Abstract
Timothy Syndrome (TS) arises from a point mutation in the human voltage-gated L-type Ca2+ channel (Cav1.2). TS is associated with cardiac arrhythmias and sudden cardiac death, as well as congenital heart disease, impaired cognitive function, and autism spectrum disorders. TS results from a de novo gain-of-function mutation which affects the voltage dependent component of Cav1.2 inactivation. We created a knock-in TS mouse. No homozygous TS mice survived, but heterozygous TS2-NEO mice (with the mutation and the neocassette in situ) had a normal outward appearance and survived to reproductive age. Previously, we have demonstrated that these mice exhibit the triad of Autistic traits. In this paper we document other aspects of these mice including Cav1.2 isoform expression levels, normal physical strength, brain anatomy and a marked propensity towards self-injurious scratching. Gross brain anatomy was not markedly different in TS2-NEO mice compared to control littermates, and no missing structures were noted. The lack of obvious changes in brain structure is consistent with theTS2-NEO mice may provide a significant tool in understanding the role of calcium channel inactivation in both cardiac function and brain development.
Keywords: Autism, channels, calcium, inactivation, brain
INTRODUCTION
Timothy syndrome (TS) is an extremely rare human multisystem disorder associated with life-threatening cardiac arrhythmias, autism, and neurological dysfunction. The first cases of TS were described in 1992 and 1995 as sporadic cases of long QT syndrome, congenital heart disease, and syndactyly.1-3 TS is associated with phenotypic abnormalities in multiple organ systems resulting from a common de-novo genetic mutation in Cav1.2.4 All humans with TS exhibit proarrhythmic prolongation of the cardiac action potential, which is a fundamental diagnosis of TS. In addition, all TS patients have arrhythmias including bradycardia, atrioventricular block, torsades de pointes ventricular tachycardia, and ventricular fibrillation. In humans, the TS mutation generally results in sudden cardiac death at a young age.5 Additional features associated with Timothy Syndrome include syndactyly, small teeth, congenital heart disease, cardiomegaly, dysmorphic facial features, myopia, immune deficiency, recurrent infections, intermittent hypoglycemia, hypothermia, and developmental delays, including language, motor, generalized cognitive impairment, and autism spectrum disorders.
All humans so far identified with TS have been heterozygous for the mutation. Given this, it is not easy to study the basic mechanisms of TS in humans, and an animal model of this syndrome is needed. We therefore developed a genetically engineered knock-in mouse with a heterogeneous G406R TS mutation in exon 8 of Cav1.2, the L-type calcium channel (see Figure 1F). Homozygous mice do not survive. Similarly, heterozygous TS2 mice did not survive. This is likely due likely due to cardiac abnormalities since in the human condition, TS children have congenital heart defects and cardiac arrhythmias. Only TS2 mice that retained the inverted neomycin cassette (TS2-NEO) survived to adulthood. Previously, we have shown that the TS2-NEO mouse exhibits the triad of autistic traits including repetitive stereotypical behavior, decreased social interaction and decreased vocalizations.6 Here we report largely normal physical attributes of these mice including largely normal brain size and structures and an additional scratching phenotype that the TS mouse shares in common with other mice expressing ASD related genetic defects.7-9
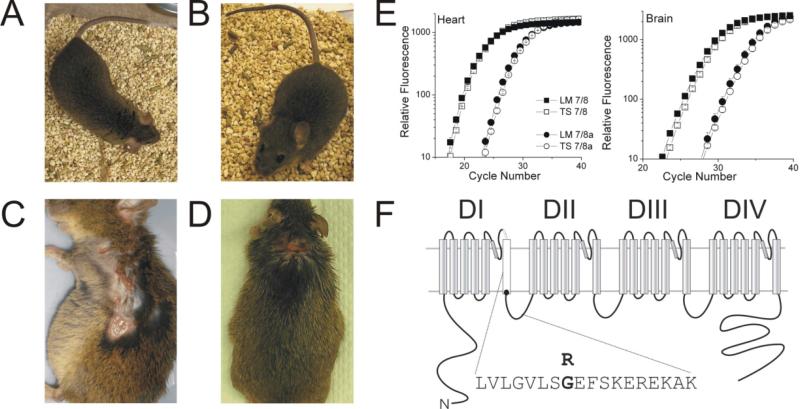
Figure 1. The TS2-NEO mouse.
A: The TS2-NEO mouse is outwardly normal in appearance, compared to B: negative control littermates. C: TS2-NEO mice were extremely susceptible to dermatitis, with a tendency to scratch on one side. D: Littermates suffered dermatitis less frequently and had smaller more symmetrical lesions. E: Representative quantitative real-time PCR data showing there is no change in relative expression of exon 8 and 8a in TS2-NEO mice compared to littermates in both heart and brain. F: Topology of the Cav1.2 channel showing the location of the G to R mutation in exon 8 at the intracellular side of S6 in domain I.
The alpha-subunit of the Cav1.2 (alpha1c, CACNA1C) L-type voltage gated calcium channel consists of four domains (I, II, III and IV), each of which is composed of six transmembrane segments (S1–S6).10 Timothy Syndrome (TS) results from a point mutation in the intracellular part of the S6 transmembrane segment of domain 1 of Cav1.2. This region is encoded in a mutually exclusive manner by exons 8 or 8a (Figure 1F). Interestingly, the identical de novo gain of function missense glycine to arginine mutation (G406R) has been found in both the 8 and 8a human splice variants.5 The TS point mutation results in severe disruption of calcium dependent Cav1.2 inactivation, which plays a key role in determining transmembrane calcium flux. In turn, this has a strong effect on intracellular calcium homeostasis, and may lead to calcium overload in some cell types. Entry of calcium through the voltage gated L-type calcium channel has been implicated in transcriptional regulation of gene expression (e.g., CAM Kinase, CREB and MEF-2), heart failure, circadian rhythms, learning, memory, and neuronal survival/death.11-13
The TS2-NEO knock-in mouse model parallels many of the more important human phenotypes and may provide an important platform for understanding the role of calcium channel inactivation and calcium signaling in such diverse areas as cardiac arrhythmias, heart failure, brain development, and autism spectrum disorders.
METHODS
Construction of the knock-in mouse
TS2-NEO is a heterozygous mouse that has the TS2 (G406R) mutation in the L-type calcium channel and contains a neomycin resistance cassette, used to construct the mouse, in the open reading frame of the downstream alternatively spliced exon. Heterozygous TS2-NEO mice were generated using homologous recombination in mouse embryonic stem cells. A ~10kb region used to generate the targeting vector was first sub cloned from a positively identified C57BL/6 BAC clone. The region was designed such that the long homology arm (LA) extends ~7.6kb 5’ to exon 8 and the Neo cassette is inserted 301bps 3’ to the G-A point mutation engineered into the end of exon 8. The short homology arm extends 1.9kb 3’ to exon 8. The exon denoted 8a in the human by the original TS paper by Splawski et al is designated 8 in mouse.
The targeting vector was constructed using Red/ET recombineering technology. The point mutation was engineered by overlap extension PCR including a site for insertion of the Neo cassette (BsiWI). The targeting vector was confirmed by restriction analysis after each modification step and by sequencing using primers designed to read from the selection cassette. T7 and P6 primers anneal to the vector sequence and read into the 5’ and 3’ ends of a BAC sub clone. All vectors were constructed and introduced into mouse stem cells to produce TS2-NEO mice by In-Genious Targeting Laboratory, Stony Brook, NY. In these mice, the NEO cassette was very effective in suppressing defective TS2 mutant channels in heart, but allowed more abundant expression in brain (approx. 26% 6). It is possible, but highly unlikely that the neocassette plays a role in the abnormal phenotype. Neocassettes are frequently used to confer neomycin resistance during the construction of genetically modified mice. It is therefore present in a wide variety of mice, which have not been demonstrated to have the triad of autistic behaviors of this mouse.
Quantitative RT-PCR Estimation of ratio of splice variants
Total RNA was obtained from mouse heart and brain, using Trizol (Invitrogen). RNA concentration was measured by UV spectrophotometer. RNA was reverse transcribed to cDNA using QuantiTect Reverse Transcription Kit (Qiagen). Each 20 μl reaction mixture contained 1 μl cDNA (1 ng/μl total RNA) plus 12.5 μl 2×Platinum SYBR Green qPCR SuperMix (Invitrogen), 0.5 μl sense primer (10 μM), 0.5 μl antisense primer (10 μM). Primers were designed with a forward primer in exon 7, and a reverse primer specific to either exon 8 or exon 8a. Primers were: 7/8F: GCTGACGGTGTTCCAGTGTA; 7/8 R: TCAAAACACCGAGAACCAGA; and 7/8a F: GCATCACCAACTTCGACAAC; 7/8a R: GCTAAGAACACCGAGAACCAA. Commercially available mGAPDH primers were used (Qiagen: QT00309099). qRT-PCR was performed on the iCycler5 Thermal Cycler (BioRad) with the intercalating dye SYBR Green as follows: initial hold at 95 °C for 3 min, followed by 45 cycles of 20 s at 95°C, 30 s at 60°C and 30 s at 72°C. Melt curve analysis was performed to ensure a single product. Triplicates were performed for each cDNA sample. Efficiencies and CT values were determined using real time PCR Miner.14 Relative expression was quantified using Relative Expression Software Tool (REST2008, v2.0.7).15
Brain Histology
Mice were deeply anesthetized with ketamine/xylazine (0.1cc/10g) and perfused through the heart with 0.9% saline followed by 4% paraformaldehyde (PFA) in phosphate buffered saline (PBS), The brains were removed and postfixed overnight in 4% PFA. They were then cryoprotected in 15% and then 30% sucrose in PBS. Frontal 40 μm thick sections were cut on a cryostat and stored in tissue culture wells in a solution of 30% ethylene glycol and 25% glycerol in PB. Sections were mounted on gelled glass slides and stained for Nissl substance with Cresyl Violet. Slides were examined with a Leitz Dialux 20 light microscope. Images of selected sections were captured with a SPOT insight camera mounted on that microscope. The brightness and contrast of the whole image were adjusted with Adobe Photoshop software.
Basic Sensorimotor Function
Tactile reflexes, vision, hearing, coordination, and muscular strength were tested using well-established protocols.16-18 For basic olfaction analysis, mice were deprived of food overnight (10h). A small piece of a food item (a cookie) was hidden beneath bedding in an unfamiliar cage. The mouse was placed in the cage, and the latency to find the food item recorded. The subject was returned to its home cage and fed.
RESULTS
TS2-NEO
We introduced the TS mutation into exon 8 in a C57BL/6 background. No viable homozygous TS mice were born, but heterozygous TS2-NEO (with the inverted cassette in situ) mice grew to adulthood with body weights and gross sensorimotor function similar to littermate controls (Table 1). Cav1.2 channels exon 8 and 8a are mutually exclusively expressed in both heart and brain, so we designed primers specific to exon 8 and 8a. We used quantitative real time PCR to determine if the expression of exon 8 was affected by the presence of the mutation (Figure 1E). Using GAPDH as a reference gene, the expression of exon 8 mRNA was unchanged by the presence of the mutation in brain (n = 5, p > 0.5), or in heart (n = 6, p > 0.4). In both TS2-NEO mice and control littermates, expression of exon 8 was higher than exon 8a in both heart and brain. The relative expression of exon 8 to 8a mRNA was not significantly different between littermates and TS2-NEO mice in either brain (n = 4, p > 0.7) or heart (n = 6, p > 0.7). The lack of change in transcript levels of channel mRNA suggests that the defective channel does not generally cause preferential premature cell death or hyperplasia relative to cells expressing the alternate splice form and confirms the previous analysis of Bader et al. that in the adults of this strain of mice the exon 8a that in humans carries the TS1 mutation is virtually absent relative to exon 8 in the hearts and brains of the these mice.6
Table 1.
General characteristics of TS mice. Mice carrying the TS2-NEO mutation are normal in most of the phenotypes measured. Unless otherwise noted, both sexes are included in the analysis and there is no significant difference between genotypes. Methods not already cited are as described in Crawley, 2003.22
| Appearance | Body Weight | Vision (reaching for ledge) | Olfactory Function | Motor Strength (Hanging Wire test) | Water Consumption | Flavor preference | ||
|---|---|---|---|---|---|---|---|---|
| Saline vs. water | Saccharin vs. water | |||||||
| TS (+/+) Littermate controls | Normal | 26 ± 1 g, n=12 | Normal | Normal | 58 ± 1 s | 9 ± 3 ml/24 hr | Normal | Normal |
| TS (+/-) | Normal | 26 ± 2 g n = 5 | Normal | Normal | 57 ± 2 s | 10 ± 4 ml/24 hr | Normal | Normal |
| p > 0.9 | p > 0.9 | p > 0.6 | ||||||
Dermatitis
The main cause of premature demise in our TS2-NEO mice was susceptibility to severe dermatitis. The C57BL/6 background strain for the TS2-NEO mouse has a predisposition to dermatitis, but the TS2-NEO mutation appears to aggravate this susceptibility with 10 out of 76 littermate control mice vs. 23 out of 66 TS2-NEO mice (p < 0.05, X2) succumbing to dermatitis. Qualitatively, the progression of dermatitis in the TS2-NEO mice was rapid and covered a larger area that tended to be on one side, compared to the smaller and more symmetrical area of dermatitis on back of the neck in littermates (Figure 1A-1D). It is not clear if TS2-NEO mice have an increased tendency to contract dermatitis, an inability to recover from dermatitis once initiated, or an inability to stop scratching once started. This final possibility is raised by the observation that many TS2-NEO mice have a peculiar scratching pattern along one side and towards the face, often resulting in self destruction of whiskers. Speculatively, such scratching might be similar to self-injurious, repetitive stereotypical behavior observed in autistic individuals. An equally likely possibility is that the dermatitis may reflect an altered immune response in autism,8 as TS individuals often have compromised immune responses.
Brain Phenotype
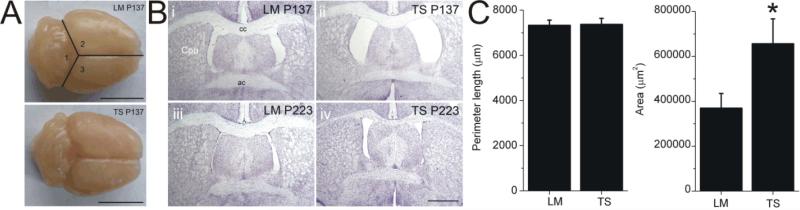
Timothy Syndrome is linked to deficits in mental function in humans, including seizures, autism, and autism spectrum disorders. This suggests that the TS mutation is associated with abnormal brain development. To examine this possibility, we first looked at gross brain shape. Overall, brains from littermate and TS2-NEO mice appear similar. The dimensions of the cerebral cortex and the cerebellum showed no systematic differences in shape or size. Similarly, wet weights of TS2-NEO brains (0.433 ± 0.011 g, n = 16) were not significantly different from control brains (0.448 ± 0.014 g, n = 13 p > 0.3). A subtle difference was noted in brain gross anatomy. TS2-NEO brains were often observed to be slightly misshapen. In order to quantify this subjective difference the angles between the midline and the cerebellum were analyzed (Figure 2). The absolute values of the angle differences were 2.8 ± 0.5 deg (n = 20) in littermates, which increased to 6.1 ± 1.0 deg (n = 24, p < 0.01) in TS2-NEO mice. Brains from TS2-NEO mice were slightly but significantly more asymmetric than littermates. Analysis of brain slices showed that overall gross mouse brain anatomy was fairly normal. No structures were missing, and all major landmarks were present. Although grossly normal, TS2-NEO brains were subtly distinct from littermate control brains with respect to the lateral ventricles. Brain cross sectional slices were analyzed on coronal sections at bregma +0.14, the level at which the anterior commissure crosses the midline. The ventricular perimeter and ventricular cross sectional area and were found to be highly variable. On average, the total cross sectional area of the lateral ventricles were larger in TS2-NEO mice (656,000 ± 111,000 μm2, n = 15) compared to control mice at (370,000 ± 65,000 μm2, n = 13, p < 0.05). The circumference of the ventricular perimeter was insensitive to the presence of the TS2-NEO mutation: 7366 ± 270 μm for TS2-NEO (n = 15) vs. 7324 ± 232 μm for littermate control (n = 13, p > 0.9). This suggests there may be a subtle effect of the TS2-NEO mutation on the development of the brain.
Figure 2. Brain Sections.
A: Asymmetry in perfusion fixed mouse brains. The angles between the midline and the cerebellum were determined and assigned as angles 1, 2 and 3. The ratio of the absolute difference between angles 2 and 3 was found to be significantly different between littermates and TS2-NEO mice. Angle 1 was unchanged. B: Cross section of brain at bregma +0.14 stained with cresyl violet from 2 age matched pairs of TS2-NEO mice and LM controls from postnatal days 137 (i, ii) and 223 (iii, iv).. Scale bar 1 mm, for all panels. Abbreviations: ac, anterior commissure; cc, corpus callosum; Cpu, caudate-putamen. C: The perimeter length of the brain ventricles are not different in TS2-NEO and LM mice, but the area of the ventricles in the TS2-NEO mice (656,000 ± 111,000 μm2, n=13) was larger than in littermate controls (370,000 ± 65,000 μm2, n = 15, p < 0.05).
CONCLUSION
Timothy syndrome results in alteration of a crucial point in cellular signaling. By changing inactivation, the TS mutation is predicted to alter the normal amount of calcium that enters the cell during stimulation. Calcium entry is the major signaling pathway by which electrical events are transduced into chemical events such as cell growth and differentiation. Consequently, this mutation may provide insight into a “missing link” in the genetic and environmental factors that influence the time course and outcomes of autism and autism spectrum disorders.
The TS defect alters calcium channel inactivation. Therefore, cells whose growth, development, or function is altered by the TS mutation must undergo sufficient sustained depolarization for calcium entry to play an important role in these cell types. The pathologies associated with TS may be sensitive to the history and pattern of electrical activity of cells expressing the defect. Altered calcium channel inactivation has the potential to alter the ability of neurons to integrate information properly by changing electrical signaling. In neurons expressing the splice variant carrying the TS defect, membrane potential responses and ability to integrate high frequency components of information may be altered. We suggest that rapid stimulation will result in abnormal calcium entry, possible calcium overload, and the consequent destabilization of responses during periods of rapid repetitive stimulation and stress leading to abnormal learning and neuronal connectivity. Thus calcium, in addition to its direct effects, may have indirect effects by altering multiple molecular pathways.
The TS2-NEO mouse is useful in studying the role of calcium channel inactivation on brain function, as the mouse recapitulates the triad of autistic traits.6 TS individuals had an 80% rate of autism or autism spectrum disorder, in addition to other cognitive disabilities. On a macroscopic level, the brains of TS2-NEO mice were relatively normal. Only a minor increase in lateral ventricle size was observed, and this occurred on a background of high variability, such that it might be difficult to detect in a human population. Changes in brain ventricle volume have been reported in autistic people, but both enlargement and decreased lateral ventricular volume have been reported.19,20 However, autism is a complex problem with few consistent anatomical findings. It seems likely that in TS, and in the majority of autistic individuals, changes may lie either in the microscopic organization of neurons or in their functional behavior. This possibility has been suggested for modulation of dendritic retraction in cultured neurons expressing the TS defect in heterologously expressed calcium channels.21 TS2-NEO mice may provide a model for autism in the context of a grossly normal brain in which microscopic organization and cellular function are affected by altered calcium channel kinetics.
The cellular calcium handling system is complex and has multiple targets in which defects can alone, or in conjunction with other defects, cause a similar predicted increase in intracellular calcium transients. This makes the TS2-NEO mouse a particularly useful tool for understanding the role of aberrant calcium handling in promoting autism.
ACKNOWLEDGEMENTS
This work was funded in part by the Dean's Fund and an Interdisciplinary Research Development Fund (IRDF) grant from the University at Buffalo, SUNY, and grants from the Simons Foundation (9103-634133), the NIH (R21HL 08805801), and the Department of Defense (CDMRP AR080107). The authors have no competing financial interests. The authors would like to thank Gloria Andolina for technical assistance.
FUNDING SOURCES
NIH, Simons Foundation, Department of Defense CDMRP, University at Buffalo IRDF and Dean's funds.
Footnotes
CONFLICT OF INTEREST
None.
REFERENCES
- 1.Marks ML, Trippel DL, Keating MT. Long QT syndrome associated with syndactyly identified in females. Am J Cardiol. 1995;76(10):744–745. doi: 10.1016/s0002-9149(99)80216-1. [DOI] [PubMed] [Google Scholar]
- 2.Marks ML, Whisler SL, Clericuzio C, Keating M. A new form of long QT syndrome associated with syndactyly. J Am Coll Cardiol. 1995;25(1):59–64. doi: 10.1016/0735-1097(94)00318-k. [DOI] [PubMed] [Google Scholar]
- 3.Reichenbach H, Meister EM, Theile H. [The heart-hand syndrome. A new variant of disorders of heart conduction and syndactylia including osseous changes in hands and feet]. KinderarztlPrax. 1992;60:54–56. [PubMed] [Google Scholar]
- 4.Splawski I, Timothy KW, Sharpe LM, et al. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119(1):19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Splawski I, Timothy KW, Decher N, et al. Severe arrhythmia disorder caused by cardiac L-type calcium channel mutations. Proc Natl Acad Sci USA. 2005;102(23):8089–8096. doi: 10.1073/pnas.0502506102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bader PL, Faizi M, Kim LH, et al. Mouse model of Timothy syndrome recapitulates triad of autistic traits. Proc Natl Acad Sci U S A. 2011;108(37):15432–15437. doi: 10.1073/pnas.1112667108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peca J, Feliciano C, Ting JT, et al. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472(7344):437–442. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun. 2012;26(4):607–616. doi: 10.1016/j.bbi.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7(2):152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- 10.Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57(4):411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- 11.Deisseroth K, Mermelstein PG, Xia H, Tsien RW. Signaling from synapse to nucleus: the logic behind the mechanisms. Curr Opin Neurobiol. 2003;13(3):354–365. doi: 10.1016/s0959-4388(03)00076-x. [DOI] [PubMed] [Google Scholar]
- 12.Dolmetsch RE, Pajvani U, Fife K, Spotts JM, Greenberg ME. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science. 2001;294(5541):333–339. doi: 10.1126/science.1063395. [DOI] [PubMed] [Google Scholar]
- 13.Moosmang S, Haider N, Klugbauer N, et al. Role of hippocampal Cav1.2 Ca2+ channels in NMDA receptor-independent synaptic plasticity and spatial memory. J Neurosci. 2005;25(43):9883–9892. doi: 10.1523/JNEUROSCI.1531-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao S, Fernald RD. Comprehensive algorithm for quantitative real-time polymerase chain reaction. J Comput Biol. 2005;12(8):1047–1064. doi: 10.1089/cmb.2005.12.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30(9):e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wersinger SR, Caldwell HK, Martinez L, Gold P, Hu SB, Young WS., III Vasopressin 1a receptor knockout mice have a subtle olfactory deficit but normal aggression. Genes Brain Behav. 2007;6(6):540–551. doi: 10.1111/j.1601-183X.2006.00281.x. [DOI] [PubMed] [Google Scholar]
- 17.Wersinger SR, Ginns EI, O'Carroll AM, Lolait SJ, Young WS., III Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol Psychiatry. 2002;7(9):975–984. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- 18.Crawley JN. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 1999;835(1):18–26. doi: 10.1016/s0006-8993(98)01258-x. [DOI] [PubMed] [Google Scholar]
- 19.Vidal CN, Nicolson R, Boire JY, et al. Three-dimensional mapping of the lateral ventricles in autism. Psychiatry Res. 2008;163(2):106–115. doi: 10.1016/j.pscychresns.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piven J, Arndt S, Bailey J, Havercamp S, Andreasen NC, Palmer P. An MRI study of brain size in autism. Am J Psychiatry. 1995;152(8):1145–1149. doi: 10.1176/ajp.152.8.1145. [DOI] [PubMed] [Google Scholar]
- 21.Krey JF, Dolmetsch R. The Timothy Syndrome Mutation In Cav1.2 Causes Dendritic Retraction Through Calcium-independent Activation Of The Rhoa Pathway. Biophysical Journal. 2009;96:221a–222a. [Google Scholar]
- 22.Crawley JN. Behavioral phenotyping of rodents. Comp Med. 2003;53(2):140–146. [PubMed] [Google Scholar]