Abstract
Background
The prognostic nutritional index (PNI) is related to the prognosis in many cancers; however, its role in esophageal cancer is still controversial. Further, controversy exists concerning the optimal cut-off points for PNI to predict survival. The aim of this study was to determine the prognostic value of PNI and propose the optimal cut-off points for PNI in predicting cancer-specific survival (CSS) in esophageal squamous cell carcinoma (ESCC).
Methods
This retrospective study included 375 patients who underwent esophagectomy for ESCC. The PNI was calculated as 10 × serum albumin (g/dL) + 0.005 × total lymphocyte count (per mm3). With the help of the fit line on the scatter plot, we classified the patients into three categories according to the PNI, ie, >52, 42–52, and <42.
Results
Our study showed that PNI was associated with tumor length (P=0.007), T grade (P=0.001), and N staging (P<0.001). The 5-year CSS in patients with PNI <42, 42–52, and >52 were 11.0%, 39.1%, and 55.2%, respectively (P<0.001). Multivariate analysis showed that PNI was a significant predictor of CSS (42–52 versus >52, P=0.011; <42 versus PNI >52, P<0.001).
Conclusion
PNI is a predictive factor for long-term survival in ESCC. The survival rate of ESCC can be discriminated between three groups, ie, PNI <42, 42–52, and >52.
Keywords: esophageal squamous cell carcinoma, prognostic nutritional index, prognostic factor, survival
Introduction
Esophageal cancer is the eighth most common cancer worldwide, with 482,000 new cases estimated in 2008, and the sixth most common cause of death from cancer, with 407,000 deaths.1 In the People’s Republic of China, esophageal cancer is the fourth most common cause of mortality, with 16.24 deaths per 100,000 in 2008.2 Although advances have occurred in multidisciplinary treatment, surgical resection remains the modality of choice. The 5-year overall survival after surgery is poor, the reason being the relatively late stage of diagnosis and rapid progression.3,4 Therefore, assessing the prognostic factors in esophageal cancer will become more and more important.
The preoperative nutritional status have been demonstrated to be associated not only with postoperative complications, but also with the long-term outcomes in patients with malignant tumors.5,6 The prognostic nutritional index (PNI), which is calculated based on serum albumin and total lymphocyte count in peripheral blood was originally proposed to assess the perioperative nutritional conditions and postoperative complications in patients with gastrointestinal tumors.7 Recently the PNI has been shown to be a prognostic marker for various malignancies.8–10 However, few studies are available regarding PNI in esophageal cancer, and the clinical significance and prognostic value of this marker remain uncertain.11,12 Further, controversy exists concerning the optimal cut-off points for PNI to predict overall survival. In addition, esophageal squamous cell carcinoma (ESCC) is the most common pathological type in the People’s Republic of China, in contrast with the predominance of adenocarcinoma in the Western world.13 Thus, the aim of this study was to determine the prognostic value of the PNI and propose an optimal cut-off point for PNI in predicting cancer-specific survival (CSS) in patients with ESCC.
Materials and methods
Patients
From January 2006 to December 2008, a retrospective analysis was conducted in 375 patients with ESCC who underwent curative esophagectomy at Zhejiang Cancer Hospital in Hangzhou, People’s Republic of China. All of the patients included in the analysis met the following inclusion criteria: ESCC confirmed by histopathology; surgery with curative esophagectomy; at least six lymph nodes examined for pathological diagnosis; surgery neither preceded nor followed by chemotherapy and/or radiotherapy; and preoperative serum albumin and lymphocyte count obtained within 1 week before esophagectomy. All subjects gave their written informed consent to the study protocol, which was approved by the ethics committee of Zhejiang Cancer Hospital, Hangzhou, People’s Republic of China.
Surgery
All patients were treated by radical resection. The standard surgical approach consisted of a limited thoracotomy on the right side and intrathoracic gastric reconstruction (the Ivor Lewis procedure) for lesions at the middle/lower third of the esophagus. Upper third lesions were treated by cervical anastomosis (the McKeown procedure). The majority of patients at our institution underwent two-field lymphadenectomy. In this cohort of patients, thoracoabdominal lymphadenectomy was performed, including the subcarinal, paraesophageal, pulmonary ligament, diaphragmatic, and paracardial lymph nodes, as well as those located along the lesser gastric curvature, the origin of the left gastric artery, the celiac trunk, the common hepatic artery, and the splenic artery. Three-field lymphadenectomy was performed only if the cervical lymph nodes were thought to be abnormal upon preoperative evaluation. All of the patients included in the study were restaged according to the seventh edition of the American Joint Committee on Cancer staging manual.14
Follow-up
At our institution, patients were followed up in our outpatient department every 3–6 months for the first 2 years after resection, then annually. Recording of medical history, physical examination, and computed tomography of the chest were performed during follow-up. Endoscopy was obtained in cases of clinically indicated recurrence or metastasis. Given that this series described the prognosis of patients with ESCC, a cancer-specific survival (CSS) analysis was deemed to be appropriate. Therefore, the CSS was ascertained in this study. The last follow-up was November 30, 2011.
Statistical analysis
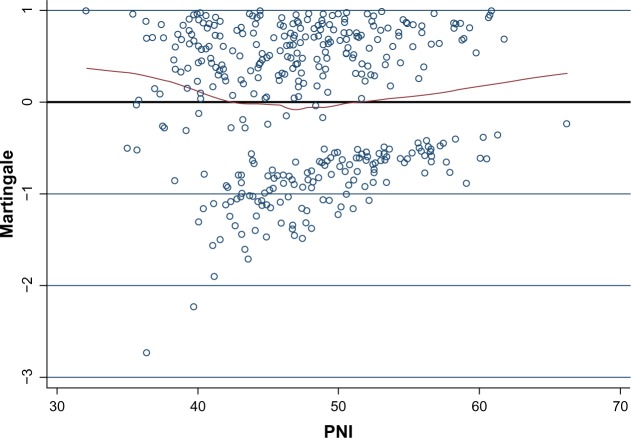
The statistical evaluation was done using the Statistical Package for the Social Sciences version 17 software (SPSS Inc., Chicago, IL, USA) and Stata version 12.0 (Statacorp, College Station, TX, USA). The PNI was calculated using the following formula: 10 × serum albumin (g/dL) + 0.005 × total lymphocyte count (per mm3).7 To determine the optimal cut-off points for the PNI as a predictor of CSS, the relationship between the PNI and death from ESCC was investigated using a scatter plot of the variable versus Martingale residuals from a Cox proportional hazard regression model without the variable of interest.15 A smoothed line fit of the scatter was applied to detect the optimal cut-off point. To determine the optimal cut-off points for PNI, the Martingale residuals of the Cox model (including sex, age, tumor location, tumor length, differentiation, T grade, and N staging) were calculated and then plotted against the PNI. With the help of the fit line on the scatter plot, we classified the patients into three categories according to their PNI, ie, <42, 42–52, and >52 (Figure 1). The Pearson chi-squared test was used to determine the significance of differences between dichotomous variables. CSS was calculated by the Kaplan–Meier method, and the difference was assessed by the log-rank test. A univariate analysis was used to examine the association between various prognostic predictors and CSS. Multivariate analyses were performed using the Cox regression proportional hazard model to evaluate the prognostic parameters for CSS. Hazard ratios with 95% confidence intervals were used to quantify the strength of the association between predictors and survival. A P-value less than 0.05 was considered to be statistically significant.
Figure 1.
Scatter plot of PNI on the x-axis versus Martingale residuals (n=375). Patients above the horizontal line (zero) were at an increased risk for death compared with the expected risk from the Cox proportional hazards regression model. The curved line represents a smoother scatter plot. The point at which the smoother line crosses the horizontal line (zero) occurs at 42, indicating that this would be the best cut-off point to predict death from esophageal squamous cell carcinoma based on PNI. The point at which the curve line crosses the horizontal line (zero) is at a PNI of 52, indicating that this would be another cut-off point. With the help of the fit line on the scatter plot, we classified the patients into three categories according to PNI, ie, <42, 42–52, and >52.
Abbreviation: PNI, prognostic nutritional index.
Results
Patient characteristics
The baseline characteristics are shown in Table 1. Among the 375 patients, 49 (13.1%) were women and 326 (86.9%) were men. The mean age was 59.1±7.8 years, with an age range of 36–80 years. The most common tumor location was the middle and lower esophagus (94.7%). The most common differentiation was the moderate type (65.6%).
Table 1.
Baseline characteristics of 375 patients with ESCC
| Cases, n (%) | |
|---|---|
| Age, years (mean ± SD) | 59.1±7.8 |
| Sex | |
| Female | 49 (13.1) |
| Male | 326 (86.9) |
| Tumor length, cm (mean ± SD) | 4.3±1.8 |
| Tumor location | |
| Upper | 20 (5.3) |
| Middle | 180 (48.0) |
| Lower | 175 (46.7) |
| Differentiation | |
| Well | 52 (13.9) |
| Moderate | 246 (65.6) |
| Poor | 77 (20.5) |
| T grade | |
| T1 | 63 (16.8) |
| T2 | 62 (16.5) |
| T3 | 210 (56.0) |
| T4a | 40 (10.7) |
| N staging | |
| N0 | 201 (53.6) |
| N1 | 97 (25.8) |
| N2 | 48 (12.8) |
| N3 | 29 (7.8) |
Abbreviations: ESCC, esophageal squamous cell carcinoma; SD, standard deviation.
Analysis of PNI and clinicopathological characteristics
The relationship between PNI and the clinicopathological characteristics of the 375 patients who underwent surgery for ESCC are shown in Table 2. Our study showed that PNI was associated with tumor length (P=0.007), T grade (P=0.001), and N staging (P<0.001).
Table 2.
Relationship between PNI and clinicopathological characteristics
| Cases, n (%) | PNI <42, n (%) | PNI 42–52, n (%) | PNI >52, n (%) | P-value | |
|---|---|---|---|---|---|
| Age (years) | 0.372 | ||||
| ≤60 | 214 (57.1) | 38 (52.5) | 119 (60.4) | 57 (54.3) | |
| >60 | 161 (42.9) | 35 (47.5) | 78 (39.6) | 48 (45.7) | |
| Sex | 0.834 | ||||
| Female | 49 (13.1) | 8 (11.0) | 27 (13.7) | 14 (13.3) | |
| Male | 326 (86.9) | 65 (89.0) | 170 (86.3) | 91 (86.7) | |
| Smoking | 0.962 | ||||
| No | 190 (50.7) | 36 (49.3) | 100 (50.8) | 54 (51.4) | |
| Yes | 185 (49.3) | 37 (50.7) | 97 (49.2) | 51 (48.6) | |
| Drinking | 0.097 | ||||
| No | 234 (62.4) | 42 (57.5) | 133 (67.5) | 59 (56.2) | |
| Yes | 141 (37.6) | 31 (42.5) | 64 (32.5) | 46 (43.8) | |
| Tumor length (cm) | 0.007 | ||||
| ≤3 | 99 (26.4) | 9 (12.3) | 56 (28.4) | 34 (32.4) | |
| >3 | 276 (73.6) | 64 (87.7) | 141 (71.6) | 71 (67.6) | |
| Tumor location | 0.354 | ||||
| Upper | 20 (5.3) | 5 (6.8) | 8 (4.1) | 7 (6.7) | |
| Middle | 180 (48.0) | 41 (56.2) | 92 (46.7) | 47 (44.8) | |
| Lower | 175 (47.3) | 27 (37.0) | 97 (49.2) | 51 (48.5) | |
| Vessel involvement | 0.089 | ||||
| Negative | 310 (82.7) | 59 (80.8) | 157 (79.7) | 94 (89.5) | |
| Positive | 65 (17.3) | 14 (19.2) | 40 (20.3) | 11 (10.5) | |
| Perineural invasion | 0.217 | ||||
| Negative | 304 (81.1) | 55 (75.3) | 159 (80.7) | 90 (85.7) | |
| Positive | 71 (18.9) | 18 (24.7) | 38 (19.3) | 15 (14.3) | |
| Differentiation | 0.228 | ||||
| Well | 52 (13.9) | 10 (13.7) | 29 (14.7) | 13 (12.4) | |
| Moderate | 246 (65.6) | 41 (56.2) | 132 (67.0) | 73 (69.5) | |
| Poor | 77 (20.5) | 22 (30.1) | 36 (18.3) | 19 (18.1) | |
| T grade | 0.001 | ||||
| T1 | 63 (16.8) | 4 (5.5) | 33 (16.8) | 26 (24.8) | |
| T2 | 62 (16.5) | 7 (9.6) | 38 (19.3) | 17 (16.2) | |
| T3 | 210 (56.0) | 48 (65.8) | 105 (53.3) | 57 (54.3) | |
| T4a | 40 (10.7) | 14 (19.1) | 21 (10.6) | 5 (4.7) | |
| N staging | <0.001 | ||||
| N0 | 201 (53.6) | 21 (28.8) | 114 (57.9) | 66 (62.9) | |
| N1 | 97 (25.8) | 26 (35.6) | 49 (24.9) | 22 (21.0) | |
| N2 | 48 (12.8) | 14 (19.2) | 24 (12.2) | 10 (9.5) | |
| N3 | 29 (7.8) | 12 (16.4) | 10 (5.0) | 7 (6.6) | |
Abbreviation: PNI, prognostic nutritional index.
Analysis of CSS and prognostic factors
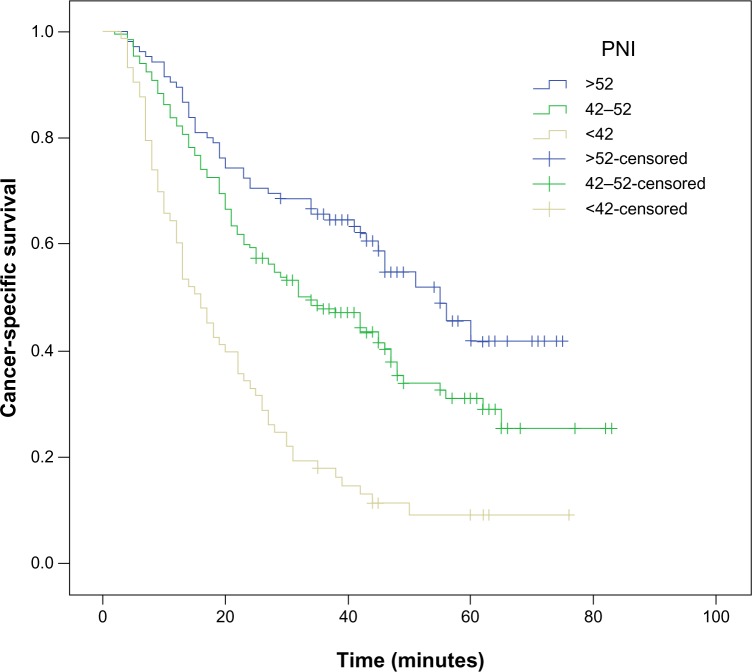
The 5-year CSS was 38.1% in our study. The 5-year CSS in patients with PNI <42, 42–52, and >52 was 11.0%, 39.1%, and 55.2%, respectively (P<0.001, Figure 2). By univariate analysis, we found that seven clinicopathological variables had a significant association with 5-year CSS (Table 3). All of confounding factors were then included in a multivariate Cox proportional hazards model (enter procedure) to adjust for the effects of covariates. In that model, we demonstrated that PNI was a significant predictor of CSS (42–52 versus >52, P=0.011; <42 versus >52, P<0.001, Table 3).
Figure 2.
Kaplan–Meier survival curves stratified by PNI.
Notes: The 5-year CSS in patients with PNI <42, 42–52, and >52 was 11.0%, 39.1%, and 55.2%, respectively (P<0.001). Thus, the PNI was able to clearly classify such patients into three independent groups.
Abbreviations: CSS, cancer-specific survival; PNI, prognostic nutritional index.
Table 3.
Univariate and multivariate analyses in patients with ESCC
| 5-year CSS (%) | Univariate analysis
|
Multivariate analysis
|
|
|---|---|---|---|
| Chi-square, P-value | HR (95% CI) P-value | ||
| Age, years | 0.074, 0.785 | ||
| ≤60 | 38.3 | 1.000 | |
| >60 | 37.9 | 0.932 (0.705–1.231) 0.620 | |
| Sex | 0.761, 0.383 | ||
| Female | 44.9 | 1.000 | |
| Male | 37.1 | 0.877 (0.575–1.337) 0.542 | |
| Smoking | 2.686, 0.101 | ||
| No | 42.1 | 1.000 | |
| Yes | 34.1 | 1.112 (0.844–1.467) 0.450 | |
| Drinking | 1.335, 0.248 | ||
| No | 38.5 | 1.000 | |
| Yes | 37.6 | 1.317 (0.988–1.755) 0.060 | |
| Tumor length (cm) | 16.001, <0.001 | ||
| ≤3 | 53.5 | 1.000 | |
| >3 | 32.6 | 1.159 (0.799–1.682) 0.437 | |
| Tumor location | 0.776, 0.678 | ||
| Upper | 50.0 | 1.000 | |
| Middle | 38.9 | 1.145 (0.589–2.227) 0.690 | |
| Lower | 36.0 | 1.213 (0.621–2.369) 0.572 | |
| Vessel involvement | 11.874, 0.001 | ||
| Negative | 41.6 | 1.000 | |
| Positive | 21.5 | 1.089 (0.766–1.547) 0.635 | |
| Perineural invasion | 6.453, 0.011 | ||
| Negative | 41.4 | 1.000 | |
| Positive | 25.4 | 1.180 (0.846–1.646) 0.329 | |
| Differentiation | 7.464, 0.024 | ||
| Well | 46.2 | 1.000 | |
| Moderate | 38.6 | 0.854 (0.553–1.319) 0.476 | |
| Poor | 31.2 | 1.250 (0.765–2.042) 0.373 | |
| T grade | 34.797, <0.001 | ||
| T1 | 66.7 | 1.000 | |
| T2 | 43.5 | 1.527 (0.876–2.663) 0.136 | |
| T3 | 31.0 | 1.736 (1.043–2.890) 0.034 | |
| T4a | 22.5 | 2.414 (1.302–4.475) 0.005 | |
| N staging | 60.136, <0.001 | ||
| N0 | 53.2 | 1.000 | |
| N1 | 25.8 | 1.677 (1.198–2.347) 0.003 | |
| N2 | 16.7 | 1.992 (1.301–3.050) 0.002 | |
| N3 | 10.3 | 2.455 (1.484–4.059) <0.001 | |
| PNI | 54.344, <0.001 | ||
| >52 | 55.2 | 1.000 | |
| 42–52 | 39.1 | 1.574 (1.111–2.229) 0.011 | |
| <42 | 11.0 | 2.558 (1.718–3.809) <0.001 |
Abbreviations: CI, confidence interval; CSS, cancer-specific survival; ESCC, esophageal squamous cell carcinoma; HR, hazard ratio; PNI, prognostic nutritional index.
Discussion
In the present study, we used Martingale residuals for survival prediction to verify the optimal cut-off points for PNI, so ours was different from previous studies.11,12 Our results show that PNI is a predictive factor for long-term survival in ESCC. We conclude that the survival rate of ESCC can be discriminated between three groups, ie, PNI <42, 42–52, and >52. In our study, PNI was associated with tumor size (P=0.007), T grade (P=0.001), and N staging (P<0.001). This observation is in line with data from Nozoe et al.11
PNI is related to prognosis in many cancers; however, its role in esophageal cancer is still controversial. Nozoe et al11 demonstrated that PNI is associated with tumor progression and survival in patients with esophageal cancer. However, Sun et al12 showed that PNI does not correlate with prognosis in patients with esophageal cancer. Further, controversy exists concerning the optimal cut-off points for PNI to predict survival in cancers. The cut-off value is usually set at 45, because a PNI <45 is defined as moderate to severe malnutrition. However, the optimal cut-off value for the PNI to predict the long-term outcome in esophageal cancer remains unclear. Sun et al12 showed that PNI was not a predictive factor when they used 50 as a cut-off point for PNI. However, Nozoe et al11 showed that PNI was a predictive factor when they used 46 as a cut-off point for PNI. In our study, with the help of the fit line on the scatter plot, we classified the patients into three categories according to PNI, ie, <42, 42–52, and >52. Multivariate analysis showed that PNI was a significant predictor of CSS. The 5-year CSS in patients with PNI <42, 42–52, and >52 was 11.0%, 39.1%, and 55.2%, respectively (P<0.001).
The mechanism of the independent correlation between PNI and postoperative survival in patients with esophageal cancer is not clear. Previous studies have suggested that albumin and lymphocyte levels have a close relationship with the presence of an inflammatory response in cancer patients.16,17 There is a strong link between inflammation and cancer.18,19 Therefore, although initially thought of purely as a reflection of the nutritional status of a patient, it is likely, given its prognostic association, that PNI is a reflection of systemic inflammation.20
The PNI is easy to measure routinely because of its low cost and convenience. Moreover, in comparison with tumor markers, the PNI may have higher applicability for estimation of the systemic inflammation response. Therefore, the PNI is a simple, reliable, cheap, and reproducible method, the interpretation of which is relatively easy in the context of a nonseptic neoplasia. In our study, the PNI was a significant predictor of CSS. Thus, we suggest that the PNI should be included in the routine assessment of ESCC patients.
There are several potential limitations to our study. The data were obtained from charts and reviewed retrospectively, so many data points may not be accurately recorded. Moreover, the PNI was not necessarily from the same date, although most were within 1 week. In addition, because the study used data from a single institution, but involving different pathologists and different surgeons, there may have been a lack of uniformity. Further, we excluded patients who had adjuvant chemotherapy and/or radiotherapy, which may have influenced our analysis. Therefore, larger prospective studies will need to be performed to confirm these preliminary findings.
In conclusion, the PNI is a simple and useful marker for predicting the long-term outcome in patients with ESCC. The survival rate of ESCC can be discriminated between three groups, ie, PNI <42, 42–52, and >52. Therefore, we suggest that the PNI should be included in the routine assessment of ESCC patients.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Center . Cancer Registration Annual Report 2011. Beijing, People’s Republic of China: Military Medical Sciences; 2012. [Google Scholar]
- 3.Feng JF, Zhao Q, Chen QX. Prognostic value of subcarinal lymph node metastasis in patients with esophageal squamous cell carcinoma. Asian Pac J Cancer Prev. 2013;14(5):3183–3186. doi: 10.7314/apjcp.2013.14.5.3183. [DOI] [PubMed] [Google Scholar]
- 4.Tachibana M, Kinugasa S, Hirahara N, et al. Lymph node classification of esophageal squamous cell carcinoma and adenocarcinoma. Eur J Cardiothorac Surg. 2008;34(2):427–431. doi: 10.1016/j.ejcts.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 5.Schwegler I, von Holzen A, Gutzwiller JP, et al. Nutritional risk is a clinical predictor of postoperative mortality and morbidity in surgery for colorectal cancer. Br J Surg. 2010;97(1):92–97. doi: 10.1002/bjs.6805. [DOI] [PubMed] [Google Scholar]
- 6.Ray-Coquard I, Cropet C, Van Glabbeke M, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009;69(13):5383–5391. doi: 10.1158/0008-5472.CAN-08-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984;85(9):1001–1005. Japanese. [PubMed] [Google Scholar]
- 8.Nozoe T, Minomiya M, Maeda T, et al. Prognostic nutritional index: a tool to predict the biological aggressiveness of gastric carcinoma. Surg Today. 2010;40(5):440–443. doi: 10.1007/s00595-009-4065-y. [DOI] [PubMed] [Google Scholar]
- 9.Kanda M, Fujii T, Kodera Y, et al. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg. 2011;98(2):268–274. doi: 10.1002/bjs.7305. [DOI] [PubMed] [Google Scholar]
- 10.Pinato DJ, North BV, Sharma R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI) Br J Cancer. 2012;106(8):1439–1445. doi: 10.1038/bjc.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nozoe T, Kimura Y, Ishida M, et al. Correlation of preoperative nutritional condition with post-operative complications in surgical treatment for esophageal carcinoma. Eur J Surg Oncol. 2002;28(4):396–400. doi: 10.1053/ejso.2002.1257. [DOI] [PubMed] [Google Scholar]
- 12.Sun P, Zhang F, Chen C, et al. Comparison of the prognostic values of various nutritional parameters in patients with esophageal squamous cell carcinoma from Southern China. J Thorac Dis. 2013;5(4):484–491. doi: 10.3978/j.issn.2072-1439.2013.08.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng JF, Huang Y, Zhao Q. Tumor length in elderly patients with esophageal squamous cell carcinoma: is it a prognostic factor? Ups J Med Sci. 2013;118(3):145–152. doi: 10.3109/03009734.2013.792887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rice TW, Rusch VW, Ishwaran H, et al. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Staging Manuals. Cancer. 2010;116(16):3763–3773. doi: 10.1002/cncr.25146. [DOI] [PubMed] [Google Scholar]
- 15.Grambsch PM, Therneau TM, Fleming TR. Diagnostic plots to reveal functional form for covariates in multiplicative intensity models. Biometrics. 1995;51(4):1469–1482. [PubMed] [Google Scholar]
- 16.Koike Y, Miki C, Okugawa Y, et al. Preoperative C-reactive protein as a prognostic and therapeutic marker for colorectal cancer. J Surg Oncol. 2008;98(7):540–544. doi: 10.1002/jso.21154. [DOI] [PubMed] [Google Scholar]
- 17.Crumley AB, Stuart RC, McKernan M, et al. Is hypoalbuminemia an independent prognostic factor in patients with gastric cancer? World J Surg. 2010;34(10):2393–2398. doi: 10.1007/s00268-010-0641-y. [DOI] [PubMed] [Google Scholar]
- 18.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 19.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 20.Moyes LH, Leitch EF, McKee RF, et al. Preoperative systemic inflammation predicts postoperative infectious complications in patients undergoing curative resection for colorectal cancer. Br J Cancer. 2009;100(8):1236–1239. doi: 10.1038/sj.bjc.6604997. [DOI] [PMC free article] [PubMed] [Google Scholar]