Abstract
Purpose
Urothelial cell carcinoma (UCC) rapidly progresses from superficial to muscle-invasive tumors. The key molecules involved in metastatic progression and its early detection require clarification. The present study defines a seminal role of the metastasis-associated gene MDA-9/Syntenin in UCC progression.
Experimental Design
Expression pattern of MDA-9/Syntenin was examined in 44 primary UCC and the impact of its overexpression and knock down was examined in multiple cells lines and key findings were validated in primary tumors.
Results
Significantly higher (p= 0.002–0.003) expression of MDA-9/Syntenin was observed in 64% (28/44) of primary tumors and an association was evident with stage (p=0.01), grade (p=0.03) and invasion status (p=0.02). MDA-9/Syntenin overexpression in non-tumorigenic HUC-1 cells increased proliferation (p=0.0012), invasion (p=0.0001) and EGFR, AKT, PI3K and c-Src expression. Alteration of Beta-catenin, E-Cadherin, Vimentin, Claudin-1, ZO-1 and TCF4 expression were also observed. MDA-9/Syntenin knock down in 3 UCC cell lines reversed phenotypic and molecular changes observed in the HUC-1 cells and reduced in vivo metastasis. Key molecular changes observed in the cell lines were confirmed in primary tumors. A physical interaction and co-localization of MDA-9/Syntenin and EGFR was evident in UCC cell lines and primary tumors. A logistic regression model analysis revealed a significant correlation between MDA-9/Syntenin:EGFR and MDA-9/Syntenin: AKT expressions with stage (p=0.04, EGFR), (p=0.01, AKT). A correlation between MDA-9/Syntenin: β-catenin co-expression with stage (p=0.03) and invasion (p=0.04) was also evident.
Conclusions
Our findings indicate that MDA-9/Syntenin might provide an attractive target for developing detection, monitoring and therapeutic strategies for managing UCC.
Keywords: Urothelial cancer, MDA-9/Syntenin, invasion, EGFR signaling
Introduction
Urothelial cell carcinoma (UCC) ranks ninth in worldwide cancer with cigarette smoking being the most important risk factor (1). It is more frequent in men than in women (1). In the United States, UCC is the fourth most commonly diagnosed cancer in men and eighth most common cancer in women (2). It is estimated that 72,570 cases of UCC will be diagnosed and 15,210 deaths will occur in 2013 (3). Of all newly diagnosed cases of UCC, about 70% are superficial tumors (non- invasive) (4). Approximately, 50–70% of those superficial tumors will recur and roughly 10–20% will progress to muscularis propria invasive (muscle-invasive) disease (4). Predicting progression from superficial to muscularis propria invasive disease and delineating approaches for early detection remain major challenges (4). Several genetic alterations including losses on chromosomal regions 3p, 5q, 10q, 11p, 13q, 14q, 17p and 18q and activation of several proto-oncogenes, such as EGFR, H-Ras, Bcl-2, mdm-2, FGFR3, and c-myc, have been implicated in UCC progression (5–6). Epithelial-mesenchymal transition (EMT) has also been implicated in the development of muscle-invasive disease (6). However, the key molecules and genetic pathways controlling the development of invasive disease remain largely unknown.
MDA-9/Syntenin is a member of the family of PDZ domain-containing scaffold proteins displaying a diverse array of biological functions, including binding to syndecans, clustering of membrane receptors, inducing pseudopodia formation and intracellular trafficking (7–13). Overexpression of MDA-9/Syntenin occurs in multiple human cancer cell lines including melanoma, breast and gastric cancer cells and is associated with increased cell migration and invasion (7–15). Cross talk between MDA-9/Syntenin, c-Src and NF-kB in mediating invasion and metastasis has also been demonstrated (12). However, a comprehensive analysis of the expression pattern of MDA-9/Syntenin and its precise role in UCC progression remains to be determined.
In the present study, we examined the expression pattern of MDA-9/Syntenin in primary UCC tumors and examined the impact of its gain-of-function (GOF) and loss-of-function (LOF) in several UCC cell lines. Overexpression of MDA-9/Syntenin was evident in primary tumors and we demonstrate a direct relationship between MDA-9/Syntenin overexpression and UCC progression and elucidate the molecular mechanism underlying these changes. MDA-9/Syntenin could represent a useful target for diagnosis and potential therapy of UCC.
Materials and Methods
Tissue specimens
Pre-constructed tissue microarray slides containing primary tumors and corresponding adjacent normal tissues in duplicate from 44 UCC patients were collected from commercial sources (US Biomax Inc). All tumors were transitional cell carcinoma (TCC) and graded according to the current WHO system. The demographic data along with MDA-9/Syntenin alteration in expression patterns are represented in Table S1.
Cell lines and reagents
Uothelial cell lines SV-HUC-1 (indicated as HUC-1), T24, SW780 and SCaBER were purchased from ATCC and cultured in ATCC-recommended medium. HEK293 cells were also procured from ATCC and cultured in ATCC recmmended medium. The cell lines were periodically checked for mycoplasmal contamination (Sigma, # MP-0025). All tissue culture media and reagents were purchased either from ATCC or Invitrogen.
Immunohistochemistry
Immunohistochemistry (IHC) was performed using specific antibody in paired primary UCC specimens of different stages as desccribed earlier (16). Each experiment was repeated at least 2 times for counting positively stained cells and intensity measurements using Metamorph software (Universal Imaging, Downington, PA) were performed. Minimums of at least 10-fields were chosen at random for counting and data were expressed as mean ± S.E. For intensity measurements, the lowest value (≤50.00) was represented by a single + sign and each fold increase was represented by an additional + sign (16). MDA-9/Syntenin antibody was a mouse monoclonal antibody obtained from Abnova Corporation (H00006386-MOI, dilution 1:200) and detects a single band at 33KDa. The EGFR (#4267, clone D38B1, rabbit monoclonal), AKT (#4691, clone C67E7, rabbit monoclonal), AKT-S473 (#4060, clone D9E, rabbit monoclonal), E-cadherin (#3195, clone 24E10, rabbit monoclonal) and CTNNB1 (#9582, clone 6B3, rabbit monoclonal) antibodies were obtained from Cell Signaling Inc. (dilution 1:200) and C-Src-Y418, (#44660G, rabbit polyclonal) (dilution 1:200) from Invitrogen. Anti-mouse (#115-065-003) and anti-rabbit (#111-065-003) secondary antibodies were obtained from Jackson Immunoresearch (dilution 1:1000).
Plasmid Constructions and transfections
The wild type (wt) MDA-9/Syntenin cDNA (Gene ID: 6386) was subcloned into SalI and NotI sites of the phosphorylated cytomegalovirus pCMV/myc/cyto plasmid, which has an N-terminal myc tag (Invitrogen). The resultant plasmid was resequenced using the ABI BigDye cycle sequencing kit (Applied Biosystems) for verification of the insert sequence. SV-HUC-1 cells were transfected with MDA-9/Syntenin expression plasmid using FuGene 6 transfection reagent (Roche Diagnostics). An empty vector was used as control. Transfected cells were selected in 300 mg/ml of G418. The expression of c-myc-tagged MDA-9/Syntenin fusion protein was confirmed by Western blotting or Immunofluorescence (IF) analysis using the anti-c-myc antibody (Invitrogen) in the stable clones. For knock down (KD) experiments, MDA-9/syntenin-specific shRNA was constructed and cloned into the BamI-HindIII sites of pRNA3.1 vector (Genescript Corporation, Piscataway, NJ). The sequence of the shRNA is GGATCCGCCTAATGGACCACACCATTTCAAGAGAATGGTGTGGTCCATTAGGCC GAAGCTT. Stable clones of T24, SCaBER and SW780 cells with MDA-9/Syntenin KD were generated using the above plasmid. Transfected cells were selected in 200 mg/ml of G418. Western blotting analysis determined the level of KD. We have also constructed full length MDA9/Syntenin with a C terminal HA tag into pcDNA3.1/Hygro(+) (Invitrogen) between BamHI and XhoI, after PCR using the following primers: sense 5’-CGCGGATCCGCCACCATGTCTCTCTATCCATCTCTC-3’ and antisense 5’-CCGCTCGAGCGGTTAAGCGTAATCTGGAACATCGTATGGGTAAACCTCA GGAATGGTGTG-3’ (Figure S4). Another deletion construct containing only PDZ1-PDZ2 domains (Figure S4) was also constructed in the same vector as above after PCR using the sense 5’-CGCGGATCCGCCACCATGTCTCTCTATCCATCTCTC-3’ and antisense 5’-CCGCTCGAGCGGTTAAGCGTAATCTGGAACATCGTATGGGTACCTGTCACGAATGGTCATG-3’ primers. All the constructs generated were verified by sequencing. In transfection, HEK293 cells were transfected with the HA-tagged plasmids. Pooled clones selected in the presence of 50 mg/ml of hygromycin after one week were further analyzed by Western blotting analysis for the detection of HA (Sigma, dilution 1:1000).
Cell Proliferation Assay
Proliferation of the transfected cells plated in triplicate wells was determined by a BrdU incorporation assay kit (Roche Diagnostics) as per the manufacturer’s protocol. Data are presented as mean ± S.E. of duplicate experiments.
Dual color fluorescence in situ hybridization (FISH)
Dual color fluorescence in situ hybridization (FISH) was performed on formalin-fixed paraffin-embedded (FFPE) sections obtained from 44 UCC patients. All tumors had corresponding normal tissue. Following deparaffinization, pretreatment consisted of 10 min steam cooking in 10 mM citrate Acid solution followed by pepsin (4 mg/ml) digestion at 45°C for 30 minutes. Bacterial artificial chromosome (BAC)-derived test probe targeting mda-9/syntenin (8q12, RP11–23K11; BACPAC Resources Center) was labeled with Spectrum Orange; this was paired for dual-target hybridization diluted 1:50 in DenHyb hybridization buffer (Insitus Laboratories, Albuquerque, NM) with control probe CEP8 (probe targeting centromeric region of chromosome 8; Abbott Laboratories, Abbott Park, Illinois). The CEP probe provided enumeration of chromosome copy number for chromosome 8. 10 ml of the hybridization mix was applied to the sections, with simultaneous denaturing of probe and target at 80°C for 2 minutes, and 50°C for 45 minutes. Overnight hybridization at 37°C occurred in a humidified chamber and post-hybridization washes included 50% formamide/1X SSC (5 minutes) and 2X SSC (5 minutes). DAPI (0.125ng/ml) (Abbott Laboratories) served as a nuclear counterstain. Sections showing sufficient hybridization efficiency (majority of nuclei with signals) were considered informative and were scored. Non-neoplastic brain specimens served as the controls. Specimens were considered amplified for mda-9/syntenin when they demonstrated nuclei containing numerous red test probe signals with a test probe: control (CEP) probe ratio >2. Cases showing an increased number of test to control probe signals, but in a ratio of >1.2 but < or equal to 2 were scored as a low level gain for that respective test probe. Cases, in which both the test and control probes were equally increased in number, were considered to show polysomy for chromosome 8.
Cell Invasion Assay
Cell invasion capacity was assessed using the Cell Invasion Assay Kit (BD Biosciences) (17). The assay was performed in triplicate. Briefly, 1 × 104 cells in 500 µl of serum-free medium were plated in triplicate in the invasion chambers (24-well format). The bottom wells of the 24-well plate containing the individual chamber were supplemented with 750 µl of serum-containing medium. Plates were incubated for 24 hours in a tissue culture incubator followed by fixation in methanol for 5 minutes and staining with 0.5% crystal violet. At least 10 fields were randomly selected for counting the cells that invaded through the membrane from each group. Data presented as mean ± S.E. of duplicate experiments.
Immunofluorescence
Cells were cultured in tissue culture-treated chamber slides and fixed in 4% paraformaldehyde as described (18) and stained with the EGFR (Cell signaling # 4267, 1:200 dilution) overnight at 4 °C followed by staining with FITC or Texas red-tagged secondary antibody (Jackson Immunoresearch, 1:1000) for 1 hour at room temperature. Cells were then washed thoroughly with PBS and mounted with Prolong Gold antifade reagent (Invitrogen) and observed immediately under a fluorescent microscope. At least 10-fields were randomly selected for examining the staining intensity and distribution pattern of the proteins.
Western blotting (WB) and Co-Immunoprecipitation (co-IP) analysis
Preparation of whole cell lysates and Western blotting analysis was performed following standard protocols as described earlier (19). The Co-IP analysis was performed using Dynabead Protein G kit (#100.07D) and DynaMag-2 system (# 123.21D) and protocols from Invitrogen Corporation. Antibodies used for Co-IP analysis are described above. We obtained whole cell lysates of primary UCC tumors and corresponding normal tissues from Proteinbiotechnologies Inc. (http://www.proteinbiotechnologies.com/).
Colony focus formation assay
Stably transfected cells were plated in duplicate at low density for determining formation of single cell colonies in 10-cm plates. Cells were cultured in the presence of appropriate complete medium containing 200 mg/ml of G418 for 3-weeks. Plates were then stained with 5% crystal violet, photographed and colonies were counted from at least 10-randomly selected fields. Data represents the mean ± S.E. of duplicate experiments.
In vivo metastasis analysis
For tumor growth, 1 × 105 cells in 100 µl PBS were injected through the intra-cardiac route into athymic, 4–6 week-old female nude mice (Charles River). All experiments were performed in accordance with the Animal Care and Use Committee guidelines of Virginia Commonwealth University. Each group consisted of 6 mice and each experiment was repeated two times. Mice were examined every day and mice showing signs of morbidity were immediately sacrificed according to University guidelines. After 12-weeks, mice were sacrificed and lungs were removed. Focal tumor lesions in the lungs were counted in each mouse and FFPE sections were prepared and stained with standard H&E method for visualization of focal metastases and IHC analysis. Data presented as mean ± S.E. of duplicate experiments.
MDA-9/Syntenin promoter methylation analysis
Twelve paired (matched normal and tumor) UCC samples showing overexpression of MDA-9/Syntenin was analyzed. For bisulfite treatment and genomic sequencing, EpiTect Bisulfite kit (Qiagen, Valencia, CA) was used according to the manufacturer’s instructions. To span CpG islands in the promoter region of MDA-9/Syntenin, specific sets of primers were designed by MethPrimer (20). The primer sequences did not contain any CG dinucleotides. Methprimer's default criteria used for CpG islands were: 1) an Observed/Expected CpG ratio over 0.6, 2) the percentage of G plus C over 50%, and 3) a window size of at least 100 bp. Utilizing these criteria, clustered CpG islands within −315 to +621 nucleotide (nt) region relative to the transcription start site of MDA-9/Syntenin were identified. We designed two sets of non-overlapping bisulfite sequencing primers within these clustered CpG islands region. The first primer set includes forward 5'-GTATATAGTAGTGAGTGGGTGGTA −3' and reverse 5'-TAACCTCTCACCTAACCCACAAA −3'; the second primer set includes forward 5'- TTTGTGGGTTAGGTGAGAGGTTA −3' and reverse 5'- AACCAAAAACTCTTTCTTATATC −3'. Bisulfite-treated DNA was analyzed by touch-down PCR using the following conditions: 95°C for 5 min, followed by cycles consisting of a 30 second denaturation step at 95°C, holding at an annealing temperature for 30 sec, extension at 72°C for 1 min, with a final extension at 72°C for 5 min. The annealing temperature was then gradually decreased as two cycles at 64°C, 62°C, 60°C, 58°C and then 35 cycles at 56°C. The PCR products were purified using the QIAquick 96 PCR Purification Kit (Qiagen, Valencia, CA), according to the manufacturer’s instructions. Purified PCR products were then subjected to direct sequencing (Genewiz Inc, Germantown, MD) for the detection of the methylation pattern.
Statistical Analysis
Chi-square, Fisher’s exact or Student's t test tests were used when appropriate. Ordinal logistic regression analysis was also performed to determine the impact of various gene signature alterations alone and in various combinations. All p-values were two-sided and all confidence intervals were at the 95% level. Computation for all the analysis was performed using the Statistical Analysis System (SAS).
Results
MDA-9/Syntenin expression is elevated in UCC and correlates with disease progression
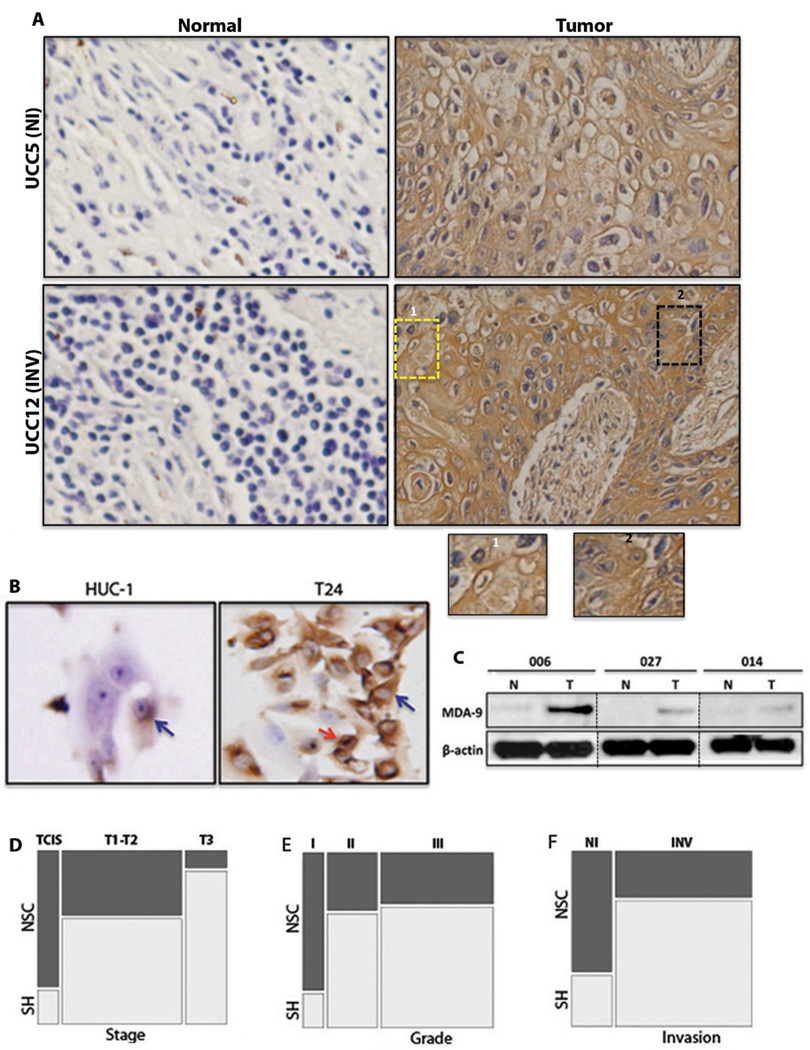
The expression pattern of MDA-9/Syntenin was examined in 44 primary UCC tumors containing both non-invasive and invasive counterparts. All cases were TCC and had corresponding adjacent normal tissue. IHC analysis confirmed significantly higher (p= 0.002–0.003) expression of MDA-9/Syntenin in 64% (28/44) of the primary tumors (Table S1 and Figure 1A). The non-cancer normal individual with reactive hyperplasia (UCC34) did not show increased expression of MDA-9/Syntenin (Table S1). Membranous or cytoplasmic expression of MDA-9/Syntenin was evident in both non-invasive and invasive tumors (Figure 1A, box 1 and inset 1). MDA-9/Syntenin expression was also noticeable in the nucleus of approximately 5% of the majority of the invasive primary tumors (Figure 1A, box 2 and inset 2). IHC of uroepithelial cell lines also revealed high level of membranous (blue arrow) and nuclear expression (red arrow) of MDA-9/Syntenin in tumorigenic T24 cells compared to a low level or undetectable level of membranous MDA-9/Syntenin expression (blue arrow) in non-tumorigenic HUC-1 cells (Figure 1B). Tumor-specific elevated expression of MDA-9/Syntenin was also confirmed by Western blotting analysis in 3 primary UCC tumors (Figure 1C). We further compared the expression pattern of MDA-9/Syntenin expression pattern with different clinical parameters. MDA-9/Syntenin expression was higher in each stage such as TCIS, T1-T2 and T3 compared to their corresponding normal (p= 0.002–0.003) and 20% (1/5) of TCIS, 62% (18/29) of T1-T2 and 90% (9/10) of the T3 staged tumors had higher expression of MDA-9/Syntenin. When compared across the stages, grades and with invasion status, MDA-9/Syntenin expression was significantly associated with progressive stages (p=0.01, Figure 1D), grades (p=0.03, Figure 1E) and invasion (p=0.02, Figure 1F) (Table S1).
Figure 1. Expression pattern of MDA-9/Syntenin in primary UCC tumors and its association with UCC progression.
A, MDA-9/Syntenin expression was significantly higher (p= 0.002–0.003) in primary tumors and was visible in the membrane (box 1 and inset 1) and nucleus (box 2 and inset 2). B, Markedly elevated levels of MDA-9/Syntenin expression were evident in the membrane (blue arrow) and nucleus (red arrow) in the tumorigenic T24 cells compared to the low level of membranous MDA-9/Syntenin expression in the non-tumorigenic HUC-1 cells. C, Elevated tumor-specific expression of MDA-9/Syntenin confirmed by Western blotting analysis in 3 primary urothelial cell tumors. All cases were transitional cell carcinomas. The expression of MDA-9/Syntenin significantly correlated with stage (p=0.01, D), grade (p=0.03, E) and invasion status (p=0.02, F) of the disease. N: Normal; T: Corresponding tumor; TCIS: Transitional cell carcinoma in situ, NI: Non-invasive carcinoma; INV: Invasive carcinoma; SH: Significantly higher; NSC: No significant changes. In all cases, ≥70% tumor cells were positive for MDA-9 expression compared to 10–20% positive cells in the corresponding normal. The mean intensity value in tumors (ranges between 107–213) and corresponding normal (ranges between 0–50) was represented by single or multiple + signs. Representative field values were then compared between matched normal and tumor by student t test to infer significance.
MDA-9/Syntenin enhances proliferation and invasion of non-tumorigenic uroepithelial HUC-1 cells
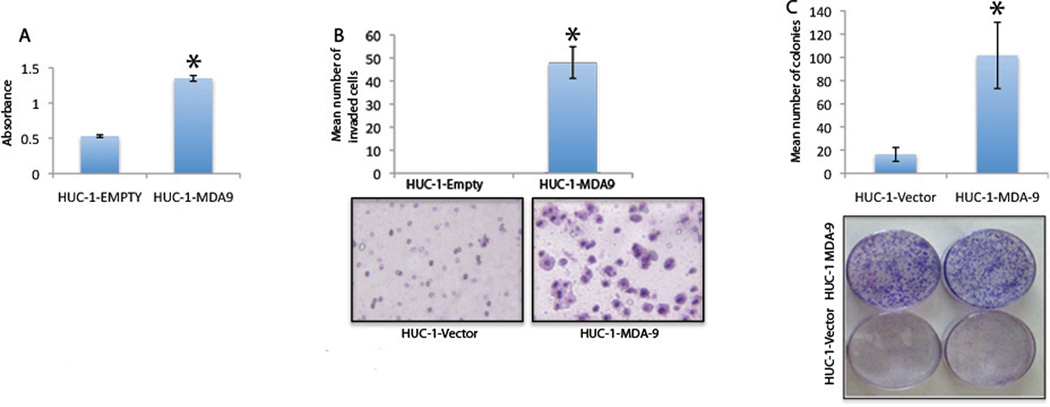
Elevated expression of MDA-9/Syntenin in invasive tumors and its direct association with disease progression suggests a potential role of this gene in invasive progression of UCC. To understand its precise role in UCC proliferation and progression, we stably overexpressed MDA-9/Syntenin in a non-tumorigenic uroepithelial HUC-1 cell line, which expresses low levels of endogenous MDA-9/Syntenin protein (Figure 1B and S1A). Western blotting analysis detected the c-myc tagged MDA-9/Syntenin protein in the transfected cells (Figure S1B). Since both clones displayed comparatively similar patterns of changes, we performed all subsequent studies with clone number 7. A significant increase was found in proliferation (p=0.0012, Figure 2A), invasion (p=0.0001, Figure 2B), and colony focus formation (p=0.0002, Figure 2C) in the MDA-9/Syntenin-overexpressed cells compared to empty vector-transfected cells.
Figure 2. Effect of MDA-9/Syntenin overexpression on non-tumorigenic uroepithelial HUC-1 cells.
Overexpression of MDA-9/Syntenin in HUC-1 cells results in a significant increase in proliferation (p=0.0012, A), invasion (p=0.0001, B), and colony focus formation (p=0.0002, C) as compared with the empty vector-treated group. Representative photomicrographs showing invasion and colony focus formation, respectively, are shown in panels B–C.
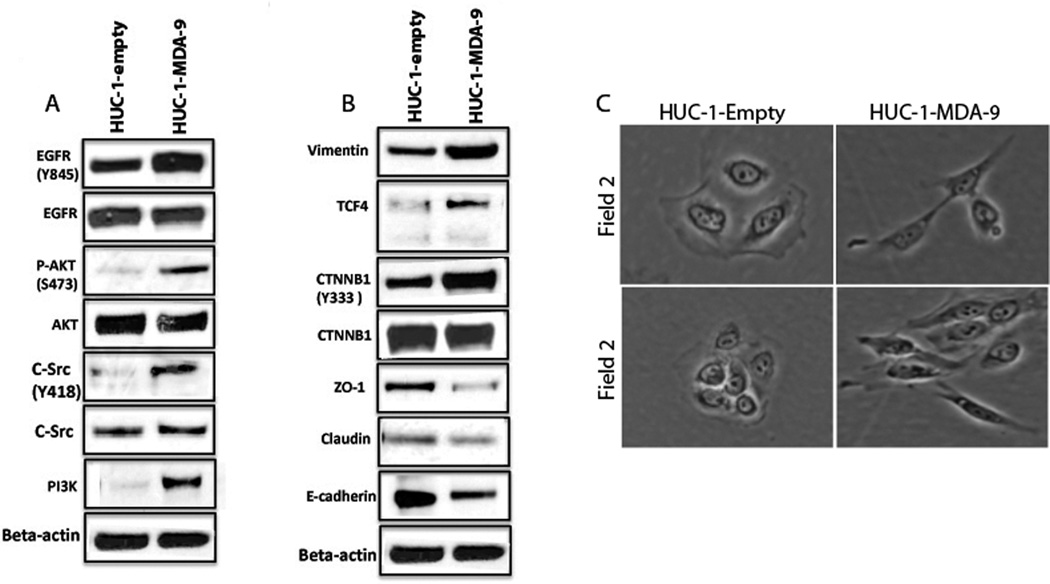
In addition to EGFR overexpression, EMT was implicated in UCC progression to muscle invasive disease (6). Since MDA-9/Syntenin overexpression was associated with invasive UCC progression and resulted in increased proliferation and invasion of HUC-1 cells, we examined the expression pattern of EGFR, its associated signaling and EMT molecules by Western blotting analysis. As shown in Figure 3A, we observed pronounced upregulation of activated EGFR (Tyr845), AKT (Ser473), c-Src (Tyr418) and PI3K (p101) in the MDA-9/Syntenin-transfected cells compared to empty vector-transfected cells. Among the various EMT molecules examined, we observed marked upregulation of β-catenin (Tyr333), vimentin and TCF4 (Figure 3B). In contrast, we observed discernible downregulation of ZO-1, Claudin-1 and E-cadherin in the MDA-9/Syntenin-transfected cells (Figure 3B). We also observed morphological changes corresponding to a mesenchymal phenotype in the non-tumorigenic uroepithelial cells overexpressing MDA-9/Syntenin (Figure 3C). Thus, forced overexpression of MDA-9/Syntenin significantly increases cellular growth, invasion and EMT that is accompanied with alterations of key EGFR-signaling and EMT-associated molecules.
Figure 3.
Marked upregulation of activated EGFR (Tyr845), AKT (Ser473), c-Src (Tyr418) and PI3K in the wt-MDA-9/Syntenin-transfected cells compared to the empty vector-treated cells (A). Discernible upregulation of β-catenin (CTNNB1) (Tyr333), vimentin and TCF4 was observed in the wt-MDA-9/Syntenin-transfected cells (B). Reduced expression of ZO-1, Claudin-1 and E-cadherin was also apparent in the wt-MDA-9/Syntenin-transfected cells (B). 1: Empty vector transfected- and 2: wt-MDA-9/Syntenin-transfected HUC-1 cells. β-actin was used as a loading control. Morphological changes corresponding to EMT phenotype in MDA-9/Syntenin overexpressing HUC-1 cells at two different microscopic fields. HUC-1-Empty: Empty vector transfected; HUC-1-MDA-9: MDA-9 transfected. Magnification X 200.
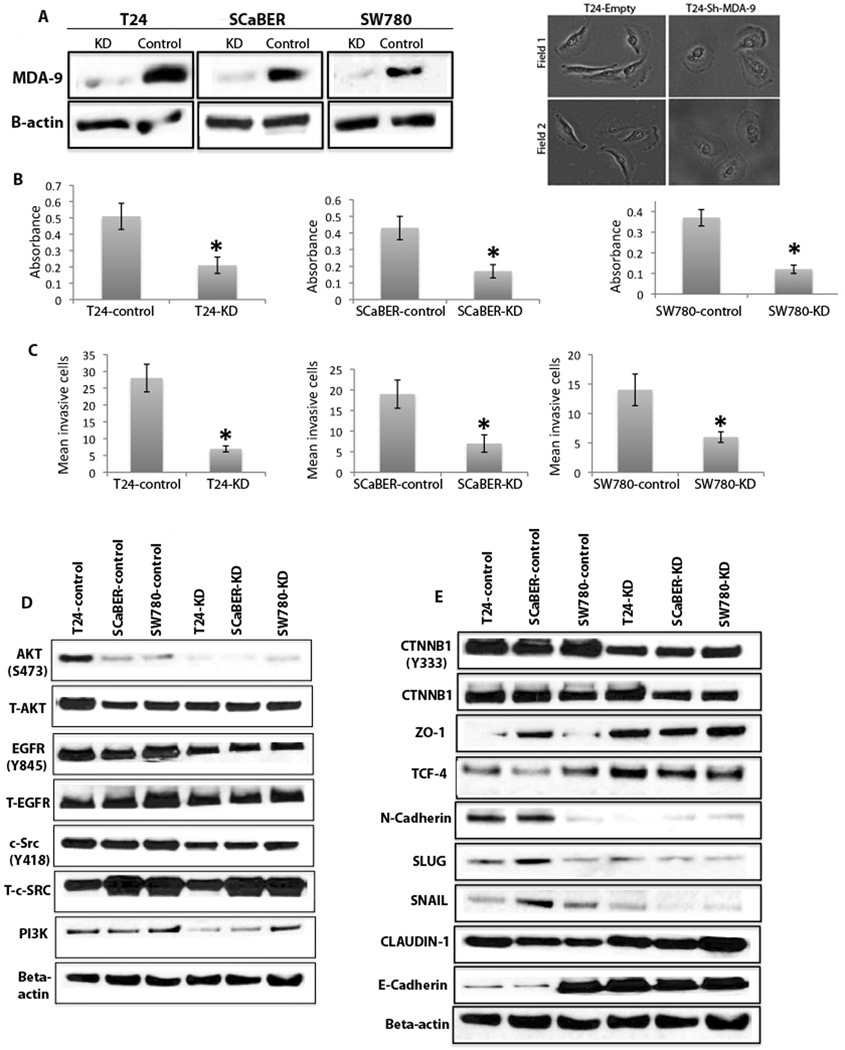
Knockdown (KD) of MDA-9/Syntenin inhibits cellular growth and invasion by inhibiting EGFR-signaling and key EMT-associated molecules
Since forced overexpression of MDA-9/Syntenin significantly modified cellular growth and altered expression of EGFR- and specific EMT-associated molecules, we examined the impact of KD of MDA-9/Syntenin in 3 UCC TCC cell lines, T24, SCaBER and SW780. Transfected cells displayed 70–80% stable KD of MDA-9/Syntenin (Figure 4A, left panel) and marked transition from a mesenchymal to epithelial phenotype in MDA-9/Syntenin depleted T24 cells (Figure 4A, right panel). Since comparable patterns of changes were observed in different clones, a single clone was selected from each UCC TCC cell line for all subsequent analyses. In the MDA-9/syntenin KD clones from the 3 UCC TCC cell lines there was significant inhibition of cellular proliferation (Figure 4B, p=0.002–0.003) and invasion (Figure 4C, p=0.001–0.002). Marked downregulation of activated EGFR (Tyr845) and its downstream and interacting signaling molecules AKT (Ser473), PI3K (p101) and c-Src (Tyr418) (Figure 4D) were also evident in these clones. In the MDA-9/Syntenin depleted T24 cells, we observed cytoplasmic/perinuclear localization of EGFR and low level of activated C-Src (Figure S2, white arrows) compared to the control T24 cells exhibiting predominantly membranous and cytoplasmic localization (Figure S2, yellow arrows). Notably, the membranous expression level of both of these molecules was comparatively low in the MDA-9/Syntenin-depleted cells compared to the control cells (Figure S2, yellow arrows). Notably, a decreased level of total AKT expression was also evident in the MDA-9/Syntenin KD T24 cells (Figure 4D). Of the various EMT molecules analyzed, we observed pronounced downregulation of β-catenin (CTNNB1) (Tyr333) in all 3 MDA-9/Syntenin-KD UCC cell lines (Figure 4E). Significant downregulation of N-cadherin, Slug and Snail with increased expression of ZO-1, TCF4, Claudin-1 and E-cadherin were observed in all but one of the UCC clones (Figure 4E). Accordingly, in general a similar pattern of molecular changes, except for TCF4, was observed following overexpression or KD of MDA-9/Syntenin in the UCC cells.
Figure 4. Impact of knockdown (KD) of MDA-9/Syntenin in UCC cells.
A, Effective KD of MDA-9/Syntenin in UCC cells (left panel). We observed 70–80% KD of MDA-9/Syntenin in the UCC cell lines. β-actin was used as a loading control. 1: KD: shMDA-9 transfected; 2: control: control-shRNA transfected; right panel: mesenchymal to epithelial transition in MDA-9 KD T24 cells at two different fields. T24-Empty; empty vector transfected; T24-ShMDA-9: MDA-9 transfected. Magnification X 200. B and C, Effect of KD of MDA-9/Syntenin on proliferation and invasion. Significant inhibition of cellular proliferation (p=0.002–0.003) and invasion (p=0.001–0.002) was observed in the MDA-9/Syntenin-KD cells compared to the empty vector transfected cells, respectively. C: control: control shRNA transfected; KD: shMDA-9 transfected. D, Marked downregulation of activated EGFR (Tyr845) and its downstream and interacting signaling molecules including AKT (Ser47), c-Src (Tyr418) and PI3K (p101) (left panel) following KD of MDA-9/Syntenin in UCC cells. E, Reduced expression of β-catenin (Tyr333) was evident in all MDA-9/Syntenin KD UCC cells and increased expression of ZO-1 and TCF4 was also evident in all but one MDA-9/Syntenin-KD cell line compared to control cells (right panel). Low expression of N-cadherin, Slug and Snail was also evident in all but one MDA-9/Syntenin KD UCC cell line. Markedly higher expression of claudin-1 was observed in all cell lines except for T24 cells. Markedly higher expression of E-cadherin was observed in all cell lines except for SW780. β-actin was used as a loading control.
MDA-9/Syntenin interacts and co-localizes with EGFR in UCC cells
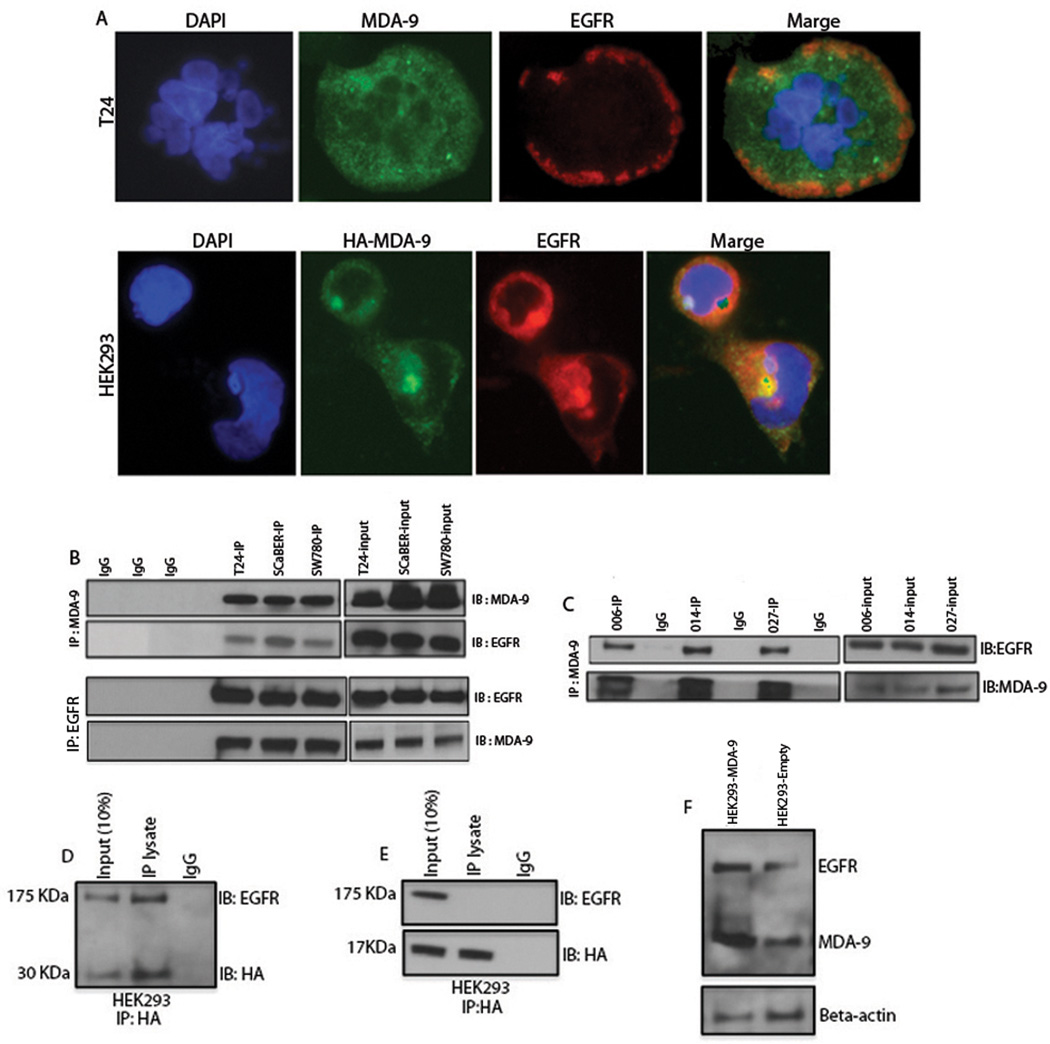
Since overexpression or KD of MDA-9/Syntenin significantly affected cellular proliferation and EGFR signaling in UCC cells, we examined whether EGFR- and MDA-9/Syntenin co-localize or physically interact. Immunofluorescence analysis was performed using both EGFR- and MDA-9/Syntenin-specific antibodies. Both membranous and cytoplasmic co-localization of EGFR and MDA-9/Syntenin were detected in the T24 cell line (Figure 5A, upper panel and Figure S3). We also confirmed co-localization of MDA-9/Syntenin and EGFR in HEK-293 cells transfected with HA-tagged MDA-9/Syntenin (Figure 5A, lower panel). In parallel, we performed co-immunoprecipitation analysis to determine whether MDA-9/Syntenin could pull down EGFR and vise versa. As shown in Figure 5B, MDA-9/Syntenin or EGFR interacted in all the 3 UCC cell lines. MDA-9/Syntenin could also pull down EGFR from 3 primary UCC tumors (Figure 5C) showing elevated levels of MDA-9/Syntenin (Figure 1C). We further utilized a HA-tagged wild type MDA-9/syntenin construct and confirmed MDA-9/Syntenin and EGFR interaction in HEK-293 cells (Figure 5D). MDA-9/Syntenin is known to interact through the PDZ domains (12). To identify the potential binding site(s) of MDA-9/Syntenin necessary to interact with EGFR, we utilized HA-tagged wild type MDA-9/Syntenin and a deletion mutation construct with intact PDZ1/PDZ2 domains but lacking both N and C terminal domains (Figure S4). However, MDA-9/Syntenin lacking both N and C terminal domains (17KDa) could not pull down EGFR, suggesting that N or C terminal domains of MDA-9/Syntenin contain potential binding sites for EGFR (Figure 5E). To further demonstrate MDA-9/Syntenin and EGFR interactions, we performed immunoprecipitation and Western blotting analysis with both MDA-9/Syntenin and EGFR antibodies in the HEK-293 cells overexpressing wild type MDA-9/Syntenin. The C terminal HA tag was evident in these cells (Figure 5D). Since MDA-9/Syntenin antibody uses a C terminal epitope, the overexpression of MDA-9/Syntenin was also detectable using the MDA-9/Syntenin antibody (Figure 5F, lane 1). A concomitant and appreciable increase in EGFR expression was also evident in these cells compared to the control cells (lane 2).
Figure 5. Co-localization and interaction of MDA-9/Syntenin with EGFR in UCC cells.
A, Immunofluorescence analysis using EGFR and MDA-9/Syntenin specific antibodies showing co-localization of both EGFR and MDA-9/Syntenin in the membrane of T24 cells (upper panel). Co-localization of MDA-9/Syntenin and EGFR was also confirmed in HEK-293 cells transfected with HA-tagged MDA-9/Syntenin (lower panel). B, Reciprocal interaction between MDA-9/Syntenin and EGFR in the 3 UCC cell lines was detected as shown using either MDA-9/Syntenin (upper panel) or EGFR (lower panel) antibody. Whole cell lysates were immunoprecipitated and immunoblotted with anti-MDA-9/Syntenin or EGFR antibody. C, MDA-9/Syntenin pulls down EGFR in 3 primary UCC tumors showing high levels of MDA-9/Syntenin expression. D, Confirmation of MDA-9/Syntenin and EGFR interaction in HEK293 cells transfected with HA-tagged MDA-9/Syntenin. Anti-HA antibody successfully pulled down EGFR. E, Ha-tagged MDA-9/Syntenin lacking both N and C terminal domains (17KDa) fails to immunoprecipitate EGFR in HEK293 cells. F, MDA-9/Syntenin overexpression in HEK-293 cells resulted in an marked increase in EGFR expression (lane 1) compared to the control (empty) cells (lane 2).
Co-expression signature of MDA-9/Syntenin, EGFR, AKT, β-catenin, E-cadherin and c-Src in primary UCC tumors
To determine whether the molecular changes observed in the cell lines was recapitulated in human samples, we performed immunohistochemical analysis with EGFR, β-catenin (CTNNB1) (Tyr333), AKT (Ser473), c-Src (Tyr418) and E-cadherin antibodies in the same cohort of 44 primary tumors, described above. Analogous to the cell lines, we observed a significantly higher expression of EGFR (p=0.004) in 41% (18/44) of the primary UCC tumors (Figure S5 and Table S2 and S3). Significantly higher co-expression of EGFR and MDA-9/Syntenin was observed in 72% (13/18) of these primary tumors (Table S2 and S3, Figure S5). Significantly higher expression of β-catenin (CTNNB1) (Tyr333, p=0.002), AKT ((Ser473, p=0.001), c-Src (Tyr418, p=0.002) and lower expression of E-cadherin (p=0.006) was also evident in 43%, (19/44), 48% (21/44), 48% (21/44) and 50% (22/44) of primary tumors, respectively, compared to their corresponding normal counterparts (Table S2 and S3, Figure S5). Altered co-expression of β-catenin-Tyr333:MDA-9/Syntenin; AKT-Ser473:MDA-9/Syntenin; c-Src-Tyr418:MDA-9/Syntenin and E-cadherin:MDA-9/Syntenin was observed in 84% (16/19), 62% (13/21), 90% (19/21) and 77% (17/22) of cases, respectively (Table S2).
A two-gene expression signature is associated with UCC progression
We observed alterations of some key regulatory molecules, such as EGFR, β-catenin (CTNNB1) (Tyr333), AKT (Ser473), c-Src (Tyr418) and E-cadherin, following GOF/LOF analysis in the UCC cell lines that was recapitulated in the primary tumors. To determine whether their alteration in concert with MDA-9/Syntenin was associated with disease progression, we performed a logistic regression model analysis utilizing these alterations alone and in various combinations with MDA-9/Syntenin overexpression. We observed a significant correlation between MDA-9/Syntenin and EGFR co-expression with clinical stage (p=0.04), but not with grade (p=0.28) or invasion (p=0.05) (Table S2). A significant correlation was also observed between MDA-9/Syntenin and β-catenin co-expression with clinical stage (p=0.03) as well as with invasion (p=0.04), but not with grade (p=0.29). There was a significant correlation between MDA-9/Syntenin and AKT co-expression with clinical stage (p=0.01), but not with grade (p=0.29) or invasion (p=0.06). No significant association of these gene alterations was evident at a single gene level (except for MDA-9/Syntenin alone) or in a combination of all of the gene changes with any clinical parameter (p=0.92-0.08).
MDA-9/Syntenin KD UCC cells display reduced metastasis in vivo
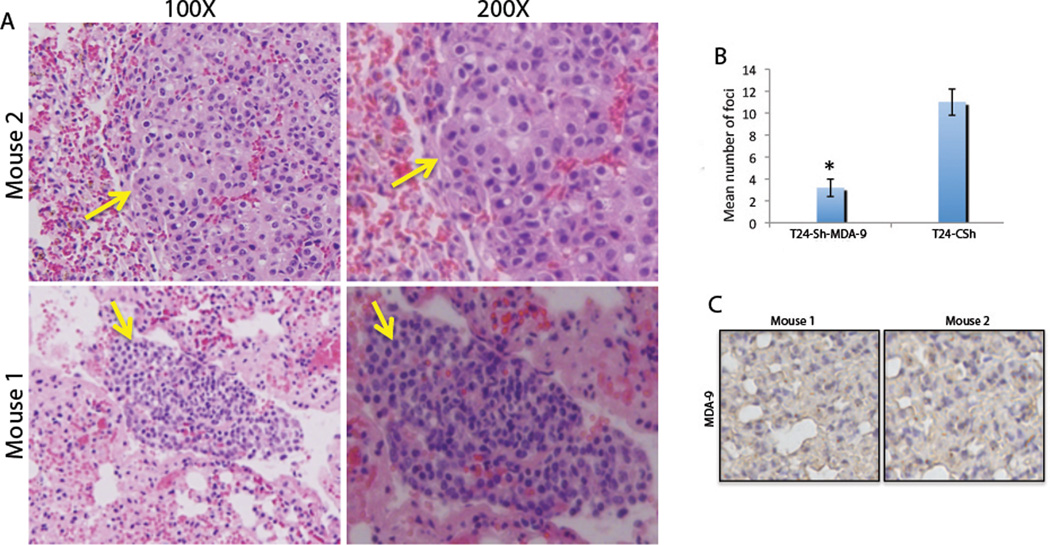
Both GOF and LOF analysis of MDA-9/Syntenin affected in vitro proliferation and invasion potential of UCC cells. A significant association between MDA-9/Syntenin overexpression and the progression of primary UCC tumors was also found. To examine the impact of MDA-9/Syntenin KD in vivo, we implanted empty vector transfected control T24 and MDA-9/Syntenin-KD-T24 cells through the intra-cardiac injection route into nude mice. As shown in Figure 6A, lung metastases caused by control T24 cells was evident at 12 weeks in cells that express steady-state levels of MDA-9/Syntenin (Figure S6). The number of metastatic foci was significantly reduced (p=0.0016) in the MDA-9/Syntenin-KD group compared to the control T24 cells (Figure 6B). In the surrounding normal lung tissues of the metastasized T24 cells, expression of MDA-9/Syntenin was barely detectable (Figure 6C).
Figure 6. Impact of knockdown of MDA-9/Syntenin on in vivometastasis of UCC cells.
A, Effect of KD of MDA-9/Syntenin on lung metastases. Lung metastases (left panel, yellow arrow) of the control shRNA transfected T24 cells was evident at 12 weeks as shown in several mice. B, The number of metastatic foci was significantly lower (p=0.0016) in the MDA-9/Syntenin KD-T24 group compared to the control group. C, Barely detectable levels of MDA-9/Syntenin expression were observed in the metastatic tumor’s surrounding normal lung tissues. Magnification X100 and X200.
Discussion
The precise role of MDA-9/Syntenin in UCC progression to muscle-invasive disease and its clinical relevance is mostly unknown. In the present study, progressive increase of MDA-9/Syntenin across the stages, grades and its association with invasion supports a potential contribution of MDA-9/Syntenin in UCC progression. A progressive increase of mda-9/syntenin mRNA from normal urothelium to carcinoma in situ to muscle invasive disease and its association with the development of therapeutic resistance and stable disease are evident in various expression databases (Figures S7 and S8). Notably, MDA-9/Syntenin overexpression was detectable as early as stage-I, thereby indicating its alteration at the initial stage of progression. Due to the lack of pre-cancer urothelial lesions, we could not assess the true earliest time point of MDA-9/Syntenin overexpression in development of UCC. In vivo metastasis of highly invasive T24 cells following MDA-9/Syntenin is strongly inhibited by LOF (knockdown) studies providing functional support for a definitive role of MDA-9/Syntenin in disease progression. Other studies reported association of MDA-9/Syntenin overexpression with metastatic progression of cutaneous and uveal melanoma patients (13). The nuclear localization of MDA-9/Syntenin in the UCC tumors could be necessary for interaction with several growth promoting molecules including CTNNB1, c-Src, which were upregulated following MDA-9/Syntenin overexpression. Nuclear localization of MDA-9/Syntenin was also observed in a recent study on uveal melanoma (13).
To define the mechanism underlying increased expression of MDA-9/Syntenin, we performed FISH analysis. However, no amplification of MDA-9/Syntenin was evident in any of these primary tumors (data not shown). Similarly, Gangemi et al. (13) did not detect any concomitant amplification of mda-9/syntenin in their cohort of 29 uveal melanoma samples, despite significantly higher expression levels of MDA-9/Syntenin. We also performed promoter methylation analysis hypothesizing hypomethylation as another potential mechanism of MDA-9/Syntenin activation. However, no significant increase in the level of hypomethylation was observed in the UCC cases (data not shown). Thus, overexpression of MDA-9/Syntenin may not be associated with gene amplification or promoter hypomethylation in these tumors. Other potential mechanism(s) including activating mutation, microRNA regulation, changes in promoter expression and/or alterations in the processing of mda-9/syntenin mRNA into protein, may contribute to enhanced MDA-9/Syntenin expression. These possibilities are currently being explored.
Earlier studies showed that MDA-9/Syntenin-mediated activation of AKT and c-Src, and MDA-9/Syntenin/c-Src interactions in breast cancer and melanoma cells, respectively (11, 21). When phosphorylated at Tyr418, c-Src can phosphorylate EGFR at Tyr845 and Tyr1101 and thereby regulate EGFR functions and tumor progression (22). We observed increased phosphorylation of both c-Src-Tyr418 and EGFR-Tyr845 in MDA-9/Syntenin overexpressing HUC-1 cells with concomitant increases in PI3K and AKT, which were reversed by KD of MDA-9/Syntenin in multiple UCC cell lines. Notably, MDA-9/Syntenin depletion altered localization of EGFR and activated C-Src in the UCC cells, which could be associated with their functional impairment. It is worth noting that in the majority of primary UCC tumors, we could detect simultaneous higher expression of EGFR, c-Src (Tyr418) and MDA-9/Syntenin. It appears that MDA-9/Syntenin overexpression activates c-Src, which in turn upregulates EGFR by phosphorylation at Tyr845 with concomitant activation of PI3K/AKT and resulted in increased proliferation. Co-localization and co-immunoprecipitation of MDA-9/Syntenin and EGFR in uroepithelial, HEK-293 cells and primary tumors further support this relationship. Moreover, the association between MDA-9/Syntenin:EGFR and MDA-9/Syntenin:AKT expression with progression implicates EGFR signaling as one of the key signaling pathways required for progression. To our knowledge, this is first report demonstrating that MDA-9/Syntenin mediates upregulation of cellular proliferation via EGFR signaling in UCC.
Epidermal growth factor receptor mediated activation of PI3K/AKT promotes key Wnt signaling molecule CTNNB1 transactivation and invasion (23). Recently, c-Src mediated phosphorylation of β-catenin at Y333 upon EGFR activation was implicated in tumorigenesis (24). In another study, MDA-9/Syntenin was shown to co-localize with β-catenin, Syndecan-1 and E-cadherin and co-immunoprecipitated with these proteins (25). We observed activation of CTNNB1) in MDA-9/Syntenin overexpressing HUC-1 cells with a concomitant increase in EGFR, PI3K, AKT and c-Src, as described above, with reversal of their expression following KD of MDA-9/Syntenin. Thus, CTNNB1 in concert with EGFR signaling may also be involved in UCC progression by physically interacting with each other. The association between MDA-9/Syntenin: CTNNB1 expression and progressive disease stage and invasion of primary UCC tumors strongly supports this assumption. Activation of the Wnt signaling pathway also requires association of CTNNB1 and T cell factor-4 (TCF4), which induces transcription of target genes, such as c-myc, Cyclin D1, VEGF and MMP-7 involved in cellular proliferation and invasion (24, 26). We observed a markedly increased TCF-4 expression in MDA-9/Syntenin overexpressing HUC-1 cells in concert with CTNNB1 activation. However, we did not observe the expected decrease of TCF-4 in UCC cells following KD of MDA-9/Syntenin, rather we observed a moderate increase of TCF4. A recent study demonstrated a tumor suppressor role for TCF4 in colon tumorigenesis (27). Thus, there could be a CTNNB1 independent role of TCF4 in concert with MDA-9/Syntenin to regulate UCC progression.
Alterations of adherens junctions and EMT play a crucial role in the development of muscle-invasive urothelial tumor (6). Progression of EMT correlates with the disintegration of cell-cell adhesion and alteration of various key junctional proteins including E-cadherin, ZO-1 and Claudin-1 (28). In the EMT, E-cadherin and ZO-1 are among the key molecules associated with the “epithelial phenotype”, whereas vimentin, CTNNB1 and N-cadherin with the “mesenchymal phenotype” (29). Selective overexpression of vimentin is observed in various cancers and correlates with increased migration and invasion of cancer cells (30). We observed decreased E-cadherin, ZO-1 and Claudin-1 and increased vimentin expression in the MDA-9/Syntenin overexpressing HUC-1 cells exhibiting characteristic EMT phenotype. Noticeably, reversal of the mesenchymal to epithelial transition phenotype was associated with increased E-cadherin, Claudin-1 and ZO-1 expression and concomitant decrease of Snail, Slug and N-cadherin expression was evident following KD of MDA-9/Syntenin. The altered pattern of E-cadherin was also recapitulated in UCC tumors further supported the relationship between MDA-9/Syntenin expression and metastasis through EMT regulation. E-cadherin is well established as a “suppressor of invasion” and is a common target for transcriptional repression in epithelial malignancies and its regulation is considered a pivotal step in the metastasis of various carcinomas (31, 32). In this cascade of events, the transcriptional repressors Snail and Slug are direct repressors of E-cadherin and Claudin-1 (32). Along this pathway, induction of aberrant N-cadherin expression and silencing of E-cadherin transcription and an E-cadherin to N- cadherin switch during metastatic progression are also evident in various cancers (33).
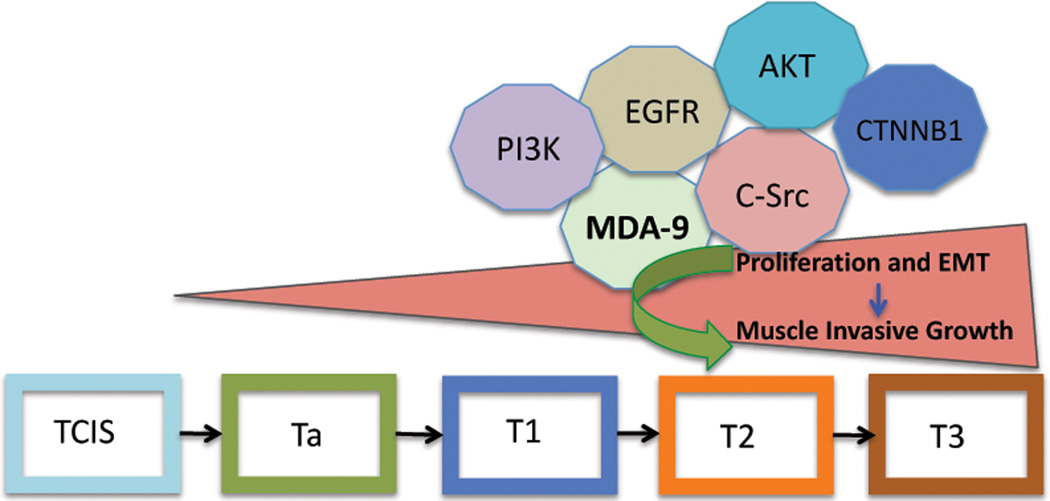
In summary, we demonstrate a marked alteration of cellular growth and invasion following overexpression or KD of MDA-9/Syntenin in uroepithelial cells. These phenotypes correlate with altered expression of EGFR and its downstream signaling partners, necessary for the progression from non-invasive to invasive disease (Figure 7). Conversely, alteration of CTNNB1 and other key EMT-associated molecules in concert with EGFR results in the disruption of the adherens junctions and leads to muscle invasive disease (Figure 7). MDA-9/Syntenin appears to be a fundamental molecule involved in UCC progression and could provide an attractive target to develop novel strategies for therapeutic management and monitoring UCC progression.
Figure 7. Schematic representation of MDA-9/Syntenin-mediated UCC progression.
Increased MDA-9/Syntenin expression during the pathological progression of UCC (TCIS to T3) and its cross talk with c-Src (Tyr418) can activate EGFR (Tyr845). The activated EGFR-PI3K–AKT can provide a continuous proliferation signal to the UCC cells and in turn transactivate β-catenin, a key molecule implicated in cellular growth and metastasis. On the other hand, disruption of adherens junctions and EMT by the simultaneous alteration of key regulatory molecules such as E-cadherin ZO-1, caludin-1, TCF4, and vimentin may lead to metastatic initiation and progression of UCC to invasive disease.
Supplementary Material
Translational Relevance.
Urothelial cell carcinoma (UCC) is an unpredictable disease that rapidly progresses from superficial to muscle invasive cancer, which poses significant obstacles to current treatment approaches. Identifying the key genes and relevant regulatory pathways leading to rapid spread of UCC is mandatory if one hopes to develop novel disease management strategies. The present study identifies MDA-9/Syntenin (human chromosome 8q12) as an abundantly overexpressed molecule from early stages of UCC development and associates with disease progression. MDA-9/Syntenin regulates UCC growth and metastatic progression in concert with various growth and epithelial to mesenchymal transition (EMT) regulatory molecules. This protein directly interacts with EGFR, which is a therapeutic target for many solid tumors, in UCC cell lines and patient samples. MDA-9/Syntenin alone or in combination with EGFR may provide a viable target to develop novel therapeutics as well as monitoring strategies for UCC.
Acknowledgments
Grant Support:
The present study was supported in part by National Institutes of Health grant CA097318 and the National Foundation for Cancer Research (PBF), and the Elisa U Pardee Foundation (SD). Funding also provided by the VCU Massey Cancer Center (PBF). We are grateful to Dr. Manny Bacolod, Department of Human and Molecular Genetics, VCU Institute of Molecular Medicine, Virginia Commonwealth University for the TCGA and GEO dataset analysis of the UCC patients.
Footnotes
Disclosure of Potential Conflict of Interest
No potential conflicts of interest.
Authors Contributions:
Conception and design: S. Dasgupta and P.B. Fisher
Development of methodology: S. Dasgupta
Acquisition of data: S. Dasgupta
Analysis and interpretation of Data: S. Dasgupta and P.B. Fisher
Writing, review, and/or revision of the manuscript: S. Dasgupta, D. Sarkar, P.B. Fisher
Administrative, technical or material support: S. Dasgupta, M. Menezes, S.K. Das, L. Emdad, C. Shao, Aleksandar Janjic, N.D. Mukhopadhyay.
Study supervision: S. Dasgupta and P. B. Fisher.
References
- 1.Murta-Nascimento C, Schmitz-Drager BJ, Zeegers MP, Steineck G, Kogevinas M, Real FX, et al. Epidemiology of urinary bladder cancer: from tumor development to patient’s death. World J Urol. 2007;25:285–295. doi: 10.1007/s00345-007-0168-5. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs BL, Lee CT, Montie JE. Bladder cancer in 2010: How far have we come. CA Cancer J Clin. 2010;60:244. doi: 10.3322/caac.20077. [DOI] [PubMed] [Google Scholar]
- 3. [(data accesses 6/21/2013)]; www.cancer.gov.
- 4.Proctor I, Stoeber K, Williams GH. Biomarkers in bladder cancer. Histopathology. 2010;57:1–13. doi: 10.1111/j.1365-2559.2010.03592.x. [DOI] [PubMed] [Google Scholar]
- 5.Habuchi T, Marberger M, Droller MJ, Hemstreet GP, 3rd, Grossman HB, Schalken JA, et al. Prognostic markers for bladder cancer: International consensus panel on bladder cancer markers. Urology. 2005;66:64–74. doi: 10.1016/j.urology.2005.08.065. [DOI] [PubMed] [Google Scholar]
- 6.McConkey DJ, Choi W, Marquis L, Martin F, Williams MB, Shah J, et al. Role of epithelial-to-mesenchymal transition (EMT) in drug sensitivity and metastasis in bladder cancer. Cancer Metastasis Rev. 2009;28:335–344. doi: 10.1007/s10555-009-9194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarkar D, Boukerche H, Su ZZ, Fisher PB. mda-9/syntenin: recent insights into a novel cell signaling and metastasis-associated gene. Pharmacol Ther. 2004;104:101–115. doi: 10.1016/j.pharmthera.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Boukerche H, Su ZZ, Emdad L, Baril P, Balme B, Thomas L, et al. mda-9/Syntenin: a positive regulator of melanoma metastasis. Cancer Res. 2005;65:10901–10911. doi: 10.1158/0008-5472.CAN-05-1614. [DOI] [PubMed] [Google Scholar]
- 9.Boukerche H, Su ZZ, Emdad L, Sarkar D, Fisher PB. mda-9/Syntenin regulates the metastatic phenotype in human melanoma cells by activating nuclear factor-kappaB. Cancer Res. 2007;67:1812–1822. doi: 10.1158/0008-5472.CAN-06-3875. [DOI] [PubMed] [Google Scholar]
- 10.Sarkar D, Boukerche H, Su ZZ, Fisher PB. mda-9/Syntenin: more than just a simple adapter protein when it comes to cancer metastasis. Cancer Res. 2008;68:3087–3093. doi: 10.1158/0008-5472.CAN-07-6210. [DOI] [PubMed] [Google Scholar]
- 11.Boukerche H, Aissaoui H, Prévost C, Hirbec H, Das SK, Su ZZ, et al. Src kinase activation is mandatory for MDA-9/syntenin-mediated activation of nuclear factor-kappaB. Oncogene. 2010;29:3054–3066. doi: 10.1038/onc.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das SK, Bhutia SK, Kegelman TP, Peachy L, Oyesanya RA, Dasgupta S, et al. MDA-9/syntenin: a positive gatekeeper of melanoma metastasis. Front Biosci. 2012;17:1–15. doi: 10.2741/3911. [DOI] [PubMed] [Google Scholar]
- 13.Das SK, Bhuttia SK, Sokhi UK, Azab B, Su ZZ, Boukerche H, et al. Raf kinase inhibitor RKIP inhibits MDA-9/syntenin-mediated metastasis in melanoma. Cancer Res. 2012;72:6217–6226. doi: 10.1158/0008-5472.CAN-12-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das SK, Bhuttia SK, Azab B, Kegelman TP, Peachy L, Santhekadur PK, et al. MDA-9/syntenin and IGFBP-2 promote angiogenesis in human melanoma. Cancer Res. 2013;73:844–854. doi: 10.1158/0008-5472.CAN-12-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gangemi R, Mirisola V, Barisione G, Fabbi M, Brizzolara A, Lanza F, et al. Mda-9/syntenin is expressed in uveal melanoma and correlates with metastatic progression. PLoS One. 2012;7(1):e29989. doi: 10.1371/journal.pone.0029989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dasgupta S, Bhattacharya-Chatterjee M, O'Malley BW, Jr, Chatterjee SK. Tumor metastasis in an orthotopic murine model of head and neck cancer: possible role of TGF-Beta 1 secreted by the tumor cells. J Cell Biochem. 2006;97:1036–1051. doi: 10.1002/jcb.20647. [DOI] [PubMed] [Google Scholar]
- 17.Dasgupta S, Hoque MO, Upadhyay S, Sidransky D. Mitochondrial cytochrome B gene mutation promotes tumor growth in bladder cancer. Can Res. 2008;68:700–706. doi: 10.1158/0008-5472.CAN-07-5532. [DOI] [PubMed] [Google Scholar]
- 18.Dasgupta S, Hoque MO, Upadhyay S, Sidransky D. Forced Cytochrome B gene mutation expression induces mitochondrial proliferation and prevents apoptosis in human uroepithelial SV-HUC-1 cells. Int J Cancer. 2009;125:2829–2835. doi: 10.1002/ijc.24701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boukerche H, Su-Z-z, Prevot C, Sarkar D, Fisher PB. mda-9/Syntenin promotes metastasis in human melanoma cells by activating c-Src. Proc Natl Acad Sci USA. 2008;105:15914–15919. doi: 10.1073/pnas.0808171105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li LC, Dahiya R. MethPrimer: designing primers for methylation PCRs. Bioinformatics. 2002;18:1427–1431. doi: 10.1093/bioinformatics/18.11.1427. [DOI] [PubMed] [Google Scholar]
- 21.Hwangbo C, Park J, Lee JH. mda-9/Syntenin protein positively regulates the activation of Akt protein by facilitating integrin-linked kinase adaptor function during adhesion to type I collagen. J Biol Chem. 2011;286:33601–33612. doi: 10.1074/jbc.M110.206789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biscardi JS, Maa MC, Tice DA, Cox ME, Leu TH, Parsons SJ. c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J Biol Chem. 1999;274:8335–8343. doi: 10.1074/jbc.274.12.8335. [DOI] [PubMed] [Google Scholar]
- 23.Hu T, Li C. Convergence between Wnt-β-catenin and EGFR signaling in cancer. Mol Cancer. 2010;9:236–243. doi: 10.1186/1476-4598-9-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang W, Xia Y, Ji H, Zheng Y, Liang J, Huang W, et al. Nuclear PKM2 regulates β-catenin transactivation upon EGFR activation. Nature. 2011;480:118–122. doi: 10.1038/nature10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimmermann P, Tomatis D, Marcela R, Grootjans J, Leenaerts I, Degeest G, et al. Characterization of syntenin, a syndecan-binding PDZ protein as a component of cell adhesion sites and microfilaments. Mol Biol Cell. 2001;12:339–350. doi: 10.1091/mbc.12.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang LH, Xu HT, Han Y, Li QC, Liu Y, Zhao Y, et al. Axin downregulates TCF-4 transcription via beta-catenin, but not p53, and inhibits the proliferation and invasion of lung cancer cells. Mol Cancer. 2010;9:25–39. doi: 10.1186/1476-4598-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Angus-Hill ML, Elbert KM, Hidalgo J, Capecchi MR. T-cell factor 4 functions as a tumor suppressor whose disruption modulates colon cell proliferation and tumorigenesis. Proc Natl Acad Sci U S A. 2011;108:4914–4919. doi: 10.1073/pnas.1102300108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh AB, Sharma A, Dhawan P. Claudin family of proteins and cancer: an overview. J Oncology. 2010 doi: 10.1155/2010/541957. ID541957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kusinska RU, Kordek R, Pluciennik E, Bednarek AK, Piekarski JH, Potemski P. Does vimentin help to delineate the so-called ‘basal type breast cancer? J Exp Clin Can Res. 2009;28:118–127. doi: 10.1186/1756-9966-28-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan AOO. E-cadherin in gastric cancer. World J Gastroenterol. 2006;12:199–203. doi: 10.3748/wjg.v12.i2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez-Estrada OM, Culleres A, Soriano FX, Peinado H, Bolos V, Martinez FO, et al. The transcription factors Slug and Snail act as repressors of Claudin-1 expression in epithelial cells. Biochem J. 2006;394:449–457. doi: 10.1042/BJ20050591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maret D, Gruzglin E, Sadr MS, Siu V, Shan W, Koch AW, et al. Surface Expression of Precursor N-cadherin Promotes Tumor Cell Invasion. Neoplasia. 2010;12:1066–1080. doi: 10.1593/neo.10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.