Abstract
Objective
To evaluate associations between neonatal intensive care unit (NICU) room type (open ward and private room) and medical outcomes; neurobehavior, electrophysiology and brain structure at hospital discharge; and developmental outcomes at two years of age.
Study design
In this prospective longitudinal cohort study, we enrolled 136 preterm infants born <30 weeks gestation from an urban, 75-bed level III NICU from 2007-2010. Upon admission, each participant was assigned to a bedspace in an open ward or private room within the same hospital, based on space and staffing availability, where they remained for the duration of hospitalization. The primary outcome was developmental performance at two years of age (n=86 infants returned for testing, which was 83% of survivors) measured using the Bayley Scales of Infant and Toddler Development, 3rd Edition. Secondary outcomes were 1) medical factors throughout the hospitalization, 2) neurobehavior, and 3) cerebral injury and maturation (determined by magnetic resonance imaging and electroencephalography).
Results
At term equivalent age, infants in private rooms were characterized by a diminution of normal hemispheric asymmetry and a trend toward having lower amplitude integrated electroencephalography cerebral maturation scores [p= 0.02; β=−0.52 (CI −0.95, −0.10)]. At age two years, infants from private rooms had lower language scores [p= 0.006; β=−8.3 (CI −14.2, −2.4)] and a trend toward lower motor scores [p= 0.02; β=−6.3 (CI −11.7, −0.99)], which persisted after adjustment for potential confounders.
Conclusion
These findings raise concerns that highlight the need for further research into the potential adverse effects of different amounts of sensory exposure in the NICU environment.
Keywords: Single Patient Room, Single Family Ward, Open Bay, Open Ward, Development, Outcome, MRI, Room Type, Sensory Deprivation, Motor, Language, Cerebral Maturation, Sensory Exposure, Surface Based Morphometry, Hemispheric Asymmetry, Sound Abatement
Advanced medical technologies have improved the rates of survival among preterm infants, although long term morbidities remain common. Perinatal factors, including cerebral injury, have been implicated in adverse development but fail to fully characterize outcomes. More recently, attention has shifted to the impact of the neonatal intensive care unit (NICU) environment on developmental trajectories. For example, the NICU environment frequently exceeds noise recommendations from the American Academy of Pediatrics.1, 2 The bright and noisy environment is thought to adversely affect growth and development of the very preterm infant.3, 4 Developmental care is a system of NICU care, which aims to protect the baby and ameliorate the negative effects of noxious stimuli and includes decreasing visual and auditory stimulation to a minimum.5 The impact of developmental care on outcomes has been mixed,6 but better outcomes have been reported.7
In support of developmental care principles, efforts have been made to reduce the exposure of high risk infants to modifiable stimuli in the NICU,1 using practices such as noise abatement.3 Further, current recommendations for NICU design include construction of private space to reduce environmental stimulation to the infant and support family involvement.8 Positive staff and caregiver perceptions,9-11 decreased noise levels and nosocomial infections,12, 13 and lower rates of readmission in the months after discharge14 were reported following NICU renovation to private rooms. A randomized clinical trial conducted in Sweden reported reductions in length of stay among infants assigned to single family rooms, consisting of private space for the family and infant, and in which parents were required to be present from admission to discharge.15 Although many hospitals world-wide are undergoing major renovations of NICU space to create private rooms, there is minimal research evaluating the neurodevelopmental outcomes for infants hospitalized in a private room environment.
To address this issue, we explored the relationship between NICU room type (private room versus open ward) and the primary outcome of neurodevelopmental performance at age two years. Secondary outcomes consisted of infant medical factors and testing at the time of discharge from the hospital (neurobehavior, electrophysiology, and brain MRI). Based on current practice recommendations, we hypothesized that infants hospitalized in the private NICU room would have better neurodevelopmental outcome than infants in the open ward.
METHODS
The sample consisted of 136 infants born ≤30 weeks gestation with no documented congenital anomaly. Consecutive admissions were recruited within the first 72 hours of life from 2007-2010. The investigation was contained within an overarching study prospectively investigating the factors influencing brain development in preterm infants. As part of this larger study, infants received serial neurobehavioral assessment, amplitude integrated electroencephalography (aEEG) and magnetic resonance imaging (MRI) during their NICU stay. Development was assessed at age two years and is planned for five years. The study was approved by the Human Research Protection Office, and parents signed informed consent.
The study site was a 75-bed, level III NICU in the Midwestern United States, which includes 39 open ward beds and 36 private rooms. Bedspace assignment for anticipated admissions was made at shift change by the charge nurse. It was based on bedspace and staffing availability and was done without knowledge of family factors, acuity level, or gestational age at birth. Any infant admitted could be cared for in either part of the NICU, and infants remained in the assigned room type until discharge. Hospital policies (including 24 hour visitation for families) were the same in both room types. Physician care rotated through both areas of the NICU. The NICU housed a diverse urban population with low parental visitation.16
Private Rooms
Each private room was approximately 168 square feet (16 square meters) and enclosed by three solid walls plus a sliding glass wall. Lighting was individually controlled, and external sounds were buffered by the walls and distance between beds. Each private room had a lounge chair for parents, a sink, and personal storage space.
Open Ward
The open ward comprised four large rooms (range 802-1375 square feet; 75-128 square meters) with eight to twelve beds in each. The distance between beds was approximately two meters. The open ward was lit by centrally controlled fluorescent overhead lights. Environmental measures used to reduce stimuli on the open ward included dimming lights, covering incubators, lowering voices, minimizing loud noises in the unit, and educating parents about the need for sensory minimization.
Baseline infant and socio-demographic factors
Gestational age at birth, birth weight, multiple birth, Critical Risk Index for Babies (CRIB) score, type of delivery (vaginal or cesarean), sex, and race were analyzed to determine group homogeneity. The CRIB score, a measurement of initial medical severity, was calculated from medical records. CRIB scores range from 0 to 24, with higher scores indicating more medical compromise.17 Insurance type, prenatal illicit drug exposure (from toxicology reports at delivery), maternal age, marital status, and education were investigated for differences across groups. Insurance type (Medicaid versus private insurance) provided a measure of socioeconomic status.
Medical factors
During NICU hospitalization the following were recorded: patent ductus arteriosis (PDA; treated with indomethacin or surgical ligation), necrotizing enterocolitis (all stages), retinopathy of prematurity (all stages), cerebral injury, confirmed sepsis, fentanyl dosage, postnatal steroid use, days of total parenteral nutrition, inotropic support, maximum amount of oxygen, days of intubation, days of continuous positive airway pressure (CPAP), hours of oxygen therapy, oxygen requirement at 36 weeks postmenstrual age (PMA), PMA at discharge, and length of stay. Parent visitation and holding was also collected throughout the NICU stay.
Infant neurobehavior
Neurobehavioral performance was evaluated within two weeks of birth and at 30 weeks PMA using the Premie Neuro;18 at 34 weeks PMA and term equivalent age (between 37-41 weeks) using the NICU Network Neurobehavioral Scale (NNNS);19 and at term equivalent age also using the Dubowitz Neurological Exam20 and Neonatal Oral Motor Assessment Scale.21 The Premie Neuro is a 24 item neurological examination appropriate for infants between 23 and 37 weeks PMA, which yields a total score between 24-120. The NNNS is a 115-item test with 13 summary scores: habituation, orientation, hypertonicity, hypotonicity, arousal, lethargy, asymmetry, sub-optimal reflexes, excitability, tolerance of handling, stress, quality of movement and self regulation. The Dubowitz Neurological Exam is a 34-item evaluation of tone, tone patterns, reflexes, movement, abnormal signs/patterns, and orientation/behavior with scores ranging from 0-34. The NOMAS is a 28-item observational checklist of tongue and jaw movement, conducted during the first two minutes of oral feeding, which classifies feeding as either normal, disorganized or dysfunctional. Evaluations were performed by a single examiner who was trained and certified in all techniques.
Amplitude integrated electroencephalography (aEEG) assessment
The BrainZ Monitor (™Natus) was used for recordings at four time points; within two weeks of birth, at 30 and 34 weeks PMA, and at term equivalent for four continuous hours. A single, trained and blinded member of the research team determined a cerebral maturation score using methodologies described previously.22 aEEG cerebral maturation scores range from 0-13 and have been shown to progress from 1-2 shortly after birth in the extremely preterm infant (24-25 weeks gestation) to 13 by term equivalent age.22 Due to the sensitivity of the aEEG measure to PMA, PMA at the time of aEEG tracing was controlled for in all analyses.
Brain imaging
MRI was acquired from each infant at 37-41 weeks PMA using a 3-T TIM Trio system (Siemens, Erlangen). MRI scanning included magnetization-prepared rapid gradient echo (MPRAGE) T1-weighted images (TR/TE 1500/3 ms, voxel size 1×0.7×1 mm3) and turbo spin echo (TSE) T2-weighted images (TR/TE 8600/160 ms, voxel size 1×1×1 mm3, echo train length 17). Functional images were collected utilizing an echo-planar-image (EPI) sequence sensitized to T2* BOLD signal changes (TR/TE 2910/28 ms; voxel size 2.4×2.4×2.4 mm3). Diffusion imaging was obtained using a single-shot EPI sequence (TR/TE 13300/1266, 48 b directions with amplitudes ranging up to 1200 s/mm2, voxel size 1.2×1.2 ×1.2 mm3). MRI was assessed using the following:
Qualitative assessments
MRI findings were combined with routine cranial ultrasound (CUS). A single neuroradiologist, blinded to room assignment, interpreted the MRI images. In addition to specific types of injury, a dichotomous variable was used to control for cerebral injury in the statistical model. The presence of cerebral injury was defined as having either grade III-IV intraventricular hemorrhage (IVH), cystic periventricular leukomalacia (PVL) or cerebellar hemorrhage. A standardized scoring evaluation of brain growth and development was also applied.23
Brain metrics
Regional brain measures, including bifrontal, biparietal, and transcerebellar diameters, ventricular size, and interhemispheric distance were obtained.24
Diffusion assessments
Regions of interest were placed manually in frontal, temporal white matter, corpus callosum and posterior limb of internal capsule using fractional anisotropy (FA), mean diffusivity, and red green blue maps to identify anatomic structures. These were sampled for mean diffusivity and FA using ANALYZE 10.0 software. Voxelwise statistical analysis of the FA data was also conducted using tract based spatial statistics (TBSS) methods.25
Volumetry
Volumetry was conducted with the Advanced Normalization Tools software (ANTS) using methodologies described previously.26
Functional connectivity MRI (fcMRI)
fcMRI data were analyzed as described previously.27 Thirteen subjects in each room type satisfied strict motion criteria. The BOLD time series for seed regions important for language (posterior superior temporal sulcus and left posterior middle temporal, superior temporal, inferior frontal, and middle frontal gyri) and motor (motor cortex and thalamus) function were cross-correlated with all other voxels in the brain.28, 29
Surface Based Morphometry
The language finding prompted investigation of hemispheric sulcal asymmetry to determine whether asymmetries previously reported in term controls30 were evident in the infants in this cohort. Twenty infants in the open ward and 23 infants in private rooms were selected for having good quality scans and no evidence of cerebral injury (see qualitative assessments above). The LIGASE method was used for cortical segmentation.30 The lack of term controls enrolled specifically for this study precluded direct comparison of left-right depth asymmetries across groups. Instead, to facilitate visual contrast with previous term control results, depth asymmetries were analyzed using methods described previously30 except that an updated PALS-term12 atlas was used as the registration target for both groups (in house software).
Home environment
When participants reached two years of age, parents completed a questionnaire for a social risk score and a measure of family functioning. A social risk score, used in parallel research studies and modified for this study, was used (Table I; available at www.jpeds.com). The general functioning scale of the McMaster Family Assessment Device (FAD) was used, which is a valid and reliable measure of family dynamics and functioning.31 In addition, due to the well established relationship between siblings and language outcome,32 the number of siblings in the home at age 2 years was captured.
Table I.
Modified social risk score used in cohort to assess family environment after NICU discharge.
| Family Structure | 0 = family intact, 1 = separated/dual custody or cared for by other intact family such as grandparents, 2 = single caregiver or foster care |
| Education of Primary Caregiver | 0 = college education, 1 = completed year 11 or 12 of high school, 2 = completed <year 11 of high school |
| Occupation of Primary Income Earner | 0 = professional/skilled, 1 = semiskilled, 2 = unskilled |
| Employment Status of Primary Income Earner | 0 = full time, 1 = part-time, 2 = unemployed/pension/money from government |
| Language Spoken at Home | 0 = English only, 1 = some English, 2 = no English |
| Maternal Age at Birth | 0 ≥ 21 years, 1 = 18-21 years, 2 ≤ 18 years |
| Total Social Risk Score | 0-12 |
Developmental assessment
Participants returned for developmental testing at age two years and were assessed with the Bayley Scales of Infant and Toddler Development®, 3rd edition (Bayley-III)33 by a blinded psychometrician. Primary outcomes included Bayley-III cognitive, language, and motor composite scores. Bayley-III composite scores range from 45-155 for language and motor and 55-145 for cognitive, were normed on a typically developing population, and have a mean of 100 with a standard deviation of 15. Higher scores indicate better performance. A potential meaningful difference in the primary outcome was considered to be > 5 Bayley-III points.34 Parent report assessments included the Modified Checklist for Autism in Toddlers (M-CHAT)35 and the Infant Toddler Social Emotional Assessment (ITSEA).36 Pass/fail scores on the M-CHAT and linear t scores for the domains of the ITSEA [externalizing (including hyperactivity and aggression), internalizing (including anxiety and depression), dysregulation (including emotional reactivity, sleep, and eating), and competence (including attention and prosocial behaviors)] were analyzed. Linear t scores for the ITSEA adjust for sex and age at testing, have been normed on typically developing children, and have a mean of 50 and standard deviation of 10. Lower scores indicate better performance, except for competence, in which higher scores indicate better performance. M-CHAT failure indicates the need for further diagnostic evaluation.
Statistical Analyses
SPSS 20 (IBM Corporation, 1989, 2011) and SAS 9.3 (SAS Institute, 2010) were used for statistical analyses. Statistical analysis was in four steps, by: (1) determining differences in baseline, sociodemographic, home environment and medical factors among infants in open wards compared with private rooms and determining which factors to include in the statistical model; (2) investigating differences in neurobehavior, aEEG, brain imaging, and outcomes among infants in the different room types; (3) determining differences in outcome among infants in the different room types while controlling for potential confounds; and (4) determining interactions between room type and risk, cerebral injury and visitation.
First, group homogeneity was determined by investigating associations between room type and baseline and sociodemographic factors using independent samples t-tests, Wilcoxon signed rank tests, and chi-square analyses. Associations between acquired medical factors and room type were also explored using the same statistical procedures. In addition to factors known to be associated with outcome, variables that differed between groups (α≤.05) were included as control variables in subsequent analyses. For term equivalent analyses, CRIB score (which includes a measure of gestational age), cerebral injury, and insurance status were included. For analyses investigating two-year outcomes, CRIB score, cerebral injury, FAD score, and social risk score were controlled for. All factors included in the model were investigated for co-linearity to ensure that all variables were independent. In the case of co-linearity (r≥.3 and p<.05), the variable that best represented the given construct was chosen. PMA at time of testing was also controlled for when investigating brain metrics, diffusion, volumetry and aEEG.
Second, associations between room type and: (1) neurobehavior during the NICU stay; (2) qualitative brain measures; (3) brain metrics; (4) brain volumes; (5) diffusion measures; (6) aEEG measures; and (7) short term neurodevelopmental outcome were investigated using univariable regression. Multivariable analysis was then used to investigate differences in infants in each room type while controlling for potential confounds (2-sided analyses). All findings with a p-value <0.05 are presented to note group differences. Due to the number of comparisons, p<0.01 was used as the cut-off to define strong relationships.
Last, interactions of clinical risk, cerebral injury and infant visitation with room type were evaluated with two-factor analysis of variance. High risk was defined as infants with gestational age at birth <26 weeks and CRIB score >6. Cerebral injury is defined above. Infant visitation (high and low) was operationalized as the lower and upper quartile for parent hours of visitation per week over the length of stay. Contrasts on room type were tested within each level of clinical risk, cerebral injury, and infant visitation.
In relation to developmental outcomes, we had 80% power to detect a mean difference at p<0.05 between groups when true differences were 7.2 points in language, 6.2 points in cognitive and 6.5 points in motor outcomes.
RESULTS
Baseline, socio-demographic, and medical factors
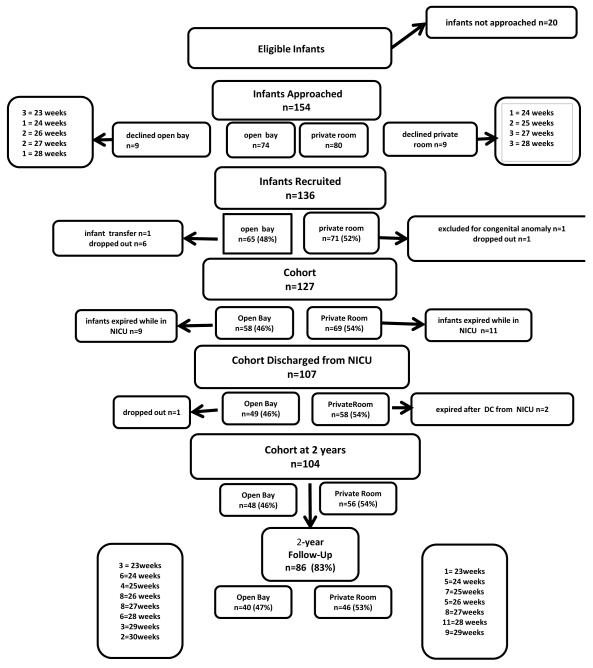
Figure 1 gives the patient flow diagram. There were no differences in baseline or medical factors among infants in private rooms compared with open wards (Table II). More infants on Medicaid were assigned to private rooms (p=0.004); therefore, insurance type was controlled for in all analyses investigating neonatal outcomes. There were no other differences in sociodemographic factors across room type during the NICU stay.
Figure 1.
Patient flow diagram.
Table II.
Baseline, sociodemographic, and acquired medical factors and investigations of differences across room type for infants in the NICU.
| Baseline factors (Total N=107*) |
N (%), Median (IQ range) or Mean (SD) |
N (%), Median (IQ Range) or Mean (SD) | p-value† | ||
|---|---|---|---|---|---|
| Range | Open ward (n=49) | Private room(n=58) | |||
| Gestational age at birth, wk | 23-30 | 26.6 (1.7) | 26.4 (1.8) | 26.8 (1.6) | 0.30 |
| Gestational age (23-25 wk) | 30 (28.0%) | 15 (30.6%) | 15 (25.8%) | 0.36 | |
| Gestational age (26-28 wk) | 62 (57.9%) | 28 (57.1%) | 34 (58.6%) | 0.36 | |
| Gestational age (29-30 wk) | 15 (14.0%) | 6 (12.2%) | 9 (15.5%) | 0.36 | |
| Birth weight, g | 480- 1600 |
945.8 (250.5) | 936.5(263.8) | 953.6(240.7) | 0.73 |
| Birth weight (<750 g) | 26 (24.3%) | 12 (24.5%) | 14 (24.1%) | 0.88 | |
| Birth weight (750-1000 g) | 35 (32.7%) | 17 (34.7%) | 18 (31.0%) | 0.88 | |
| Birth weight (1000+ g) | 64 (59.8%) | 31 (63.3%) | 33 (56.9%) | 0.88 | |
| Singleton birth | 71 (66.4%) | 33 (67.4%) | 38 (65.5%) | 0.94 | |
| Critical Risk Index for Babies** | 0-14 | 2.0 (1-6) | 4.0 (1-8) | 1.5 (1-4) | 0.11 |
| Vaginal delivery | 30 (28.0%) | 17 (34.7%) | 13 (22.4%) | 0.16 | |
| Female sex | 61 (57.0%) | 28 (57.1%) | 33 (56.9%) | 0.98 | |
| Caucasian race | 53 (49.5%) | 25 (51.0%) | 28 (48.3%) | 0.78 | |
| Prenatal steroid use | 96 (90%) | 45 (92%) | 51 (88%) | 0.37 | |
| Socio-demographic factors | |||||
| Medicaid | 71 (67.0%) | 25 (52.1%) | 46 (79.3%) | 0.003 | |
| Maternal Illicit drug use | 7 (6.5%) | 3 (6.1%) | 4 (6.9%) | 0.87 | |
| Maternal age, yr | 15-47 | 27.9 (7.3) | 28.6 (8.1) | 27.4 (6.7) | 0.51 |
| Married marital status | 39 (36.4%) | 17 (34.7%) | 22 (37.9%) | 0.73 | |
| Mother did not graduate high school (n=104) | 5 (4.8%) | 0 (0%) | 5 (8.6%) | 0.26 | |
| Mother high school education (n=104) | 56 (53.8%) | 27 (56.3%) | 29 (51.8%) | 0.26 | |
| Mother college education (n=104) | 43 (41.4%) | 19 (48.7%) | 22 (39.3%) | 0.26 | |
| Acquired medical factors | |||||
| Patent ductus arteriosus | 55 (51.4%) | 30 (61.2%) | 25 (43.1%) | 0.06 | |
| Necrotizing enterocolitis | 8 (7.5%) | 4 (8.2%) | 4 (6.9%) | 0.43 | |
| Retinopathy of prematurity | 13 (12.1%) | 7 (14.3%) | 6 (10.3%) | 0.82 | |
| Cerebral injury (n=100) | 17 (17.0%) | 9 (18.4%) | 8 (15.7%) | 0.72 | |
| Confirmed sepsis | 32 (29.9%) | 17 (34.7%) | 15 (25.9%) | 0.46 | |
| Fentanyl total dose (mcg) | 0-3900 | 1.9 (0-134) | 2.0 (0.98-57.5) | 1.6 (0-150) | 0.55 |
| Postnatal steroids (n=88) | 28 (26.2%) | 16 (32.7%) | 12 (20.7%) | 0.16 | |
| Total parenteral nutrition, days | 5-117 | 17 (11-31) | 19 (12-33.5) | 14.5 (9-26.8) | 0.11 |
| Inotrope use, yes or no | 35 (32.7%) | 17 (34.7%) | 18 (31.0%) | 0.92 | |
| Inotrope, hours | 0-432 | 0(0-21) | 0 (0-22.5) | 0 (0-19.5) | 0.68 |
| Highest oxygen >60% (n=106) | 62 (57.9%) | 28 (57.1%) | 34 (59.7%) | 0.79 | |
| Number of days ventilated | 0-89 | 2 (1-13) | 3 (1-24) | 1.5 (1-7) | 0.19 |
| Continuous positive airway pressure, days | 0-68 | 3 (1-9) | 4(1-10) | 3 (1-9.3) | 0.89 |
| Oxygen therapy, hours | 72-5592 | 1416(720-2064) | 1608(900-2076) | 1248 (589-2034) | 0.09 |
| Oxygen at 36 weeks | 53 (49.5%) | 26 (53.1%) | 27 (46.6%) | 0.50 | |
| Postmenstrual age at discharge, weeks | 34-59 | 39.3 (3.3) | 39.7 (3.6) | 38.7 (3.4) | 0.14 |
| 41+ wks at discharge | 35 (32.7%) | 16 (32.6%) | 19 (32.7%) | 0.99 | |
| Length of stay, days | 36-232 | 85(70-102) | 87 (74-104) | 79(65-102) | 0.17 |
Investigation of differences across infants in the open ward compared with infants in private rooms, using independent samples t-tests, Wilcoxon signed rank tests, and chi-square analyses.
Infants who were enrolled and discharged from the study NICU. When variables are missing data, a separate n is reported next to the variable itself.
Higher Critical Risk Index for Babies (CRIB) scores indicate more medical compromise at admission to the NICU. CRIB scores range from 0-24.
For infants discharged from the NICU (n=107), the average hours per week of parent visitation over the length of stay ranged from 1.8 hours to 104 hours with a mean of 19 ± 19 hours. The average number of days held per week over the length of stay was 0-6 days with a mean of 2.4 ± 1.5 days. The average number of days held skin to skin over the length of stay ranged from 0-4 days, with a mean of 0.7 ± 0.9 days.
Neurobehavioral assessment
There were no differences in Premie Neuro scores among infants in private rooms compared with open wards (p>.05). At 34 weeks PMA, higher levels of arousal on the NNNS were observed in infants in private rooms compared with open wards [mean open ward 2.9 ± 0.7; mean private room 3.4 ± 0.8; p=0.0004, Beta 0.5 (0.9, 0.2)], and associations remained after controlling for CRIB score, insurance type, and cerebral injury [p=0.008, Beta 0.5 (0.9, 0.1)] with good model fit (p=0.02 / r square change 0.1). NNNS arousal scores ranged from 2.2-5.9, with higher scores indicating that an infant was easily aroused, showing greater irritability during neurobehavioral testing with more associated motor activity.19 There were no differences in other NNNS summary scores at 34 weeks PMA or in NNNS, Dubowitz and NOMAS scores at term equivalent age (p>.05).
Serial aEEG
Table III shows aEEG cerebral maturation scores across hospitalization. Infants in private rooms demonstrated a trend toward lower cerebral maturation scores at term equivalent age (p=.04). After multivariable adjustment for CRIB score, insurance status, cerebral injury, and time of aEEG tracing, scores were lower at 30 weeks and trended toward being lower at term equivalent age (p=.01 and p=.02 respectively).
Table III.
Preterm aEEG cerebral maturation scores (CMS) during the NICU hospitalization.
| aEEG CMS* (n=107) |
Median (IQ range) |
Median (IQ range) | Multi- variable p-value† |
Multi variable Beta (CI) |
Multi- variable†† p-value |
Multi- variable† Beta (CI) |
||
|---|---|---|---|---|---|---|---|---|
| Open ward (n=49) |
Private room (n=58) |
Model fit/ r2 |
||||||
| CMS within 2 weeks of birth (n=106) |
3.0 (2-4) |
(n=46) 3 (2-4) |
(n=60) 3 (2-4.5) |
0.41 | −0.31 (−1.05, 0.43) |
0.17 | −0.55 (−1.34, 0.24) |
<0.001/ 0.40 |
| CMS at 30 weeks PMA (n=68) |
7.0 (6-8) |
(n=24) 8 (6.5-8) |
(n=44) 7 (6-8) |
0.17 | −0.70 (−1.71, 0.30) |
0.01 | −1.26 (−2.23, −0.29) |
0.002/ 0.30 |
| CMS at 34 weeks PMA (n=61) |
11 (10-11) |
(n=26) 11 (10-11) |
(n=35) 10 (10-11) |
0.66 | −0.12 (−0.68, 0.43) |
0.19 | −0.39 (−0.98, 0.20) |
0.02/ 0.24 |
| CMS at term age (n=56) |
12.5 (12-13) |
(n=27) 13 (12-13) |
(n=29) 12 (12-13) |
0.04 | −0.42 (−0.83, −0.02) |
0.02 | −0.52 (−0.95, −0.10) |
0.17/ 0.15 |
Multivariable linear regression investigating associations between room type and aEEG CMS score, Controlling for time of aEEG recording
Multivariable linear regression investigating associations between room type and aEEG CMS score, while controlling for CRIB score, insurance type, cerebral injury and PMA at time of aEEG recording.
Cerebral maturation scores can range from 0-13, with higher scores indicating more mature electrocortical activity and better cerebral health. Previous research has demonstrated scores of 1 -2 at early gestational ages with increasing scores as infants approach term, with infants achieving scores of 13 by 39 weeks PMA.21
Abbreviations: amplitude integrated electroencephalography (aEEG), cerebral maturation score (CMS), postmenstrual age (PMA).
Brain imaging
Qualitative brain measures, brain metrics, diffusion measures (including tract based spatial statistics), volumetry, and fcMRI did not differ at term equivalent among infants in the two room types (p>.05).
fcMRI
For participants in each room type, networks demonstrating synchronous, spontaneous neuronal activity incorporating language and motor regions were identified.28 Statistical comparison between results generated using each seed location for infants categorized by room type revealed no differences between groups (p>.05).
Morphometry
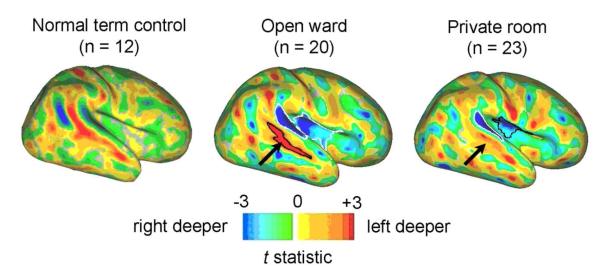
Surface-based analysis demonstrated hemispheric asymmetries in insular and lateral temporal cortex based on sulcal depth. The region of significantly asymmetric cortex was larger in the open ward group (1647 mm2) compared with the private room group (1281 mm2). Both groups showed sulcal depth asymmetry near the planum temporale, but the asymmetry in the superior temporal sulcus was muted in private room infants relative to open ward infants and term controls (Figure 2; available at www.jpeds.com) on qualitative interpretation. The open ward and private room t-statistic maps (t-maps) are shown alongside the term control asymmetry t-map from a published study.30
Figure 2.
Hemispheric sulcal depth asymmetry (left-right) t-statistic maps for term control (n=12)30; private room (n=23), and open ward infants (n=20). The superior temporal sulcus asymmetry for the term controls was significant based on the 3D trajectory of the sulcal fundus (illustrated by non-outlined color, as reproduced with permission from Journal of Neuroscience). Note the differences in left-right asymmetry of the superior temporal sulcus (arrows) across groups, with greater hemispheric asymmetry present in the open ward (1647 mm2) compared with the private room infants (1281 mm2). T-maps are scaled to a t-value of ±3.0, which corresponds to p=0.0121 for the term controls; p=0.0074 for the open ward; and p=0.0066 for the private room infants.
Functional outcome at age 2 years
Among infants who returned for developmental testing (n=86), there were trends toward higher rates of PDA (p=0.046) in infants from the open ward. Correlations revealed co-linearity between PDA and CRIB score (p<0.01, r=0.3); therefore, only CRIB score was maintained in the model. A second analysis was conducted controlling for PDA, and the findings remained unchanged. No other differences were observed across room type (p>.05; Table IV available at www.jpeds.com). There were no differences in factors related to the home environment, including social risk score, FAD score, and number of siblings among infants in private rooms compared with open wards (p>0.5; Table IV).
Table IV.
Baseline, sociodemographic, medical, and home environment factors and investigations of differences across room type for infants who returned for developmental follow up testing.
| Baseline factors (Total n=86*) |
Range | N (%), Median (IQ range) or Mean (SD) |
N (%), Median (IQ Range) or Mean (SD) | p-value† | |
|---|---|---|---|---|---|
| Open ward (n=40) |
Private room (n=46) |
||||
| Gestational age at birth, wk | 23-30 | 26.6 (1.8) | 26.3 (1.9) | 26.8 (1.8) | 0.21 |
| Birth weight, g | 480-1600 | 940.2 (266.2) | 919.8 (277.0) | 958.0 (258.1) | 0.51 |
| Singleton birth | 50 (58.1%) | 24 (60.0%) | 26 (56.5%) | 0.91 | |
| Critical Risk Index for Babies** | 0-14 | 2. (1-7) | 3.5 (1-8) | 2.0 (1-4) | 0.11 |
| Vaginal delivery | 22 (25.6%) | 11 (27.5%) | 11 (23.9%) | 0.81 | |
| Female sex | 47 (54.7%) | 22 (55.0%) | 25 (54.4%) | 1.00 | |
| Caucasian race | 48 (55.8%) | 23 (57.5%) | 25 (54.3%) | 0.83 | |
| Socio-demographic factors | |||||
| Maternal illicit drug use | 4 (4.7%) | 2 (5.0%) | 2 (4.3%) | 0.89 | |
| Maternal age, yr | 18-47 | 28.5 (7.2) | 29.5 (8.1) | 27.7 (6.3) | 0.27 |
| Married marital status | 35 (40.7%) | 16 (40.0%) | 19 (41.3%) | 1.00 | |
| Maternal college education | 37 (43.0%) | 19 (47.5%) | 18 (39.1%) | 0.20 | |
| Bilingual household (n=78) | 10 (12.7%) | 5 (13.5%) | 5 (11.9%) | 0.83 | |
| Family Assessment Device-family functioning score (n=79) |
1-2.5 | 1.5 (0.4) | 1.4(0.4) | 1.6 (0.4) | 0.14 |
| Social risk score (n=85) | 1-7 | 3.6 (1.3) | 3.7 (1.3) | 3.6 (1.4) | 0.73 |
| Number of siblings | 0-4 | 1.5 | 1.4 (1.1) | 1.6.3) | 0.34 |
| Acquired factors | |||||
| Patent ductus arteriosis | 46 (53.5%) | 26 (65.0%) | 20.0 (43.4%) | 0.046 | |
| Necrotizing enterocolitis | 6 (7.0%) | 4 (10.0%) | 2 (4.3%) | 0.09 | |
| Retinopathy of prematurity | 13 (15.1%) | 7 (17.5%) | 6 (13.0%) | 0.84 | |
| Cerebral injury (n=81) | 15 (18.5%) | 7 (17.5%) | 8 (19.5%) | 0.82 | |
| Confirmed sepsis | 29 (33.7%) | 15 (37.5%) | 14 (30.4%) | 0.56 | |
| Fentanyl total dose (mcg) | 0-3900 | 2 (1.0-150.0) | 2.0 (1.0-103.3) | 1.6 (0-171.1) | 0.56 |
| Postnatal steroids (n=73) | 24 (27.9%) | 14 (35.0%) | 10 (25.0%) | 0.17 | |
| Days on total parenteral nutrition | 5-117 | 17 (11-30.2) | 20 (13-34) | 15.5 (9-26.8) | 0.10 |
| Highest oxygen >60% | 49 (57.0%) | 24 (60.0%) | 25 (54.3%) | 0.60 | |
| Inotrope use, yes or no | 31 (36.0%) | 15 (37.5%) | 16 (34.8%) | 0.82 | |
| Inotrope hours | 0-432 | 0(0-24) | 0 (3-30.8) | 0(0-24) | 0.71 |
| Number of days ventilated | 0-89 | 3(1-18) | 4.5 (1-28.5) | 2 (1-9) | 0.21 |
| Days continuous positive airway pressure | 0-68 | 3.5 (1-10.2) | 4 (1-10.5) | 3 (1-11) | 0.96 |
| Hours on oxygen | 72-5592 | 1464 (768-2136) | 1680 (926.5-2268.0) | 1248 (552-2088) | 0.06 |
| Oxygen at 36 weeks gestation | 41 (47.7%) | 21 (52.5%) | 20 (43.4%) | 0.40 | |
| Postmenstrual age at discharge, weeks | 30-59 | 39.4 (3.7) | 40.1 (3.7) | 38.7 (3.6) | 0.08 |
| Length of stay, days | 36-232 | 88.5 (29.1) | 94.0 (31.2) | 83.8 (26.6) | 0.17 |
Investigation of differences across infants in the open ward compared with infants in private rooms, using independent samples t-tests, Wilcoxon signed rank tests, and chi-square analyses.
Total number of Infants in the cohort who returned for developmental testing at age two years. When variables are missing data points, a separate n is reported next to the variable itself.
Higher Critical Risk Index for Babies (CRIB) scores indicate more medical compromise at admission to the NICU. CRIB scores range from 0-24. Please note that the factors that were measured at two years of age are shaded gray.
At two years of age, infants from the private room environment had lower language scores (p=.006) and demonstrated a trend toward having lower motor scores (p=.02) on the Bayley-III and more externalizing behaviors (p=.04) on the ITSEA (Table V) after controlling for confounds. There were no associations between room type and cognitive scores on the Bayley-III, other domain scores of the ITSEA, or M-CHAT scores (p>.05). Further, controlling for parent visitation and holding in the NICU did not alter the findings. Controlling for number of siblings in the home did not alter the findings.
Table V.
Two year neurodevelopmental and behavioral outcomes and investigations of differences across room type.
| Outcome Variable (n=86) * |
N (%) or Mean (SD) [Range] |
N (%) or Mean (SD) by room type |
Uni variable p-value |
Uni variable Beta (CI) |
Multi- variable † p-value |
Multi-variable Beta (CI) |
||
|---|---|---|---|---|---|---|---|---|
| Open ward (n=40) |
Private room (n=46) |
Model fit/ R2 |
||||||
| Age at Developmental Testing, months |
27.4 (2.1) |
27.3 (2.9) |
27.5 (2.3) |
0.62 | 0.228 (−0.7, 1.2) |
|||
| Bayley-III Cognitive | 86.0 (9.4) |
86.8 (10.0) | 85.3 (8.8) | 0.49 | −1.4 (−5.5, 2.6) |
0.18 | −3.2 (−7.8, 1.5) |
P=0.27/ R2=0.09 |
| Bayley-III Motor (n=85) |
83.3 (110) |
86.2 (10.5) | 80.7 (10.8) | 0.02 | −5.5 (−10, −0.9) |
0.02 | −6.3 (−11.7, −0.99) |
P=0.07/ R2=0.14 |
| Bayley-III Language (n=84) |
88.0 (11.4) |
91.9 (11.4) | 84.9 (10.5) | 0.005 | −7.0 (−11.8, −2.2) |
0.006 | −8.3 (−14.2, −2.4) |
P=0.09/ R2=0.13 |
| ITSEA-Externalizing (n=80) |
54.2 (12.5) |
51.43 (11.1) | 56.5 (13.4) | 0.07 | 5.1 (−0.4, 10.6) |
0.04 | 6.4 (0.22, 12.6) |
P=0.07/ R2=0.14 |
| ITSEA Internalizing (n=80) |
50.4 (110) |
50.4 (8.2) | 50.4 (13.1) | 0.99 | 0.04 (−4.9, 5.0) |
0.90 | −0.3 (−5.5,4.8) |
P=0.16/ R2=0.11 |
| ITSEA-Dysregulation (n=80) |
49.7 (14.1) |
48.5 (14.9) | 50.8 (13.4) | 0.47 | 2.3 (−4.0, 8.6) |
0.31 | 3.5 (−3.3, 10.4) |
P=0.56/ R2=0.06 |
| ITSEA-Competence (n=80) |
41.2 (14.0) |
44.0 (12.9) | 38.8 (14.5) |
0.09 | −5.2 (−11.4, 0.92) |
0.054 | −6.3 (−12.7, −0.01) |
P=0.25/ R2=0.09 |
| M-CHAT Fail (n=77) | 19 (25%) |
7 (19%) | 12 (29%) | 0.32 | 0.5 (−0.5, 1.6) |
0.42 | 0.5 (−0.7, 1.7) |
P=0.870 R2=0.04 |
Multivariable linear regression exploring associations between room type and developmental outcome, while controlling for CRIB score, cerebral injury, social risk score, and family functioning.
Total number of infants who returned for developmental testing at age 2 years. When there are missing data points, a separate n is reported next the variable.
Abbreviations: Bayley Scales of Infant and Toddler Development®, 3rd Edition (Bayley-III), Infant Toddler Social Emotional Assessment (ITSEA)
Additional analyses revealed a trend toward an interaction between medical risk factors and room type for language outcome (p=0.04). There was no impact of room type on neurodevelopmental outcome among infants who were high risk (n=16; p>0.05). However, low risk infants (n=70) demonstrated strong associations between room type and outcome, with higher language scores by 9.9 (95%CI: 5.1-14.8, p=0.0002) among infants in the open wards. Initially, we felt this may reflect that high risk infants had greater cerebral injury, but analysis of moderate-severe cerebral injury revealed a different interaction (p=0.02) in which infants from the open wards with moderate-severe cerebral injury (n=16) displayed higher language scores by 18.6 (95% CI 9.2-28, p=0.001) compared with infants with cerebral injury in the private rooms. In contrast, infants without moderate-severe cerebral injury (n=66) displayed a smaller difference of 4.2 (95% CI −1 to 9.4, p=0.1) in language scores. There was no interaction between motor and cognitive outcomes in relation to room type and medical risk or cerebral injury (p>.05). There were no significant interactions between room type and parent visitation on any of the outcomes (p>.05).
DISCUSSION
The key finding of our study was the rejection of the hypothesis of improved neurodevelopmental outcome for infants hospitalized in private NICU rooms. Lower language scores were found at age two years amongst children hospitalized as infants in NICU private rooms, with early neuroimaging and electrophysiology findings also suggesting altered cerebral development. This finding challenges previous reports on the positive benefits of the NICU private room. In addition, we found variation of the outcome in relation to medical and infant characteristics, with a very large clinical impact on neurodevelopmental outcome in selected infant groups, such as those with moderate-severe cerebral injury. This suggests that individualized approaches to room assignment may be appropriate. The current findings highlight the importance of the NICU environment by identifying associations between room type and brain activity, brain structure and neurodevelopmental outcome in the prematurely-born infant.
The differences in outcomes may relate to the relative sensory deprivation associated with private rooms, particularly in an urban American NICU setting with low parental visitation. Private rooms have reduced noise exposure13 and longer periods of silence37 than open wards, and these differences have been observed in a separate cohort at the study site.38 The increased levels of arousal evident in infants hospitalized in the lower stimulation environment of the NICU private rooms in the current study is supported by other research that has documented decreased arousal with continuous stimulation.39 The hypothesis that our findings could relate to sensory deprivation is supported by evidence that reduced language exposure and caregiver contact after full term birth is associated with emotional disturbances including externalizing symptoms,40, 41 delayed cognitive and language skills,41, 42 and abnormalities evident on MRI.43 Although the very preterm infant differs from a child who has been institutionalized or deprived of caregiving attention, studies from preterm infants suggest an important role of early sensory exposure. Greater language exposure in the NICU is associated with more early vocalizations,44 and more noise in the NICU environment has been associated with better language and motor outcomes.45
The means by which sensory deprivation affects neurodevelopmental outcome is likely related to the critical stage of neural development that characterizes the premature infant in the NICU. For example, exposure to the mother’s voice prior to birth may be essential for typical brain development.46-48 Auditory processing has been demonstrated between the 28th -33rd week of gestation using fetal fcMRI.49 In utero exposure, despite filtering out high frequency noise, is believed to facilitate speech and language acquisition, with the fetus perceiving and learning aspects of human speech between 31 and 40 weeks PMA.47, 50-52 Studies in preterm infants indicate that the amount of language exposure as early as 32 weeks PMA53 alters the amount of vocalizations and conversational turns by the infant at 36 weeks PMA. Although we did not quantify sound and language exposure in either environment, reduced sensory exposure in the private room may have altered brain structure and the developmental trajectory. Identifying the associations between early language and sound exposure on outcomes is an important next step.
Although few studies have investigated outcome in relation to qualitative MRI assessment, brain metrics, diffusion, volumetry, fcMRI and morphometry, we attempted to clarify the functional underpinnings of a difference in outcome by analyzing electrophysiological and MRI data from this cohort. Differences in cerebral maturation scores on aEEG were not evident in infants in each room type at baseline, suggesting that the infants started on equal footing, but were delayed in infants in the private room setting by discharge from the NICU. With regard to neuroimaging, qualitative and volumetric differences were not found. Resting state functional networks incorporating language and motor regions were identified, but with no differences across room type. In contrast, structural differences in hemispheric asymmetry were detected between the infants in the two room types, particularly in the planum temporale and superior temporal sulcus - regions implicated in language function. Hemispheric asymmetry in these regions has been documented in healthy term-born infants at birth.30 Both open ward and private room infants had asymmetry of the planum temporale, but the asymmetry in the superior temporal sulcus was markedly diminished in the private room infants. The discrepancy between the presence of resting state networks in the language domains at discharge from the NICU and the reduction in the hemispheric asymmetry may be due to differences in the sensitivity between the imaging techniques for cerebral development. An alternate explanation is that language exposure during the third trimester, and in the NICU for the preterm infant, is not crucial for the early development of language networks, as seen on fcMRI, but may be necessary for regional cortical folding and subsequent functional language maturation by two years of age.
Limitations of the current study include that this was an exploratory study that did not employ a randomized design. Further, the analyses relied on multiple comparisons of outcome, which increases the risk of a Type I error. In addition, the study NICU may not be representative of other open wards (due to the smaller number of beds in four separate open ward rooms). Finally, the findings of this study should be interpreted cautiously, as they cannot necessarily be generalized. They may be of relevance only to urban populations and may have less applicability in other socio-cultural settings due to differing family visitation, holding, and interactions with infants. The poor rates of parent visitation and holding within the study site NICU private room may have been insufficient to have mediated altered outcome, but this may differ in settings in which parents are fully engaged in NICU care.
Although current theory supports the positive aspects of sound abatement and sensory minimization, this theory was conceived prior to the advent of the NICU private room. Further, the degree of stimulation which best supports the early development of the preterm infant, independent of room type, is not well understood. Our findings suggest that environmental sound and language exposure in the private room may, in some cases, be reduced to levels that are detrimental to child development. More research is needed to determine the optimal NICU environment.
Acknowledgments
We express our appreciation to the following individuals who assisted with this project: Jiajing Chen, MA (Washington University Biostatistics Department), and Ryan Colvin, MA (Washington University Biostatistics Department), who provided biostatistical support; Karen Lukas, RN (Washington University School of Medicine), Anthony Barton (Washington University School of Medicine), Jessica Conners (Washington University School of Medicine), Claudine Vavasseur, MD (National Maternity Hospital, Dublin Ireland), and Han Tjoeng, MD (University of Hawaii), who obtained informed consent and conducted patient oriented responsibilities to support the success of this project; Jim Alexopoulos, Reginald Lee, Tara Smyser, and Joe Ackermann, Jr. (all from Washington University School of Medicine) who worked on imaging data synthesis; and Katrina Stransky, MSOT, and Cori Zarem, OTD (both from Washington University Program in Occupational Therapy), who provided some editorial assistance and guidance. We also thank the families and infants who were participants and made this research possible.
Supported by the National Institutes of Health (NIH; ROI HD 057098, K12 NS001690, KL2 TR000450, and UL1 TR000448), the Intellectual and Developmental Disabilities Research Center at Washington University (NIH/National Institute of Child Health and Human Development P30 HD062171), and the Doris Duke Foundation.
Abbreviations
- (NICU)
Neonatal intensive care unit
- (aEEG)
amplitude integrated electroencephalography
- (CRIB)
Critical Risk Index for Babies
- (PMA)
postmenstrual age
- (PDA)
patent ductus arteriosis
- (CPAP)
continuous positive airway pressure
- (NNNS)
NICU Network Neurobehavioral Scale
- (MRI)
magnetic resonance imaging
- (MPRAGE)
magnetization prepared rapid gradient echo
- (TSE)
turbo spin echo
- (EPI)
echo planar image
- (CUS)
cranial ultrasound
- (IVH)
intraventricular hemorrhage
- (PVL)
periventricular leukomalacia
- (ROI)
region of interest
- (ANTS)
advanced normalization tools
- (FA)
fractional anisotropy
- (fcMRI)
functional connectivity magnetic resonance imaging
- (M-CHAT)
Modified Checklist for Autism in Toddlers
- (ITSEA)
Infant Toddler Social Emotional Assessment
- (Bayley-III)
Bayley Scales of Infant and Toddler Development, 3rd edition
- (FAD)
McMaster Family Assessment Device
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Lasky RE, Williams AL. Noise and light exposures for extremely low birth weight newborns during their stay in the neonatal intensive care unit. Pediatrics. 2009;123:540–6. doi: 10.1542/peds.2007-3418. [DOI] [PubMed] [Google Scholar]
- [2].Noise: a hazard for the fetus and newborn. American Academy of Pediatrics. Committee on Environmental Health. Pediatrics. 1997;100:724–7. [PubMed] [Google Scholar]
- [3].Liu WF, Laudert S, Perkins B, Macmillan-York E, Martin S, Graven S. The development of potentially better practices to support the neurodevelopment of infants in the NICU. Journal of perinatology : official journal of the California Perinatal Association. 2007;27(Suppl 2):S48–74. doi: 10.1038/sj.jp.7211844. [DOI] [PubMed] [Google Scholar]
- [4].Hassanein SM, El Raggal NM, Shalaby AA. Neonatal nursery noise: practice-based learning and improvement. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2012 doi: 10.3109/14767058.2012.733759. [DOI] [PubMed] [Google Scholar]
- [5].Als H, Brazelton TB. A new model of assessing the behavioral organization in preterm and fullterm infants: two case studies. Journal of the American Academy of Child Psychiatry. 1981;20:239–63. doi: 10.1016/s0002-7138(09)60987-0. [DOI] [PubMed] [Google Scholar]
- [6].Maguire CM, Veen S, Sprij AJ, Le Cessie S, Wit JM, Walther FJ. Effects of basic developmental care on neonatal morbidity, neuromotor development, and growth at term age of infants who were born at <32 weeks. Pediatrics. 2008;121:e239–45. doi: 10.1542/peds.2007-1189. [DOI] [PubMed] [Google Scholar]
- [7].Als H, Duffy FH, McAnulty G, Butler SC, Lightbody L, Kosta S, et al. NIDCAP improves brain function and structure in preterm infants with severe intrauterine growth restriction. Journal of perinatology : official journal of the California Perinatal Association. 2012;32:797–803. doi: 10.1038/jp.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].White RD, Smith JA, Shepley MM. Recommended standards for newborn ICU design, eighth edition. Journal of perinatology : official journal of the California Perinatal Association. 2013;33(Suppl 1):S2–16. doi: 10.1038/jp.2013.10. [DOI] [PubMed] [Google Scholar]
- [9].Carter BS, Carter A, Bennett S. Families’ views upon experiencing change in the neonatal intensive care unit environment: from the ‘baby barn’ to the private room. Journal of perinatology : official journal of the California Perinatal Association. 2008;28:827–9. doi: 10.1038/jp.2008.102. [DOI] [PubMed] [Google Scholar]
- [10].Bosch S, Bledsoe T, Jenzarli A. Staff Perceptions Before and After Adding Single-Family Rooms in the NICU. Herd. 2012;5:64–75. doi: 10.1177/193758671200500406. [DOI] [PubMed] [Google Scholar]
- [11].Stevens DC, Helseth CC, Thompson PA, Pottala JV, Khan MA, Munson DP. A Comprehensive Comparison of Open-Bay and Single-Family-Room Neonatal Intensive Care Units at Sanford Children’s Hospital. Herd. 2012;5:23–39. doi: 10.1177/193758671200500403. [DOI] [PubMed] [Google Scholar]
- [12].Walsh WF, McCullough KL, White RD. Room for improvement: nurses’ perceptions of providing care in a single room newborn intensive care setting. Advances in neonatal care : official journal of the National Association of Neonatal Nurses. 2006;6:261–70. doi: 10.1016/j.adnc.2006.06.002. [DOI] [PubMed] [Google Scholar]
- [13].Liu WF. Comparing sound measurements in the single-family room with open-unit design neonatal intensive care unit: the impact of equipment noise. Journal of perinatology : official journal of the California Perinatal Association. 2012;32:368–73. doi: 10.1038/jp.2011.103. [DOI] [PubMed] [Google Scholar]
- [14].Erdeve O, Arsan S, Yigit S, Armangil D, Atasay B, Korkmaz A. The impact of individual room on rehospitalization and health service utilization in preterms after discharge. Acta Paediatr. 2008;97:1351–7. doi: 10.1111/j.1651-2227.2008.00889.x. [DOI] [PubMed] [Google Scholar]
- [15].Ortenstrand A, Westrup B, Brostrom EB, Sarman I, Akerstrom S, Brune T, et al. The Stockholm Neonatal Family Centered Care Study: effects on length of stay and infant morbidity. Pediatrics. 2010;125:e278–85. doi: 10.1542/peds.2009-1511. [DOI] [PubMed] [Google Scholar]
- [16].Pineda RG, Stransky KE, Rogers C, Duncan MH, Smith GC, Neil J, et al. The single-patient room in the NICU: maternal and family effects. Journal of perinatology : official journal of the California Perinatal Association. 2012;32:545–51. doi: 10.1038/jp.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tarnow-Mordi W, Parry G. The CRIB score. Lancet. 1993;342:1365. doi: 10.1016/0140-6736(93)92276-y. [DOI] [PubMed] [Google Scholar]
- [18].Daily DK, Ellison PH. The premie-neuro: a clinical neurologic examination of premature infants. Neonatal network : NN. 2005;24:15–22. doi: 10.1891/0730-0832.24.1.15. [DOI] [PubMed] [Google Scholar]
- [19].Lester B, Tronick E. NICU Network Neurobehavioral Scale Manual. Paul H. Brookes Publishing Co.; Baltimore: 2004. [Google Scholar]
- [20].Dubowitz L, Mercuri E, Dubowitz V. An optimality score for the neurologic examination of the term newborn. The Journal of pediatrics. 1998;133:406–16. doi: 10.1016/s0022-3476(98)70279-3. [DOI] [PubMed] [Google Scholar]
- [21].Palmer MM, Crawley K, Blanco IA. Neonatal Oral-Motor Assessment scale: a reliability study. Journal of perinatology : official journal of the California Perinatal Association. 1993;13:28–35. [PubMed] [Google Scholar]
- [22].Burdjalov VF, Baumgart S, Spitzer AR. Cerebral function monitoring: a new scoring system for the evaluation of brain maturation in neonates. Pediatrics. 2003;112:855–61. doi: 10.1542/peds.112.4.855. [DOI] [PubMed] [Google Scholar]
- [23].Kidokoro H, Neil JJ, Inder TE. New MR Imaging Assessment Tool to Define Brain Abnormalities in Very Preterm Infants at Term. AJNR American journal of neuroradiology. 2013 doi: 10.3174/ajnr.A3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nguyen The Tich S, Anderson PJ, Shimony JS, Hunt RW, Doyle LW, Inder TE. A novel quantitative simple brain metric using MR imaging for preterm infants. AJNR American journal of neuroradiology. 2009;30:125–31. doi: 10.3174/ajnr.A1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- [26].Klein A, Ghosh SS, Avants B, Yeo BT, Fischl B, Ardekani B, et al. Evaluation of volume-based and surface-based brain image registration methods. NeuroImage. 2010;51:214–20. doi: 10.1016/j.neuroimage.2010.01.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, et al. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex. 2010;20:2852–62. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Turken AU, Dronkers NF. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Frontiers in systems neuroscience. 2011;5:1. doi: 10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fransson P, Skiold B, Horsch S, Nordell A, Blennow M, Lagercrantz H, et al. Resting-state networks in the infant brain. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15531–6. doi: 10.1073/pnas.0704380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hill J, Dierker D, Neil J, Inder T, Knutsen A, Harwell J, et al. A surface-based analysis of hemispheric asymmetries and folding of cerebral cortex in term-born human infants. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:2268–76. doi: 10.1523/JNEUROSCI.4682-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Epstein N, Baldwin L, Bishop D. The McMaster Family Assessment Device. Journal of Marital and Family Therapy. 1983;9:171–80. [Google Scholar]
- [32].Schjolberg S, Eadie P, Zachrisson HD, Oyen AS, Prior M. Predicting language development at age 18 months: data from the Norwegian Mother and Child Cohort Study. Journal of developmental and behavioral pediatrics : JDBP. 2011;32:375–83. doi: 10.1097/DBP.0b013e31821bd1dd. [DOI] [PubMed] [Google Scholar]
- [33].Bayley N. Bayley Scales of Infant and Toddler Development. III ed Harcourt Assessment, Inc.; San Antonio: 2006. [Google Scholar]
- [34].Makrides M, Gibson RA, McPhee AJ, Collins CT, Davis PG, Doyle LW, et al. Neurodevelopmental outcomes of preterm infants fed high-dose docosahexaenoic acid: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2009;301:175–82. doi: 10.1001/jama.2008.945. [DOI] [PubMed] [Google Scholar]
- [35].Robins DL, Fein D, Barton ML, Green JA. The Modified Checklist for Autism in Toddlers: an initial study investigating the early detection of autism and pervasive developmental disorders. Journal of autism and developmental disorders. 2001;31:131–44. doi: 10.1023/a:1010738829569. [DOI] [PubMed] [Google Scholar]
- [36].Carter A, Briggs-Gowan M. Infant Toddler Social and Emotional Assessment (ITSEA) Psychological Corporation, Harcourt Assessment; San Antonio, TX: 2006. [Google Scholar]
- [37].Caskey M, Tucker R. Vohr. Language environment in a single familly room NICU. Pediatric Academic Societies; Boston: 2012. EPAS2012: 2920314. [Google Scholar]
- [38].Dunsirn S, Mara M, Tiltges L, Crapnell T, Rogers C, Inder T, Neil J, Pineda R. Longer periods of silence in the NICU private room. Pediatric Academic Societies; Washington DC: 2013. E-PAS2013:2922. [Google Scholar]
- [39].Brackbill Y. Continuous stimulation reduces arousal level: stability of the effect over time. Child development. 1973;44:43–6. [PubMed] [Google Scholar]
- [40].Merz EC, McCall RB. Behavior problems in children adopted from psychosocially depriving institutions. Journal of abnormal child psychology. 2010;38:459–70. doi: 10.1007/s10802-009-9383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ellis BH, Fisher PA, Zaharie S. Predictors of disruptive behavior, developmental delays, anxiety, and affective symptomatology among institutionally reared romanian children. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:1283–92. doi: 10.1097/01.chi.0000136562.24085.160. [DOI] [PubMed] [Google Scholar]
- [42].Berument SK, Sonmez D, Eyupoglu H. Supporting language and cognitive development of infants and young children living in children’s homes in Turkey. Child: care, health and development. 2012;38:743–52. doi: 10.1111/j.1365-2214.2011.01314.x. [DOI] [PubMed] [Google Scholar]
- [43].Govindan RM, Behen ME, Helder E, Makki MI, Chugani HT. Altered water diffusivity in cortical association tracts in children with early deprivation identified with Tract-Based Spatial Statistics (TBSS) Cereb Cortex. 2010;20:561–9. doi: 10.1093/cercor/bhp122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Caskey M, Vohr B, Stephens B, Tucker R. Impact of language exposure in the NICU on the development of vocalizations in preterm infants. Pediatric Academic Societies; Vancouver: 2010. E-PAS2010:1467. [Google Scholar]
- [45].Stromswold K, Sheffield E. Neonatal Intensive Care Unit Noise and Language Development. Rutgers University Center for Cognitive Science Technical Report. 2004 [Google Scholar]
- [46].Fifer WP, Moon CM. The role of mother’s voice in the organization of brain function in the newborn. Acta Paediatr Suppl. 1994;397:86–93. doi: 10.1111/j.1651-2227.1994.tb13270.x. [DOI] [PubMed] [Google Scholar]
- [47].Kisilevsky BS, Hains SM. Onset and maturation of fetal heart rate response to the mother’s voice over late gestation. Developmental science. 2011;14:214–23. doi: 10.1111/j.1467-7687.2010.00970.x. [DOI] [PubMed] [Google Scholar]
- [48].Jardri R, Houfflin-Debarge V, Delion P, Pruvo JP, Thomas P, Pins D. Assessing fetal response to maternal speech using a noninvasive functional brain imaging technique. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2012;30:159–61. doi: 10.1016/j.ijdevneu.2011.11.002. [DOI] [PubMed] [Google Scholar]
- [49].Jardri R, Pins D, Houfflin-Debarge V, Chaffiotte C, Rocourt N, Pruvo JP, et al. Fetal cortical activation to sound at 33 weeks of gestation: a functional MRI study. NeuroImage. 2008;42:10–8. doi: 10.1016/j.neuroimage.2008.04.247. [DOI] [PubMed] [Google Scholar]
- [50].Granier-Deferre C, Ribeiro A, Jacquet AY, Bassereau S. Near-term fetuses process temporal features of speech. Developmental science. 2011;14:336–52. doi: 10.1111/j.1467-7687.2010.00978.x. [DOI] [PubMed] [Google Scholar]
- [51].Cheour-Luhtanen M, Alho K, Sainio K, Rinne T, Reinikainen K, Pohjavuori M, et al. The ontogenetically earliest discriminative response of the human brain. Psychophysiology. 1996;33:478–81. doi: 10.1111/j.1469-8986.1996.tb01074.x. [DOI] [PubMed] [Google Scholar]
- [52].Moon C, Lagercrantz H, Kuhl PK. Language experienced in utero affects vowel perception after birth: a two-country study. Acta Paediatr. 2013;102:156–60. doi: 10.1111/apa.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Caskey M, Stephens B, Tucker R, Vohr B. Importance of parent talk on the development of preterm infant vocalizations. Pediatrics. 2011;128:910–6. doi: 10.1542/peds.2011-0609. [DOI] [PubMed] [Google Scholar]