularly affect the prefrontal and anterior temporal lobes. Microscopically, it is distinguished by the presence of round, argyrophilic intraneuronal cytoplasmic inclusions, Pick bodies, which are composed of abnormally phosphorylated tau filaments enriched in 3 repeat (3R) tau isoforms as well as varying degrees of 4R tau and other cytoplasmic proteins (King et al., 2001; Probst et al., 1996; Zhukareva et al., 2002). To lesser degrees, Pick’s disease shows tau-immunoreactive glial inclusions, including oligodendroglia and astrocytes. Consistent with its clinical profile as a frontotemporal dementia, and also with its previously reported distribution (Hof et al., 1994a; Yoshimura, 1989) we found very high scores for cellular and neuritic tau lesions in the limbic cortices and frontal association cortex, and to a slightly lesser degree, the temporal and parietal association cortices of Pick’s disease cases. We also found modest scores of tau pathology in subcortical and brainstem regions and in cerebellum too, regions less commonly considered as affected in Pick’s disease.
CBD is associated with diverse clinical presentations, including progressive asymmetrical ideomotor apraxia, rigidity and parkinsonism, and alien limb, as well as varying frequency and severity of aphasia and behavioral-frontal-dysexecutive syndrome (Armstrong et al., 2013). Neuropathologically, CBD is characterized by abundant abnormally phosphorylated 4R tau in astroglial and neuronal processes (Dickson et al., 2002). Astrocytic “plaques” are distinctive (Feany and Dickson, 1995). Tau-immunoreactive granular cytoplasm or tangle-like or Pick body-like inclusions may be observed in neurons. In our survey we found that average ratings of tau pathology were moderate and very widespread, with little differentiation in scores among cortical, subcortical, brain stem and cerebellar regions.
PSP is clinically characterized by progressive axial rigidity, falls, vertical gaze palsy, bulbar signs and varying degrees of dementia, often with prominent frontal-executive dysfunction. Filamentous tau lesions, particularly enriched in 4R tau are found in astroglia as tufted astrocytes in gray and white matter, oligodendroglial coiled bodies in white matter, and globose-like neurofibrillary tangles in neurons. Consistently affected neuroanatomic regions in PSP include the caudate, putamen and globus pallidus, subthalamus, substantia nigra and pons (Hauw et al., 1994) with varying degrees of cortical and cerebellar involvement. In our survey, the highest ratings of tau pathologies also were recorded in the basal ganglia and thalamus and all regions of the brain stem, and the cerebellum with lower grades and more variable grades of pathology in limbic cortices and isocortical regions.
In summary, we found that the topographical distribution of tau pathology was most similar for AD and Pick’s disease among the tauopathies, consistent with the frequent difficulty in clinically differentiating these two disorders, particularly in more advanced disease. In CBD, we found ratings of pathology to be more evenly distributed across regions, likely reflecting an averaging of the diversity of involvement (and commensurate symptoms) that occurs in this disorder. PSP’s preferentially severe involvement of subcortical, brainstem and cerebellum regions is consistent with the prominent motor tone, oculomotor, facial, bulbar and balance dysfunction that clinically distinguish the disorder.
Synucleinopathies
Sporadic PD is the second most common neurodegenerative disease after AD. It is clinically characterized by varying degrees of bradykinesia, rigidity, tremor, postural instability and gait disorder which highlight the involvement of the nigrostriatal system (Gelb et al., 1999; Hughes et al., 2001) Additional symptoms and signs of autonomic, cognitive, neuropsychiatric, sensory, and sleep dysfunction invoke multifocal involvement of other subcortical, cortical and peripheral neural systems. The defining neuropathology of PD is moderate to severe neuron loss in the substantia nigra pars compacta accompanied by the presence of phosphorylated α-synuclein containing Lewy pathology (Dickson et al., 2009). Lewy bodies are the classical eosinophilc spherical inclusions in neuronal perikarya and are intensely immunoreactive for α-synuclein (Baba et al., 1998). Other pleomorphic α-synuclein inclusions may be found in neuronal perikarya as well as in axons and dendrites, including intraneuritic Lewy bodies, dots, dystrophic-appearing neurites and axonal spheroids (Dickson et al., 2009). In our series of PD cases without known dementia, Lewy pathology was greatest in the substantia nigra and other brain stem regions as expected, but moderate pathology was also evident in limbic cortices as well as the basal ganglia and thalamus, while association isocortices exhibited some, but relatively low levels.
While mild cognitive dysfunction is almost universal in PD, perhaps 30-78% of clinical PD patients progress to significant cognition-based functional decline, especially in later stages of the disease (Aarsland et al., 2003; Aarsland et al., 2005). Parkinson’s disease with dementia (PDD) is the term used when dementia emerges in people subsequent to established parkinsonism (Emre et al., 2007). In neuropathological studies, we and others have found that comorbid AD is very common in PDD (Irwin et al., 2012). However, dementia in PD also occurs in the absence of AD when limbic and cortical Lewy pathology is extensive (Compta et al., 2011; Hurtig et al., 2000; Sabbagh et al., 2009). Here, we focused only on PDD patients whose co-existent AD pathology did not rise to a threshold at which a second diagnosis of AD was made. Compared to PD (without dementia), the PDD group had generally greater ratings of Lewy pathology in all brain regions, but particularly in limbic and isocortical association cortices.
DLB is a condition in which dementia emerges first as the predominant clinical feature, with relatively mild or no accompanying motor dysfunction. Other salient features frequently include visual hallucinations, fluctuating levels of alertness, sleep disturbances and other features of PD. The parkinsonism of DLB often worsens with time so that other than the temporal sequence of cognitive and motor symptoms, there may be little to distinguish DLB and PDD (Lippa et al., 2007). Most cases of DLB in our collection were excluded for this survey analysis because they had co-morbidities.
Indeed, out of 89 cases with a primary neuropathological diagnosis of DLB, only 8 had “pure” DLB while 81 had AD as well. Neuropathologically, the Lewy pathologies in both pure DLB and PDD (without AD) are identical. In accord with this, our survey also found little difference, with regional scores for Lewy pathology virtually identical in DLB and PDD, with the exception of greater globus pallidus Lewy pathology in DLB compared to PDD or PD.
MSA is a neuropathologically distinct synucleinopathy from the Lewy body diseases considered above. Clinically, MSA is characterized by variable features of parkinsonism, autonomic dysfunction, cerebellar ataxia and corticospinal tract signs (Gilman et al., 2008). Depending on the predominance of a given feature, MSA was diagnosed as one of three disorders considered to be distinct: striatonigral degeneration (parkinsonism predominant), Shy-Drager syndrome (autonomic dysfunction) and olivo-ponto-cerebellar atrophy (ataxia and corticospinal signs). However, it was recognized that the microscopic neuropathology of all three were identical, consisting of argyrophilic glial cytoplasmic inclusions (GCI) in oligodendroglia (Papp et al., 1989), with the clinical phenotype reflecting varying GCI distribution (Inoue et al., 1997). The principal protein component of GCIs is fibrillary α-synuclein, which can also be found occasionally in neuronal intracytoplasmic, intranuclear and dystrophic neurite inclusions in MSA (Lin et al., 2004). Our survey did not differentiate clinical variants of MSA. While there was noteworthy variability in regional scores, the brain stem and cerebellum were most consistently and severely affected, along with the cerebral subcortical nuclei with modestly lower scores in limbic and isocortical association areas.
In summary, the greatest difference in the synucleinopathies was the lower burden of cortical α-synuclein deposits in PD subjects compared to PDD and DLB subjects. However, the most distinguishing feature is the cell-type specific deposition of CGI in oligodendroglia in MSA and the high abundance of deposits in cerebellum.
TDP-43 Proteinopathies
FTLD-TDP is the most common cause of frontotemporal dementia, more so than Pick disease and other tauopathies (Wider and Wszolek, 2008). Its clinical presentations are diverse and have been categorized into three principal types: behavioral variant FTD (bvFTD), primary progressive non-fluent aphasia (PNFA) and semantic dementia (SD). TDP-43 is now considered to be the signature molecular component of ubiquitinated lesions that previously defined most non-tauopathy FTLDs. TDP-43 lesions include diversely shaped intracytoplasmic inclusions, rod-like intranuclear inclusions, granular or diffuse intracytoplasmic immunoreactivity, fibrillar or skein-like curled inclusions, and dystrophic neurites (Geser et al., 2010). In our survey, TDP-43 scores in FTLD-TDP were highest in limbic and isocortical association areas, consistent with the predominantly cortically-mediated cognitive functions that are impaired in frontotemporal dementias, but also striatum, globus pallidus and thalamus while brainstem scores were low.
The overall ratings and distribution of TDP-43 pathology in FTLD-TDP contrasted with those of ALS. The clinical criteria of ALS include spreading and progressive motor weakness with clinical or electrophysiological (or neuropathological) evidence of both lower and upper motor neuron degeneration (Brooks et al., 2000). Beyond the dysfunction of the pyramidal motor system, patients with ALS commonly exhibit cognitive, extrapyramidal or autonomic dysfunction indicating spread beyond motor areas, and these are referred to as ALS-plus syndromes. It has been estimated that 22-48% of ALS patients exhibit cognitive, especially FTD-like impairments (Brooks et al., 2000), and conversely, 15% of FTD patients show ALS (Chen-Plotkin et al., 2010; Lomen-Hoerth et al., 2002). Classical neuropathological lesions of ALS include filamentous thread-like inclusions and round eosinophilc cytoplasmic inclusions (Bunina bodies) in degenerating lower and upper motor neurons (Hirano, 1996). TDP-43-immunoreactive ubiquitinated inclusions in motor neurons are now pathognomonic in most sporadic ALS cases with TDP-43 lesion morphologies identical to those described above for FTLD-TDP (Chen-Plotkin et al., 2010). As expected, TDP-43 scores in ALS were highest in motor cortex and medulla, but they were also often present in limbic, isocortical and cerebral subcortical nuclear regions assessed as well, likely reflecting the diverse non-motor clinical symptoms evident in some patients.
Strengths of this survey include the large number of cases (particularly for the less common diseases), the uniformity of autopsy, tissue processing and microscopic neuropathology assessments, the use of highly sensitive and specific antibodies for the molecular lesions of interest, the examination of multiple molecular lesions in the same cases and tissue blocks, and the strategy of comparing relatively clean cases that did not harbor significant additional co-morbid pathology.
A relative weakness is the likely selection bias of our cohort. Most cases were referred by university-based subspecialty practices and also most cases died in advanced stages of their neurodegenerative diseases. Differences in regionally selective distributions of pathology would likely have been sharper in earlier stages of disease, just as clinical features are more distinct in early compared to end-stage dementias. Another relative weakness is that our data were “global” semi-quantitative ratings of all lesions labeled for the pathological protein of interest within the tissue section. Such data allow statistical comparisons that are especially good with large sample sizes. However, the resolution of differences would likely have been improved if measures were more quantitative (Robinson et al., 2011) or detailed in their characterization of the involvement of specific layers, cell types (neurons, glia), morphologies and subcellular localization of lesions (nuclear, perikaryal, neuritic), etc. Finally, while we sampled a broad range of regions, we do not routinely sample others that would be of interest, such as primary motor and sensory cortices, basal forebrain, hypothalamus or olfactory bulb.
The pathophysiological basis for selective vulnerability at the cellular, laminar, regional and neural systems levels in different neurodegenerative diseases remains enigmatic. Why do α-synuclein aggregates arise as Lewy bodies in certain brainstem nuclei and remain relatively confined there in PD, but in DLB they predominate in cortex? How do abnormal tau aggregates form initially and selectively in certain cell layers of perirhinal/entorhinal (Braak and Braak, 1985; Hyman et al., 1984) and then spread widely but relatively selectively? Some answers are emerging in exciting work demonstrating seeding and transmissibility of protein misfolding and aggregation across synapses, at least for tau and α-synuclein (Iba et al., 2013; Luk et al., 2012; Walker et al., 2013).
Table 5. Mean Differences and Significance Values in TDP-43 Pathology Ratings Between TDP-43 Proteinopathies.
| a. Limbic Cortices | |
| Comparison | FTLD-TDP |
| ALS vs. | Mean Diff: −1.91 Pval: <0.0001 |
| b. Isocortical Association Cortices | |
| Comparison | FTLD-TDP |
| ALS vs. | Mean Diff: −2.18 Pval: <0.0001 |
| c. Subcortical Nuclei | |
| Comparison | FTLD-TDP |
| ALS vs. | Mean Diff: −1.27 Pval: <0.0001 |
| d. Brainstem | |
| Comparison | FTLD-TDP |
| ALS vs. | Mean Diff: −0.32 Pval: 0.0722 |
| e. Cerebellum | |
| Comparison | FTLD-TDP |
| ALS vs. | Mean Diff: 0.13 Pval: 0.2115 |
Note: per legend of Table 2.
ACKNOWLEDGMENT
We acknowledge the special contributions to case recruitment and evaluation of Christopher. M. Clark, Stephen J. DeArmond, Mark S. Forman, Murray Grossman, Howard I. Hurtig, Jason H. Karlawish, Leo F. McCluskey, and William Seeley as well as neuropathology fellows and staff at the University of Pennsylvania.
Grant support: National Institutes of Health R01 AG0390478, P30 AG010124, AG039510, P50 NS053488, P01 AG032953, P01 AG017586.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare that no conflict of interest is related to this work.
LITERATURE CITED
- Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. 2003;60(3):387–392. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- Aarsland D, Zaccai J, Brayne C. A systematic review of prevalence studies of dementia in Parkinson’s disease. Mov Disord. 2005;20(10):1255–1263. doi: 10.1002/mds.20527. [DOI] [PubMed] [Google Scholar]
- Alafuzoff I, Arzberger T, Al-Sarraj S, Bodi I, Bogdanovic N, Braak H, Bugiani O, Del-Tredici K, Ferrer I, Gelpi E, Giaccone G, Graeber MB, Ince P, Kamphorst W, King A, Korkolopoulou P, Kovacs GG, Larionov S, Meyronet D, Monoranu C, Parchi P, Patsouris E, Roggendorf W, Seilhean D, Tagliavini F, Stadelmann C, Streichenberger N, Thal DR, Wharton SB, Kretzschmar H. Staging of neurofibrillary pathology in Alzheimer’s disease: a study of the BrainNet Europe Consortium. Brain Pathol. 2008;18(4):484–496. doi: 10.1111/j.1750-3639.2008.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Association Alzheimer’s disease facts and figures. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2013;9(2):208–245. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, Boxer AL, Dickson DW, Grossman M, Hallett M, Josephs KA, Kertesz A, Lee SE, Miller BL, Reich SG, Riley DE, Tolosa E, Troster AI, Vidailhet M, Weiner WJ. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80(5):496–503. doi: 10.1212/WNL.0b013e31827f0fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong RA, Ellis W, Hamilton RL, Mackenzie IR, Hedreen J, Gearing M, Montine T, Vonsattel JP, Head E, Lieberman AP, Cairns NJ. Neuropathological heterogeneity in frontotemporal lobar degeneration with TDP-43 proteinopathy: a quantitative study of 94 cases using principal components analysis. J Neural Transm. 2010;117(2):227–239. doi: 10.1007/s00702-009-0350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb Cortex. 1991;1(1):103–116. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992;42(3 Pt 1):631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, Lee VM, Trojanowski JQ, Iwatsubo T. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am J Pathol. 1998;152(4):879–884. [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol. 2004;61(3):378–384. doi: 10.1001/archneur.61.3.378. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. On areas of transition between entorhinal allocortex and temporal isocortex in the human brain. Normal morphology and lamina-specific pathology in Alzheimer’s disease. Acta Neuropathol. 1985;68(4):325–332. doi: 10.1007/BF00690836. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E, Bohl J, Bratzke H. Evolution of Alzheimer’s disease related cortical lesions. J Neural Transm Suppl. 1998;54:97–106. doi: 10.1007/978-3-7091-7508-8_9. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(5):293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- Chen-Plotkin AS, Lee VM, Trojanowski JQ. TAR DNA-binding protein 43 in neurodegenerative disease. Nat Rev Neurol. 2010;6(4):211–220. doi: 10.1038/nrneurol.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compta Y, Parkkinen L, O’Sullivan SS, Vandrovcova J, Holton JL, Collins C, Lashley T, Kallis C, Williams DR, de Silva R, Lees AJ, Revesz T. Lewy- and Alzheimer-type pathologies in Parkinson’s disease dementia: which is more important? Brain. 2011;134(Pt 5):1493–1505. doi: 10.1093/brain/awr031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW. The pathogenesis of senile plaques. J Neuropathol Exp Neurol. 1997;56(4):321–339. doi: 10.1097/00005072-199704000-00001. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Bergeron C, Chin SS, Duyckaerts C, Horoupian D, Ikeda K, Jellinger K, Lantos PL, Lippa CF, Mirra SS, Tabaton M, Vonsattel JP, Wakabayashi K, Litvan I. Office of Rare Diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol. 2002;61(11):935–946. doi: 10.1093/jnen/61.11.935. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Braak H, Duda JE, Duyckaerts C, Gasser T, Halliday GM, Hardy J, Leverenz JB, Del Tredici K, Wszolek ZK, Litvan I. Neuropathological assessment of Parkinson’s disease: refining the diagnostic criteria. Lancet Neurol. 2009;8(12):1150–1157. doi: 10.1016/S1474-4422(09)70238-8. [DOI] [PubMed] [Google Scholar]
- Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, Broe GA, Cummings J, Dickson DW, Gauthier S, Goldman J, Goetz C, Korczyn A, Lees A, Levy R, Litvan I, McKeith I, Olanow W, Poewe W, Quinn N, Sampaio C, Tolosa E, Dubois B. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord. 2007;22(12):1689–1707. doi: 10.1002/mds.21507. quiz 1837. [DOI] [PubMed] [Google Scholar]
- Feany MB, Dickson DW. Widespread cytoskeletal pathology characterizes corticobasal degeneration. Am J Pathol. 1995;146(6):1388–1396. [PMC free article] [PubMed] [Google Scholar]
- Forman MS, Zhukareva V, Bergeron C, Chin SS, Grossman M, Clark C, Lee VM, Trojanowski JQ. Signature tau neuropathology in gray and white matter of corticobasal degeneration. Am J Pathol. 2002;160(6):2045–2053. doi: 10.1016/S0002-9440(10)61154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56(1):33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- Geser F, Brandmeir NJ, Kwong LK, Martinez-Lage M, Elman L, McCluskey L, Xie SX, Lee VM, Trojanowski JQ. Evidence of multisystem disorder in whole-brain map of pathological TDP-43 in amyotrophic lateral sclerosis. Arch Neurol. 2008;65(5):636–641. doi: 10.1001/archneur.65.5.636. [DOI] [PubMed] [Google Scholar]
- Geser F, Lee VM, Trojanowski JQ. Amyotrophic lateral sclerosis and frontotemporal lobar degeneration: a spectrum of TDP-43 proteinopathies. Neuropathology. 2010;30(2):103–112. doi: 10.1111/j.1440-1789.2009.01091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakopoulos P, Herrmann FR, Bussiere T, Bouras C, Kovari E, Perl DP, Morrison JH, Gold G, Hof PR. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology. 2003;60(9):1495–1500. doi: 10.1212/01.wnl.0000063311.58879.01. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VM. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron. 2002;34(4):521–533. doi: 10.1016/s0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Durr A, Fowler CJ, Kaufmann H, Klockgether T, Lees A, Poewe W, Quinn N, Revesz T, Robertson D, Sandroni P, Seppi K, Vidailhet M. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71(9):670–676. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg SG, Davies P, Schein JD, Binder LI. Hydrofluoric acid-treated tau PHF proteins display the same biochemical properties as normal tau. J Biol Chem. 1992;267(1):564–569. [PubMed] [Google Scholar]
- Hauw JJ, Daniel SE, Dickson D, Horoupian DS, Jellinger K, Lantos PL, McKee A, Tabaton M, Litvan I. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy) Neurology. 1994;44(11):2015–2019. doi: 10.1212/wnl.44.11.2015. [DOI] [PubMed] [Google Scholar]
- Hirano A. Neuropathology of ALS: an overview. Neurology. 1996;47(4 Suppl 2):S63–66. doi: 10.1212/wnl.47.4_suppl_2.63s. [DOI] [PubMed] [Google Scholar]
- Hof PR, Bouras C, Perl DP, Morrison JH. Quantitative neuropathologic analysis of Pick’s disease cases: cortical distribution of Pick bodies and coexistence with Alzheimer’s disease. Acta Neuropathol. 1994a;87(2):115–124. doi: 10.1007/BF00296179. [DOI] [PubMed] [Google Scholar]
- Hof PR, Bouras C, Perl DP, Morrison JH. Quantitative neuropathologic analysis of Pick’s disease cases: cortical distribution of Pick bodies and coexistence with Alzheimer’s disease. Acta Neuropathologica. 1994b;87:115–124. doi: 10.1007/BF00296179. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Lees AJ. Improved accuracy of clinical diagnosis of Lewy body Parkinson’s disease. Neurology. 2001;57(8):1497–1499. doi: 10.1212/wnl.57.8.1497. [DOI] [PubMed] [Google Scholar]
- Hurtig HI, Trojanowski JQ, Galvin J, Ewbank D, Schmidt ML, Lee VM, Clark CM, Glosser G, Stern MB, Gollomp SM, Arnold SE. Alpha-synuclein cortical Lewy bodies correlate with dementia in Parkinson’s disease. Neurology. 2000;54(10):1916–1921. doi: 10.1212/wnl.54.10.1916. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. Journal of Neuropathology & Experimental Neurology. 1997;56:1095–1097. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Damasio AR. Memory-related neural systems in Alzheimer’s disease: an anatomic study. Neurology. 1990;40(11):1721–1730. doi: 10.1212/wnl.40.11.1721. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer’s disease: cell-specific pathology isolates the hippocampal formation. Science. 1984;225(4667):1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- Iba M, Guo JL, McBride JD, Zhang B, Trojanowski JQ, Lee VM. Synthetic tau fibrils mediate transmission of neurofibrillary tangles in a transgenic mouse model of Alzheimer’s-like tauopathy. J Neurosci. 2013;33(3):1024–1037. doi: 10.1523/JNEUROSCI.2642-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Yagishita S, Ryo M, Hasegawa K, Amano N, Matsushita M. The distribution and dynamic density of oligodendroglial cytoplasmic inclusions (GCIs) in multiple system atrophy: a correlation between the density of GCIs and the degree of involvement of striatonigral and olivopontocerebellar systems. Acta Neuropathol. 1997;93(6):585–591. doi: 10.1007/s004010050655. [DOI] [PubMed] [Google Scholar]
- Irwin DJ, White MT, Toledo JB, Xie SX, Robinson JL, Van Deerlin V, Lee VM, Leverenz JB, Montine TJ, Duda JE, Hurtig HI, Trojanowski JQ. Neuropathologic substrates of Parkinson disease dementia. Ann Neurol. 2012;72(4):587–598. doi: 10.1002/ana.23659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA, Attems J. Prevalence and impact of vascular and Alzheimer pathologies in Lewy body disease. Acta Neuropathol. 2008;115(4):427–436. doi: 10.1007/s00401-008-0347-5. [DOI] [PubMed] [Google Scholar]
- King ME, Ghoshal N, Wall JS, Binder LI, Ksiezak-Reding H. Structural analysis of Pick’s disease-derived and in vitro-assembled tau filaments. Am J Pathol. 2001;158(4):1481–1490. doi: 10.1016/S0002-9440(10)64099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EB, Leng LZ, Zhang B, Kwong L, Trojanowski JQ, Abel T, Lee VM. Targeting amyloid-beta peptide (Abeta) oligomers by passive immunization with a conformation-selective monoclonal antibody improves learning and memory in Abeta precursor protein (APP) transgenic mice. J Biol Chem. 2006;281(7):4292–4299. doi: 10.1074/jbc.M511018200. [DOI] [PubMed] [Google Scholar]
- Lin WL, DeLucia MW, Dickson DW. Alpha-synuclein immunoreactivity in neuronal nuclear inclusions and neurites in multiple system atrophy. Neurosci Lett. 2004;354(2):99–102. doi: 10.1016/j.neulet.2003.09.075. [DOI] [PubMed] [Google Scholar]
- Lippa CF, Duda JE, Grossman M, Hurtig HI, Aarsland D, Boeve BF, Brooks DJ, Dickson DW, Dubois B, Emre M, Fahn S, Farmer JM, Galasko D, Galvin JE, Goetz CG, Growdon JH, Gwinn-Hardy KA, Hardy J, Heutink P, Iwatsubo T, Kosaka K, Lee VM, Leverenz JB, Masliah E, McKeith IG, Nussbaum RL, Olanow CW, Ravina BM, Singleton AB, Tanner CM, Trojanowski JQ, Wszolek ZK. DLB and PDD boundary issues: diagnosis, treatment, molecular pathology, and biomarkers. Neurology. 2007;68(11):812–819. doi: 10.1212/01.wnl.0000256715.13907.d3. [DOI] [PubMed] [Google Scholar]
- Litvan I, Hauw JJ, Bartko JJ, Lantos PL, Daniel SE, Horoupian DS, McKee A, Dickson D, Bancher C, Tabaton M, Jellinger K, Anderson DW. Validity and reliability of the preliminary NINDS neuropathologic criteria for progressive supranuclear palsy and related disorders. J Neuropathol Exp Neurol. 1996;55(1):97–105. doi: 10.1097/00005072-199601000-00010. [DOI] [PubMed] [Google Scholar]
- Lomen-Hoerth C, Anderson T, Miller B. The overlap of amyotrophic lateral sclerosis and frontotemporal dementia. Neurology. 2002;59(7):1077–1079. doi: 10.1212/wnl.59.7.1077. [DOI] [PubMed] [Google Scholar]
- Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, Lee VM. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338(6109):949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IR, Ansorge O, Strong M, Bilbao J, Zinman L, Ang LC, Baker M, Stewart H, Eisen A, Rademakers R, Neumann M. Pathological heterogeneity in amyotrophic lateral sclerosis with FUS mutations: two distinct patterns correlating with disease severity and mutation. Acta Neuropathol. 2011a;122(1):87–98. doi: 10.1007/s00401-011-0838-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IR, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E, Perry RH, Trojanowski JQ, Mann DM, Lee VM. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 2011b;122(1):111–113. doi: 10.1007/s00401-011-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IR, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, Kovacs GG, Ghetti B, Halliday G, Holm IE, Ince PG, Kamphorst W, Revesz T, Rozemuller AJ, Kumar-Singh S, Akiyama H, Baborie A, Spina S, Dickson DW, Trojanowski JQ, Mann DM. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol. 2010;119(1):1–4. doi: 10.1007/s00401-009-0612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Trojanowski JQ, Vinters HV, Hyman BT. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123(1):1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, Castellani RJ, Crain BJ, Davies P, Del Tredici K, Duyckaerts C, Frosch MP, Haroutunian V, Hof PR, Hulette CM, Hyman BT, Iwatsubo T, Jellinger KA, Jicha GA, Kovari E, Kukull WA, Leverenz JB, Love S, Mackenzie IR, Mann DM, Masliah E, McKee AC, Montine TJ, Morris JC, Schneider JA, Sonnen JA, Thal DR, Trojanowski JQ, Troncoso JC, Wisniewski T, Woltjer RL, Beach TG. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71(5):362–381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Rademakers R, Roeber S, Baker M, Kretzschmar HA, Mackenzie IR. A new subtype of frontotemporal lobar degeneration with FUS pathology. Brain. 2009;132(Pt 11):2922–2931. doi: 10.1093/brain/awp214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314(5796):130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Otvos L, Jr., Feiner L, Lang E, Szendrei GI, Goedert M, Lee VM. Monoclonal antibody PHF-1 recognizes tau protein phosphorylated at serine residues 396 and 404. J Neurosci Res. 1994;39(6):669–673. doi: 10.1002/jnr.490390607. [DOI] [PubMed] [Google Scholar]
- Papp MI, Kahn JE, Lantos PL. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome) J Neurol Sci. 1989;94(1-3):79–100. doi: 10.1016/0022-510x(89)90219-0. [DOI] [PubMed] [Google Scholar]
- Probst A, Tolnay M, Langui D, Goedert M, Spillantini MG. Pick’s disease: hyperphosphorylated tau protein segregates to the somatoaxonal compartment. Acta Neuropathol. 1996;92(6):588–596. doi: 10.1007/s004010050565. [DOI] [PubMed] [Google Scholar]
- Robinson JL, Geser F, Corrada MM, Berlau DJ, Arnold SE, Lee VM, Kawas CH, Trojanowski JQ. Neocortical and hippocampal amyloid-beta and tau measures associate with dementia in the oldest-old. Brain. 2011;134(Pt 12):3708–3715. doi: 10.1093/brain/awr308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbagh MN, Adler CH, Lahti TJ, Connor DJ, Vedders L, Peterson LK, Caviness JN, Shill HA, Sue LI, Ziabreva I, Perry E, Ballard CG, Aarsland D, Walker DG, Beach TG. Parkinson disease with dementia: comparing patients with and without Alzheimer pathology. Alzheimer Dis Assoc Disord. 2009;23(3):295–297. doi: 10.1097/WAD.0b013e31819c5ef4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt ML, Lee VM-Y, Hurtig H, Trojanowski JQ. Properties of antigenic determinants that distinguish neurofibrillary tangles in progressive supranuclear palsy and Alzheimer’s disease. Laboratory Investigation. 1988;59:460–466. [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58(12):1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- Toledo JB, Brettschneider J, Grossman M, Arnold SE, Hu WT, Xie SX, Lee VM, Shaw LM, Trojanowski JQ. CSF biomarkers cutoffs: the importance of coincident neuropathological diseases. Acta Neuropathol. 2012;124(1):23–35. doi: 10.1007/s00401-012-0983-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo JB, VanDeerlin VM, Lee EB, Suh E, Baek Y, Robinson JL, Xie SX, McBride J, Wood EM, Schuck T, Irwin D, Gross RG, Hurtig H, McCluskey L, Eleman L, Karlawish J, Schellenberg G, Chen-Plotkin A, Wolk D, Grossman M, Arnold SE, Shaw LM, Lee VMY, Trojanowski JQ. A platform for discovery: The University of Pennsylvania Integrated Neurodegenerative Disease Biobank. Alzheimer’s & Dementia. 2013 doi: 10.1016/j.jalz.2013.06.003. ePub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uryu K, Nakashima-Yasuda H, Forman MS, Kwong LK, Clark CM, Grossman M, Miller BL, Kretzschmar HA, Lee VM, Trojanowski JQ, Neumann M. Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J Neuropathol Exp Neurol. 2008;67(6):555–564. doi: 10.1097/NEN.0b013e31817713b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LC, Diamond MI, Duff KE, Hyman BT. Mechanisms of protein seeding in neurodegenerative diseases. JAMA Neurol. 2013;70(3):304–310. doi: 10.1001/jamaneurol.2013.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Teplow DB. Alzheimer’s disease and the amyloid beta-protein. Prog Mol Biol Transl Sci. 2012;107:101–124. doi: 10.1016/B978-0-12-385883-2.00012-6. [DOI] [PubMed] [Google Scholar]
- Wider C, Wszolek ZK. Etiology and pathophysiology of frontotemporal dementia, Parkinson disease and Alzheimer disease: lessons from genetic studies. Neurodegener Dis. 2008;5(3-4):122–125. doi: 10.1159/000113680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarchoan M, Xie SX, Kling MA, Toledo JB, Wolk DA, Lee EB, Van Deerlin V, Lee VM, Trojanowski JQ, Arnold SE. Cerebrovascular atherosclerosis correlates with Alzheimer pathology in neurodegenerative dementias. Brain. 2012;135(Pt 12):3749–3756. doi: 10.1093/brain/aws271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N. Topography of Pick body distribution in Pick’s disease: a contribution to understanding the relationship between Pick’s and Alzheimer’s diseases. Clin Neuropathol. 1989;8(1):1–6. [PubMed] [Google Scholar]
- Zhukareva V, Mann D, Pickering-Brown S, Uryu K, Shuck T, Shah K, Grossman M, Miller BL, Hulette CM, Feinstein SC, Trojanowski JQ, Lee VM. Sporadic Pick’s disease: a tauopathy characterized by a spectrum of pathological tau isoforms in gray and white matter. Ann Neurol. 2002;51(6):730–739. doi: 10.1002/ana.10222. [DOI] [PubMed] [Google Scholar]