Abstract
A shift in GABAA signaling from inhibition to excitation in primary afferent neurons appears to contribute to the inflammation-induced increase in afferent input to the central nervous system (CNS). An activity-dependent depolarization of the GABA equilibrium potential (EGABA) has been described in CNS neurons which drives a shift in GABAA signaling from inhibition to excitation. The purpose of the present study was to determine if such an activity-dependent depolarization of EGABA occurs in primary afferents and whether the depolarization is amplified with persistent inflammation. Acutely dissociated retrogradely labeled cutaneous DRG neurons from naïve and inflamed rats were studied with gramicidin perforated patch recording. Rather than a depolarization, 200 action potentials delivered at 2 Hz resulted in a ~10 mV hyperpolarization of EGABA in cutaneous neurons from naïve rats. No such hyperpolarization was observed in neurons from inflamed rats. The shift in EGABA was not blocked by 10 µM bumetanide. Furthermore, because activity-dependent hyperpolarization of EGABA was fully manifest in the absence of HCO3− in the bath solution, this shift was not dependent on a change in HCO3−-Cl− exchanger activity, despite evidence of HCO3−-Cl− exchangers in DRG neurons that may contribute to the establishment of EGABA in the presence of HCO3−. While the mechanism underlying the activity-dependent hyperpolarization of EGABA has yet to be identified, because this mechanism appears to function as a form of feedback inhibition, facilitating GABA mediated inhibition of afferent activity, it may serve as a novel target for the treatment of inflammatory pain.
Keywords: Chloride equilibrium potential, inflammatory pain, nociceptor sensitization, sodium-potassium-chloride co-transporter (NKCC1), feedback-inhibition
Pain and hypersensitivity observed in the presence of inflammation are due, at least in part, to an increase in primary afferent input to the dorsal horn. Recent evidence suggests that a shift in GABAA signaling from inhibition to excitation in primary afferents may play a prominent role in this inflammation-induced increase in afferent input. This suggestion is based on several lines of evidence. First, in the absence of inflammation, spinal application of the GABAA receptor agonist muscimol is analgesic, while in the presence of inflammation, muscimol facilitates inflammatory hyperalgesia, at least at low doses (Anseloni and Gold, 2008). Second, GABAA receptors are present in all primary afferents (White, 1990, Paul et al., 2012, Zhu et al., 2012b) with evidence that in the absence of tissue injury, receptor activation results in the inhibition of glutamate release (Yuan et al., 2009). Third, spinal application of the GABAA receptor antagonists are antinociceptive (Yaksh, 1989, Anseloni and Gold, 2008) and inhibit afferent activity initiated within the dorsal horn (Rees et al., 1995, Lin et al., 1999) following tissue injury or inflammation.
A compelling explanation for the inflammation-induced shift in GABAA signaling is that it is due to a depolarization of the GABAA current equilibrium potential (EGABA) in primary afferents (Price et al., 2009). There are several lines of evidence in support of this hypothesis. First, EGABA in primary afferents is 15~30 mV more positive to the afferent resting membrane potential (Alvarez-Leefmans et al., 1988, Rocha-Gonzalez et al., 2008, Zhu et al., 2012b). Second, this depolarized EGABA is thought to reflect the distinct pattern of Cl− co-transporter expression in primary afferent neurons that underlie anion homeostasis: a high level of Na+- K+- Cl−-cotransporter1 (NKCC1), which accumulates intracellular Cl−, and a low level of K+- Cl−-cotransporter 2 (KCC2), which extrudes Cl− (Kanaka et al., 2001). However, GABA induced excitation would result from a depolarization of EGABA above the action potential threshold. Third, tissue injury has been shown to result in the phosphorylation and membrane translocation of NKCC1 (Galan and Cervero, 2005), as well as an increase in total spinal protein levels of NKCC1 (Lagraize et al., 2010). Furthermore, inflammatory mediators are released in the spinal cord as well as the periphery following tissue injury and have also been shown to increase NKCC1 phosphorylation which was accompanied by a positive shift in EGABA in an in vitro study of dorsal root ganglion (DRG) neurons, (Funk et al., 2008). And fourth, attenuation of NKCC1 activity with a genetic deletion (Sung et al., 2000) or the use of the relatively specific NKCC1 blocker, bumetanide, results in the attenuation of touch evoked allodynia and the sensitization of spinal nociceptive neurons associated with peripheral capsaicin (Pitcher et al., 2007).
The evidence in support of an increase in NKCC1 in primary afferents as a mechanism of the inflammation-induced shift in GABAA signaling is compelling. Nevertheless, the vast majority of the data are in support of this mechanism were collected with acute models of noxious stimulation (i.e., capsaicin administration), or within hours of the induction of more persistent inflammation (Lagraize et al., 2010). Furthermore, despite evidence of a shift in GABAA signaling in the presence of persistent inflammation (Anseloni and Gold, 2008), our recent results suggest that a steady-state depolarizing shift in EGABA cannot account for the persistence of excitatory GABAA signaling (Zhu et al., 2012b). Rather, our results suggested that an increase in GABAA current density secondary to an increase in tyrosine kinase activity in combination with a decrease in low threshold K+ current density was more likely to account for the steady-state inflammation-induced shift in GABAA signaling (Zhu et al., 2012b).
Regardless of the steady-state mechanisms contributing to an inflammation-induced shift in GABAA signaling, further depolarization of EGABA in primary afferents will still result in an increase in the driving force on Cl− and therefore may still contribute to an increase in excitatory GABAA signaling. Importantly, there is evidence that the regulation of intracellular Cl− concentration is dynamic such that the impact of GABA can change in association with prolonged GABA application or ongoing neural activity. For example, prolonged GABA application can result in the emergence of an excitatory response in CNS neurons from immature animals because Cl− extrusion mechanisms are not sufficiently developed to handle large Cl− loads (Cordero-Erausquin et al., 2005). Similarly, and more relevant to afferent signaling, there is evidence of activity-dependent changes in NKCC1 (Brumback and Staley, 2008) and depolarization of EGABA (Khalilov et al., 2003, Brumback and Staley, 2008). Thus, while our data argue against a steady-state shift in EGABA as a mechanism underlying the inflammation-induced increase in GABA mediated excitation of isolated sensory neurons we hypothesized that an inflammation-induced change in the regulation of intracellular Cl− would enable a dynamic shift in EGABA to contribute to the inflammation-induced changes in GABA signaling observed in vivo. Specifically, we hypothesized that an increase in the accumulation capacity of NKCC1 would contribute to an activity-dependent depolarizing shift in EGABA. To test this hypothesis, perforated patch clamp techniques were used to study activity-dependent changes in EGABA in acutely isolated retrogradely labeled cutaneous DRG neurons from naïve and inflamed rats.
Experimental Procedures
Animals
Adult male Sprague Dawley rats (Harlan-Sprague Dawley, Indianapolis, IN) weighing between 250 and 350 g were used for all the experiments. Rats were housed two per cage in the University of Pittsburgh AAALAC approved animal facility on a 12:12 light: dark schedule with food and water available. All procedures involving animals were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and performed in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Labeling and inflammation
DRG neurons that innervate the glabrous skin of rat hind paw were retrogradely labeled with 1,1’-Dioctadecyl-3,3,3’,3’-tetramethylindocarbocyanine perchlorate (DiI, 17 mg/ml in DMSO and saline), which was injected (3–5 sites at 2–3 µl/site) with a 30g needle, 14–17 days prior to electrophysiological recording. Complete Freud’s adjuvant (CFA, Sigma-Aldrich, St Louis MO; mixed 1:1 with saline), was injected (100 µl) into the site previously injected with DiI. DRG neurons from inflamed rats were studied 72 hours after CFA injection. Both DiI and CFA were injected under isofluorane-induced anesthesia.
Preparation of isolated DRG neurons
Prior to tissue harvest, rats were deeply anesthetized with a subcutaneous injection (1 ml/kg) of a cocktail containing ketamine (55 mg/ml), xylaxine (20 mg/ml) and acepromazine (5.5 mg/ml). L4 and L5 DRG were harvested bilaterally and either pooled for non-inflamed animals or processed in parallel (for inflamed animals) so that neurons ipsilateral and contralateral to the site of inflammation could be studied. DRG were cleaned of connective tissue, enzymatically treated and mechanically dispersed as previously described (Lu et al., 2006). Isolated neurons were plated on poly-lysine coated coverslips and electrophysiology experiments were performed 2–8 h after plating.
Electrophysiology
Voltage clamp recordings were performed using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, USA) controlled with pClamp software (Molecular Devices) via a Digidata 1320A A/D converter. Data were low-pass filtered at 10 kHz and digitally sampled at 2 kHz. Capacity transients were canceled via amplifier circuitry. Borosilicate glass (WPI, Sarasota Springs, FL) patch electrodes were pulled on a Sutter P-2000 horizontal puller (Sutter Instruments) and were 1.5~2.5 MΩ in resistance when filled with electrode solution of the following composition (in mM): K-Methansulphonate 140, MgCl2.6H2O 2, CaCl2 1, EGTA 11, HEPES 10 and NaCl 5; pH was adjusted to 7.2 with Tris Base, and osmolality was adjusted to 317 mOSm with sucrose.
The gramicidin perforated patch configuration was used to measure EGABA (Akaike, 1996) as described previously (Zhu et al., 2012b). Briefly, gramicidin stock solution in DMSO (1.5 mg/100 µl) was diluted with electrode solution in a 1:300 ratio. The tip of the electrode was loaded with a small volume of gramicidin free electrode solution and gramicidin containing electrode solution was back loaded. Experiments were not started until access resistance was less than 7 MΩ. Seal resistance was continuously monitored throughout experiments, and data were excluded from further analysis if there was any evidence of a change in stability throughout the experiment or there was a decrease in seal resistance over time greater than 10%. Furthermore, neurons were repeatedly stimulated throughout each experiment with the same voltage protocol in the presence and absence of GABA and if there was any evidence of instability in the currents evoked in the absence of GABA, data from that neuron were not included in subsequent analyses. Finally, to rule out the impact of movement artifacts assessed with the fast drug application system, we assessed the presence of “current” associated with a step in the perfusion system with normal bath flowing through both capillaries; data were excluded from further analysis if there was any evidence of current evoked with this protocol.
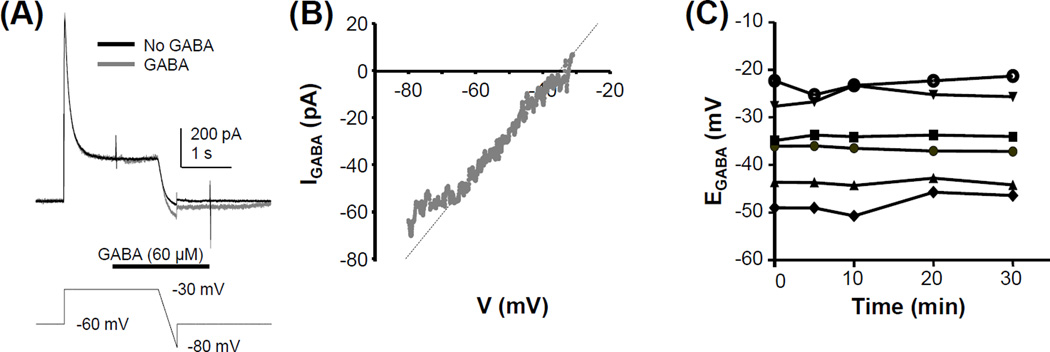
A hyperpolarizing ramp protocol was used to determine EGABA (Figure 1A). The membrane potential was depolarized to ~EGABA for two seconds to inactivate transient outward and inward currents and then hyperpolarized from this potential to −80 mV at a rate ~0.063 mV/ms. The amplitude of the depolarizing voltage step used for the start of the hyperpolarizing ramp was adjusted to EGABA. The depolarizing step to ~EGABA was used to minimize the possibility that flux of Cl− during the EGABA measurement would change the intracellular Cl− concentration. As a result, the GABA current evoked during the two second pre-pulse was always less than 20 pA. The membrane potential was then returned to rest at −60 mV. The same protocol was run once with the application of 60 µM GABA and three times in the absence of GABA application. To minimize the possibility that GABAB receptor activation influenced estimates of EGABA, the GABAB receptor antagonist CGP 55845 (1 µM) was included in the GABA solution applied to all neurons. Our previous results indicate that CGP 55845 has no influence on EGABA (Zhu et al., 2012b). EGABA was determined from the difference between the current trace evoked in the presence of GABA and the average of the 3 traces obtained in its absence as the point on the voltage ramp where the corresponding GABA current equaled 0 pA (Figure 1B). If necessary, the linear phase of the GABA current was extrapolated to 0 pA to determine EGABA. While the relatively small currents evoked with 60 µM GABA resulted in a less then optimal signal to noise ratio for the determination of EGABA from the GABA evoked currents, this low concentration was chosen for three important reasons. First, with smaller currents, we minimized the possibility of a GABA current-induced shift in EGABA (Cordero-Erausquin et al., 2005). Second, we have previously demonstrated that the desensitization of GABA evoked current in DRG neurons is concentration dependent such that there is a significant decay in current evoked with GABA concentrations greater than 100 µM (Zhu et al., 2012a). Thus, we minimized the impact of channel desensitization across the ramp protocol on the estimate of EGABA. To further ensure the stability of GABA current during the ramp, GABA was applied at least 100 ms prior to the start of the ramp. Third, full recovery from GABA-induced desensitization is also concentration dependent, such that longer intervals are needed between GABA applications to obtain stable currents. Importantly, preliminary data indicated that current evoked with 60 µM GABA was stable with an inter-stimulus interval of two minutes.
Figure 1.
Determination of EGABA. (A) Current evoked in response to a ramp protocol used to determine EGABA. The protocol was run before (black trace) and after (gray trace) the application of GABA (60 µM). GABA application was initiated during the sustained depolarizing step used to inactivate transient inward and outward currents prior to the ramp. The voltage protocol is shown beneath the current traces. Data were from a cutaneous neuron from a naïve rat (B) The difference between the current evoked during the ramp, in the presence and absence of GABA in A is plotted as function of the change in voltage. The voltage at which the linear component of the difference current is 0 pA is EGABA. (C) EGABA monitored for 30 minutes at a 5 to 10 minute interval was relatively stable in the 6 control neurons tested.
The stability of EGABA was determined with at least three measurements taken at an interval of two minutes. Neurons were then stimulated with a voltage clamp protocol based on a typical action potential wave form recorded from a DiI labeled, acutely dissociated DRG neuron from a naïve rat. While we have evidence of an inflammation-induced change in the action potential waveform of cutaneous neurons (Zhang et al., 2012), we chose to use the same stimulus for all neurons studied because we were primarily interested in changes in the dynamic regulation of EGABA rather than an interaction between changes in the action potential waveform and changes in the regulation of EGABA. The action potential waveform was applied for 200 pulses at a rate of 2 Hz. These parameters were chosen based on a series of preliminary studies on naïve neurons in which we determined the minimal number of pulses needed to produce a consistent change in EGABA within 5 minutes of the last pulse. It should be noted, however, that there was variability between neurons with respect to the minimal number of pulses needed to produce a shift in EGABA with a shift detected in one of the 14 neurons studied with as few as 50 pulses. EGABA was determined within one minute of the last pulse and again every two minutes for 20 minutes. Changes in EGABA following stimulation were compared to measurements obtained prior to stimulation. Two bath solutions were used in this study. One was a HCO3− buffered bath consisting of (in mM): NaCl 103, KCl 3, NaHCO3 26, NaH2PO4 1.25, CaCl2 2.5, MgCl2 0.6 and Glucose 10, osmolality was adjusted to 325 mOsm with sucrose, bubbled with 5% CO2 and 95% O2. The second was a HEPES buffered bath consisting of (in mM): NaCl 130, KCl 3, CaCl2 2.5, MgCl2 0.6, HEPES 10, Glucose 10; pH was adjusted to 7.4 with Tris-Base and osmolality was adjusted to 325 mOsm with sucrose. All salts were from Sigma-Aldrich (St Louis, MO).
PCR
Rats from inflamed and naive groups were anesthetized, and L4 /L5 DRG were harvested. Total RNA was extracted from DRG with Trizol (Invitrogen, Carlsbad, CA). cDNA synthesis was carried out using 1 µg of total RNA with Superscript II Reverse Transcriptase (Invitrogen), 0.1 M DTT, RNaseOUT™, dNTP Mix, 5× First-Strand Buffer, random primer (Invitrogen) at 42°C for 50 min, as described by the manufacture protocol. cDNA was then kept at −20 °C for future use. For PCR, we used hot start Taq DNA Polymerases, all the reactions were denatured at 95 °C, annealed at 58 °C and extended at 72° C. After 36 cycles the product was separated on a 2% agarose gel. Primers used for amplification of SLC4A family members are listed in Table 1. The gel was then stained with 0.5 µg/mL ethidium bromide and imaged with a LAS3000 (Fujifilm Inc, Japan).
Table 1.
Primers for HCO3− exchangers
| Gene | Sequence | Start→END | SIZE |
|---|---|---|---|
| NCBE_F | 5'-GGTGTTGGCACTGGTATTTGTGAG-3' | 3091–3362 | 272 |
| NCBE_R | 5'-TGTCAGCAGTGACTAGAAGGTTCC-3' | ||
| NDCBE_F | 5'-CGTGCTCTTCTGGTCCTGTATTCT-3' | 2122–2358 | 237 |
| NDCBE_R | 5'-ATGAACCATCCTCGATCATCCCTC-3' | ||
| AE3_F | 5'-TCCCAACGATGACAAGGACAGT-3' | 1312–1556 | 245 |
| AE3_R | 5'-TCTTCTCCAGCAGTTTCAGGCT-3' |
Test compounds
4,4′-Diisothiocyanatostilbene-2,2′-disulfonic acid disodium salt hydrate (DIDS), γ-aminobuteric acid (GABA), bumetanide and gramicidin were from Sigma-Aldrich. CGP 55845 was from Tocris Bioscience (Bristol, UK). GABA and bumetanide were dissolved in water, and CGP 55845 and DIDS were dissolved in DMSO to make stocks that would be diluted 1:1000. Stock solutions were then kept in a −20°C freezer and diluted with bath solution to their working concentration before use.
All drugs were applied with a SF-77B Perfusion Fast-Step system (Warner Inc.). The Fast-Step was controlled by the pClamp 10.0 software.
Statistics
All pooled data are presented as mean ± standard error of the mean. Student’s T-test was used for two-group comparisons and repeated measure two-way ANOVA was used for multiple group comparisons (i.e., EGABA before and after stimulus in DRG neurons from naïve and inflamed animals). The Tukey test was used for post-hoc pair wise multiple comparisons. p < 0.05 was considered statistically significant.
Results
Activity-dependent shift of EGABA
Because we are primarily interested in mechanisms underlying inflammation-induced changes the GABA regulation of nociceptive signaling and there is evidence that nociceptive cutaneous afferents are enriched in the population of DRG neurons with a small to medium cell body diameter (Fang et al., 2005), small to medium diameter retrogradely labeled DRG neurons were the focus of the present study. The membrane capacitance ranged between 17.7 to 49 pF, with an average of 32.8 ± 8.8 pF.
In the absence of stimulation, EGABA was relatively stable in cutaneous neurons from both naïve and inflamed rats prior to stimulation during the determination of baseline EGABA, as well as in control neurons studied over time in the absence of stimulation (Figure 1C). Of note, while there appears to be considerable heterogeneity in EGABA among cutaneous neurons, this degree of heterogeneity is consistent with previous results from unlabeled DRG neurons (Rocha-Gonzalez et al., 2008), as well as our previous results from cutaneous DRG neurons (Zhu et al., 2012a). While the basis for this heterogeneity has yet to be determined, we suggest it is likely a reflection of heterogeneity in afferent function.
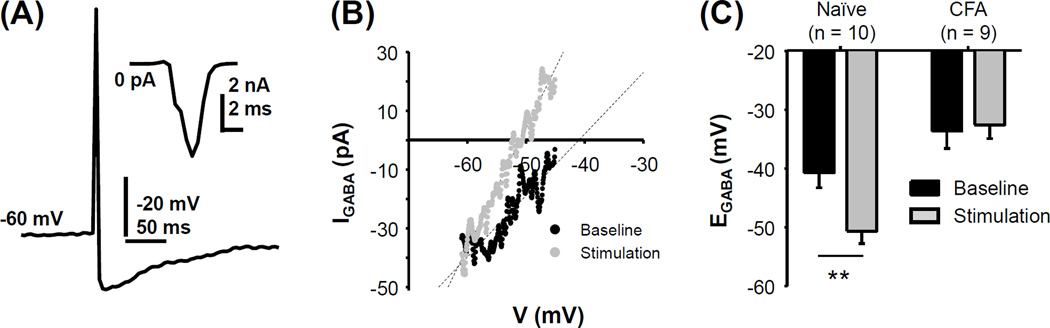
The action potential waveform used to stimulate cutaneous neurons (Figure 2A) resulted in the activation of primarily inward currents (Figure 2A inset). Stimulation of cutaneous neurons from naïve rats with the action potential waveform 200 times at 2 Hz resulted in a hyperpolarizing shift in EGABA in 10 of 10 neurons studied of −9.9 ± 0.9 mV mV (Figure 2B, C). The hyperpolarization of EGABA was fully manifest in minutes, and recovered to baseline within 16 ± 1.8 min. Consistent with our previous analysis of the impact of inflammation on EGABA in cutaneous neurons when recorded in HCO3− buffered bath (Zhu et al., 2012b), EGABA in cutaneous neurons from inflamed rats (−32.6 ± 2.3, n = 9) was significantly more depolarized than that in cutaneous neurons from naïve rats (−40.7 ± 2.5 mV, n = 10, Figure 2C, p = 0.02, Students t-test). However, in contrast to the hyperpolarization observed in cutaneous neurons from naïve rats, the average stimulation-induced shift in EGABA in the 9 cutaneous neurons tested from inflamed rats was depolarization of 1.1 ± 1.4 mV. The impact of inflammation on the activity-dependent shift in EGABA was statistically significant (Two-way mixed design ANOVA, p < 0.01, Figure 2C).
Figure 2.
The activity-dependent hyperpolarization of EGABA is absent in cutaneous neurons from inflamed rats. (A) Action potential waveform used to drive “activity-dependent” changes in EGABA was elicited in a small diameter cutaneous neuron from a naïve rat with direct current injection under current clamp configuration with gramicidin patch. Inset: current evoked in response to the action potential waveform used as a “stimulus” in voltage clamp. (B) Stimulation of a small diameter cutaneous neuron from a naïve rat with the waveform shown in A, 200 times at a frequency of 2 Hz resulted in a hyperpolarization of EGABA (black trace: before stimulus; grey trace: after stimulus). (C) Pooled EGABA data obtained before and after 200 pulse stimulation of cutaneous neurons from naïve and inflamed (CFA) rats. There was a significant interaction between group (naïve vs inflamed) and the impact of stimulation (before vs after) due to the hyperpolarizing shift in EGABA present in neurons from naïve but not inflamed rats. Number of neurons recorded is indicated in the parenthesis. ** p< 0.01.
NKCC1 does not contribute to the activity-dependent shift in EGABA
Because NKCC1 is suggested to contribute to the activity-dependent EGABA changes in CNS neurons (Khalilov et al., 2003, Balena and Woodin, 2008), and is considered as an important determinant of EGABA in primary afferent neurons we sought to determine whether a decrease in NKCC1 activity could contribute to the activity-dependent hyperpolarizing shift in EGABA in cutaneous neurons from naïve rats. To test this possibility, we used the relatively selective NKCC1 blocker bumetanide (10 µM) (Balena and Woodin, 2008) to determine whether block of NKCC1 would occlude the activity-dependent hyperpolarization of EGABA. In a second group of neurons EGABA was first determined, bumetanide was applied and EGABA was monitored until it re-stabilized, and then the impact of the action potential protocol on EGABA was determined. Consistent with the suggestion that NKCC1 contributes to the determination of EGABA, bumetanide induced a hyperpolarizing shift of EGABA from −42.3 ± 3.3 mV to −47.5 ± 2.0 mV (n = 6). This change was manifest in 5 minutes and reached a plateau within 20 minutes. Interestingly, the presence of bumetanide had no influence on the activity-dependent shift in EGABA which was −11.4 ± 1.5 mV (Figure 3). Furthermore, despite the continued presence of bumetanide, EGABA returned to baseline levels within 20 minutes in all neurons tested. The activity-dependent shift in EGABA in the presence of bumetanide is not significantly different than the shift observed in untreated neurons (p > 0.05, students t-test). These results suggested that NKCC1 does not contribute to the activity-dependent shift in EGABA in cutaneous neurons from naïve rats.
Figure 3.
Activity-dependent hyperpolarization of EGABA was fully manifest in the presence of the NKCC1 antagonist bumetanide. (A) EGABA was assessed in a cutaneous neuron from a naïve rat before (black trace) and after (gray trace) stimulation with the action potential waveform 200 times at 2 Hz, 30 minutes after applying bumetanide (10 µM). (B) Pooled data indicate that the activity-dependent hyperpolarization of EGABA in neurons from naïve rats was comparable (p > 0.05) in the presence and absence of bumetanide. Pooled data from the HCO3− group were from the naïve group in Figure 2C and are plotted again to facilitate the comparison between groups.
A HCO3−-Cl− exchanger(s) contributes to the determination of EGABA in cutaneous DRG neurons
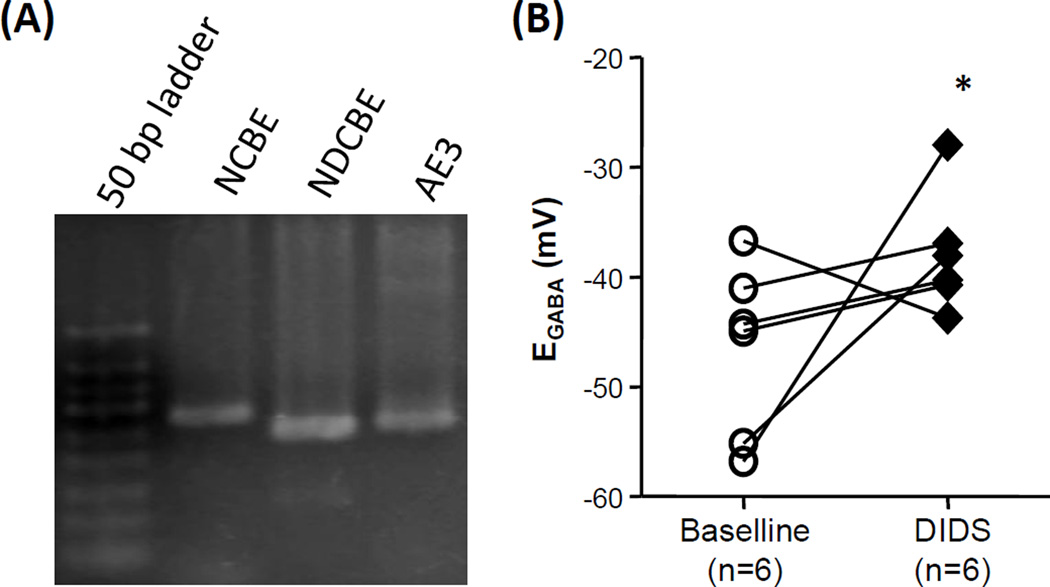
We recently reported that EGABA determined in HCO3− buffered bath solution is more hyperpolarized than that determined in HEPES buffered solution (Zhu et al., 2012b). To confirm this observation, we compared EGABA measured in HCO3−-buffered bath solution to that determined in HEPES buffered bath solution. Consistent with our previous results, EGABA was significantly (p < 0.01, students t-test) more hyperpolarized when determined in HCO3−-buffered bath solution (−43.0 ± 2.3 mV, n = 16) than that in HEPES-buffered bath solution (−26.4 ± 3.2, n = 7). This observation suggests that a HCO3−-dependent Cl− extrusion mechanism contributes to the determination of EGABA in cutaneous neurons. As there was no difference in the holding current of neurons recorded in HCO3−- and HEPES-buffered bath solutions, we predicted that an electro-neutral HCO3−-Cl− exchanger may be the underlying mechanism. There are three neuronal electro-neutral HCO3−-Cl− exchangers in the SLC4A family: Na+-dependent HCO3−-Cl− exchangers NDCBE, NCBE, and HCO3−-Cl− exchanger AE3. To determine which, if any of these family members are present in DRG and therefore potential candidates for the exchanger underlying the hyperpolarizing shift in EGABA in the presence of HCO3−, conventional PCR was used to screen for the presence of mRNA encoding these three family members in adult rat DRG. The results of this analysis indicated that mRNA encoding all three family members were detectable in DRG (Figure 4A). To further confirm the contribution of SLC4A family members to the hyperpolarizing shift in EGABA observed in HCO3− buffered bath solution, we assessed the impact of the non-selective Cl− exchanger blocker, DIDS on EGABA since all the HCO3− exchangers share a DIDS binding site and can all be inhibited by DIDS. Consistent with the contribution of a SLC4A family member to determination of EGABA in cutaneous DRG neurons, 5 minute exposure to DIDS (50 µM) resulted in a depolarizing shift in EGABA from −46.5 ± 3.2 mV to −38.0 ± 2.2 mV (Figure 4B). This shift is statistically significant (p<0.05, n=6, paired t-test).
Figure 4.
HCO3−-Cl− exchangers are present in DRG and contribute to the determination of EGABA in cutaneous neurons. (A) An ethidium bromide stained agarose gel loaded with PCR products generated with primers specific to each of the electro-neutral members of the SLC4A family of HCO3−-Cl− exchanger. mRNA was harvested from whole ganglia obtained from a naïve rats. Comparable results were obtained in ganglia from three different rats. (B) EGABA was determined in cutaneous neurons from naïve rats before and after the application of the HCO3−-Cl− exchanger blocker, DIDS (50 µM). The depolarization of EGABA in the presence of DIDS was significant (p < 0.05).
A HCO3− exchanger is not necessary for the activity-dependent shift in EGABA
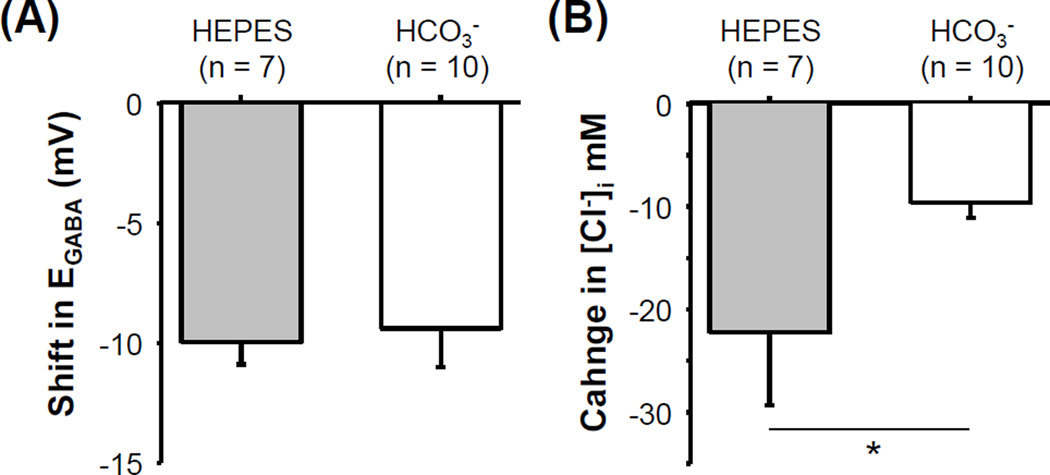
The presence of a HCO3−-Cl− exchanger in cutaneous DRG neurons raised the possibility that the activity-dependent shift in EGABA is due to an increase in exchanger activity. To test this possibility, we measured the EGABA in cutaneous neurons from naïve rats before and after stimulation in HEPES buffered solution. The results of this experiment indicated that the activity-dependent shift in EGABA in HEPES buffered bath solution was comparable to that observed in HCO3− buffered bath solution (Figure 5A). Interestingly, while the magnitude of the activity-dependent shift in EGABA was comparable in HCO3−- and HEPES-buffered bath solutions, the magnitude of the change in intracellular Cl− associated with the activity-dependent shift in EGABA should be different under these recording conditions. This is because of the significant difference in baseline EGABA under these recording conditions. To confirm this prediction, we used EGABA to estimate the concentration of intracellular Cl− ([Cl−]i) before and after stimulation, with the Goldman Hodgkin-Katz equation (with extracellular Cl ([Cl−]o) = 140 mM, [HCO3−]o=26 mM, [HCO3−]i=10 mM and the Cl−:HCO3− permeability ratio of the GABAA receptor as 4:1 (Kaila et al., 1997). The result of this analysis indicated that the change in intracellular Cl− needed to produce a 10 mV hyperpolarizing shift in EGABA in a HEPES buffered bath solution was significantly greater than that in HCO3− buffered bath solution (Figure 5B). While the mechanism underlying the activity-dependent shift in EGABA remains to be determined, the observation that the shift appears to be dependent on the driving force on Cl− suggests that the mechanism may be a channel enabling diffusion of Cl− down its electro-concentration gradient.
Figure 5.
No evidence for an increase in HCO3−-Cl− exchanger activity in the activity-dependent hyperpolarization of EGABA. (A) EGABA was determined before and after action potential stimulation of a cutaneous neurons from naïve rats in HCO3−- and HEPES-buffered bath solution as described in Figure 2C. Pooled data of the activity-dependent shift in EGABA are plotted. Data for the HCO3− group were from Figure 2C and are plotted again to facilitate the comparison between groups. (B) The decrease in the concentration of intracellular Cl− ([Cl−]i) associated with the activity-dependent hyperpolarization of EGABA was estimated based on the composition of the bath solution and measurements of EGABA before and after stimulation. * is p>0.05.
Discussion
We hypothesized that an increase in NKCC1 activity would contribute to an activity-dependent depolarizing shift in EGABA that would be larger in cutaneous neurons from inflamed rats than that in neurons from naïve rats. In contrast to our hypothesis, we observed an activity-dependent hyperpolarizing shift in EGABA in neurons from naïve rats. Interestingly, no activity-dependent shift in EGABA was detectable in cutaneous neurons from inflamed rats. Additional analysis of the mechanisms underlying the activity-dependent hyperpolarization of EGABA indicated that the shift was still fully manifest in the presence of bumetanide. Furthermore, despite evidence for the presence of a HCO3−-Cl− exchanger in cutaneous neurons that contributes to the steady-state determination of EGABA, the activity-dependent hyperpolarization of EGABA was still fully manifest in HEPES buffered bath solution.
Additional experiments will ultimately be needed to determine whether an activity-dependent hyperpolarization of EGABA is inhibitory or excitatory. In CNS neurons, the available evidence suggests a depolarizing shift in EGABA is associated with a decrease in inhibition (Prescott et al., 2006). However, in primary afferent neurons where GABA mediated membrane depolarization is the norm because of the persistent expression of NKCC1, GABAA receptor activation, and consequently membrane depolarization, is still inhibitory. Thus, the consequences of a hyperpolarizing shift in EGABA are not necessarily as straightforward as in the CNS. Nevertheless, we suggest that the available evidence indicates that even in primary afferent neurons, a hyperpolarizing shift in EGABA will facilitate GABA mediated inhibition. This suggestion is based on the observation that there is a hyperpolarizing shift in EGABA in NKCC1 knock out mice (Sung et al., 2000) and in wild type animals following application of the NKCC1 blocker, bumetanide (Pitcher et al., 2007). These hyperpolarizing shifts in EGABA are associated with an increase in nociceptive threshold (Sung et al., 2000) and the attenuation of injury-induced decreases in nociceptive threshold. Importantly, if the activity-dependent hyperpolarization of EGABA contributes to the normal inhibitory actions of GABA, the inflammation-induced decrease in the activity-dependent hyperpolarization of EGABA may contribute to the inflammation-induced pro-nociceptive shift in spinal GABAA signaling observed in the presence of inflammation (Anseloni and Gold, 2008).
Although our results suggested that NKCC1 is not responsible for the activity-dependent hyperpolarization of EGABA in DRG neurons from naïve animals, a conclusion that a decrease in NKCC1 activity does not contribute to the activity-dependent hyperpolarizing shift in EGABA should be made with caution. This is because there is evidence that activity is sufficient to down-regulate NKCC1, resulting in the hyperpolarization of EGABA (Balena and Woodin, 2008). Furthermore, despite evidence that the concentration of bumetanide used was sufficient to hyperpolarize EGABA, this concentration may have been insufficient to block enough NKCC1 activity to occlude an activity-dependent decrease in NKCC1 activity. Consistent with this suggestion, EGABA in NKCC1 knockout mice is −53. 6 mV while that in DRG of wildtype with 10 µM bumetanide block is −37.2 mV (Sung et al., 2000). Unfortunately, the concentration range over which bumetanide has selectivity for NKCC1 over the other Cl− co-transporters is limited, making the interpretation of results with higher concentrations of this compound difficult. Other strategies may ultimately be needed to rule out the contribution of NKCC1 to the activity-dependent shift in EGABA in cutaneous neurons.
Our results with HEPES buffered bath argue against a role for an increase in HCO3−-dependent Cl− transporter activity as a mechanism for the activity-dependent hyperpolarization of EGABA. Nevertheless, the presence of HCO3− exchangers in the primary afferent neurons and their contribution to the maintenance of steady-state EGABA has at least two important implications. First, since most of the EGABA data in primary afferents available in the literature were collected with HEPES buffered solutions (Alvarez-Leefmans et al., 1988, Sung et al., 2000, Gilbert et al., 2007, Rocha-Gonzalez et al., 2008), the estimates of EGABA in these studies may be artificially depolarized. Second, a more hyperpolarized EGABA under physiological conditions may contribute to the dramatic shifts in EGABA signaling observed in vivo (Anseloni and Gold, 2008). That is, if EGABA is already above action potential threshold in the absence of tissue injury as suggested by results obtained in HEPES buffered bath solution in this and previous studies, then a further depolarization of EGABA as should occur following inflammatory mediator-induced increases in NKCC1 activity (Funk et al., 2008), should have a relatively minor influence on nociceptive signaling. In contrast, if EGABA is hyperpolarized to action potential threshold as suggested by the results of the present study, an increase in NKCC1 activity sufficient to drive EGABA above the action potential threshold should have a considerably larger impact on nociceptive afferent input to the CNS.
While it is possible that a secondary active transport mechanism contributes to the activity-dependent hyperpolarization of EGABA, a more likely mechanism is an anion channel that enables the passive redistribution of Cl− in response to potential difference between EGABA and resting membrane potential. In this case, there will be Cl− efflux as long as the resting membrane potential is more negative than EGABA. As suggested, such a mechanism would account for the larger efflux observed in the HEPES buffered bath solution because of the greater potential difference between EGABA and resting membrane potential under these conditions. A Ca2+-dependent Cl− channel would be ideally suited to mediate the activity-dependent hyperpolarizing shift in EGABA, given that activity will result in an increase in intracellular Ca2+ concentration that can persist long after activity has been terminated (Lu et al., 2006). Several of these channels have been described in sensory neurons (Mayer, 1985) including TMEM16A (AKA ANO1). While it remains to be determined how TMEM16A and the other Ca2+-dependent Cl− channels are regulated in the presence of persistent inflammation, the decrease in high threshold voltage-gated Ca2+ current observed in cutaneous neurons 3 days after CFA injection (Lu et al., 2010), could account for the loss of the hyperpolarizing shift in EGABA observed in the presence of persistent inflammation.
In summary, while we have been forced to reject our hypothesis regarding the contribution of the dynamic regulation of EGABA to the inflammation-induced shift in GABAA signaling, we have described a novel feedback inhibitory mechanism that should serve to attenuate nociceptive input into the CNS in the absence injury. Furthermore, rather than a further depolarization of EGABA as a means to enable the emergence of excitatory actions of GABA, inflammation is associated with a decrease in this feedback inhibitory mechanism. Identification of this mechanism may provide a novel target for the treatment of inflammatory hypersensitivity.
Highlights.
-
-
There is a persistent inflammation-induced shift in spinal GABAA signaling
-
-
There is evidence for activity dependent depolarization of EGABA in CNS neurons
-
-
Our results indicate activity drives a hyperpolarization of EGABA in cutaneous DRG neurons.
-
-
This shift is due to neither an increase in NKCC1 or a decrease in HCO3--Cl− exchanger activity.
-
-
Persistent inflammation is associated with a loss of the activity-dependent hyperpolarization of EGABA
Acknowledgements
The authors wish to thank Drs. Ronald Dubner, Man-Kyo Chung and Steven Prescott for helpful feedback on the preparation of this manuscript.
Grants: This work was supported in part by a Grant from the National Institutes of Health, NS063010-01 (MSG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: YZ contributed to the experimental design, all data acquisition and analysis, and preparation of the manuscript. XLZ contributed to the collection of electrophysiological data. MG contributed to the experimental design, data analysis and preparation of the manuscript.
Conflict of Interest: None of the authors has any conflicts of interest with any of the data presented in this manuscript.
References
- Akaike N. Gramicidin perforated patch recording and intracellular chloride activity in excitable cells. Prog Biophys Mol Biol. 1996;65:251–264. doi: 10.1016/s0079-6107(96)00013-2. [DOI] [PubMed] [Google Scholar]
- Alvarez-Leefmans FJ, Gamino SM, Giraldez F, Nogueron I. Intracellular chloride regulation in amphibian dorsal root ganglion neurones studied with ion-selective microelectrodes. J Physiol. 1988;406:225–246. doi: 10.1113/jphysiol.1988.sp017378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anseloni VC, Gold MS. Inflammation-induced shift in the valence of spinal GABA-A receptor-mediated modulation of nociception in the adult rat. J Pain. 2008;9:732–738. doi: 10.1016/j.jpain.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balena T, Woodin MA. Coincident pre- and postsynaptic activity downregulates NKCC1 to hyperpolarize E(Cl) during development. Eur J Neurosci. 2008;27:2402–2412. doi: 10.1111/j.1460-9568.2008.06194.x. [DOI] [PubMed] [Google Scholar]
- Brumback AC, Staley KJ. Thermodynamic regulation of NKCC1-mediated Cl− cotransport underlies plasticity of GABA(A) signaling in neonatal neurons. J Neurosci. 2008;28:1301–1312. doi: 10.1523/JNEUROSCI.3378-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero-Erausquin M, Coull JA, Boudreau D, Rolland M, De Koninck Y. Differential maturation of GABA action and anion reversal potential in spinal lamina I neurons: impact of chloride extrusion capacity. J Neurosci. 2005;25:9613–9623. doi: 10.1523/JNEUROSCI.1488-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, McMullan S, Lawson SN, Djouhri L. Electrophysiological differences between nociceptive and non-nociceptive dorsal root ganglion neurones in the rat in vivo. J Physiol. 2005;565:927–943. doi: 10.1113/jphysiol.2005.086199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk K, Woitecki A, Franjic-Wurtz C, Gensch T, Mohrlen F, Frings S. Modulation of chloride homeostasis by inflammatory mediators in dorsal root ganglion neurons. Mol Pain. 2008;4:32. doi: 10.1186/1744-8069-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan A, Cervero F. Painful stimuli induce in vivo phosphorylation and membrane mobilization of mouse spinal cord NKCC1 co-transporter. Neuroscience. 2005;133:245–252. doi: 10.1016/j.neuroscience.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Gilbert D, Franjic-Wurtz C, Funk K, Gensch T, Frings S, Mohrlen F. Differential maturation of chloride homeostasis in primary afferent neurons of the somatosensory system. Int J Dev Neurosci. 2007;25:479–489. doi: 10.1016/j.ijdevneu.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Kaila K, Lamsa K, Smirnov S, Taira T, Voipio J. Long-lasting GABA-mediated depolarization evoked by high-frequency stimulation in pyramidal neurons of rat hippocampal slice is attributable to a network-driven, bicarbonate-dependent K+ transient. J Neurosci. 1997;17:7662–7672. doi: 10.1523/JNEUROSCI.17-20-07662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaka C, Ohno K, Okabe A, Kuriyama K, Itoh T, Fukuda A, Sato K. The differential expression patterns of messenger RNAs encoding K-Cl cotransporters (KCC1,2) and Na-K-2Cl cotransporter (NKCC1) in the rat nervous system. Neuroscience. 2001;104:933–946. doi: 10.1016/s0306-4522(01)00149-x. [DOI] [PubMed] [Google Scholar]
- Khalilov I, Holmes GL, Ben-Ari Y. In vitro formation of a secondary epileptogenic mirror focus by interhippocampal propagation of seizures. Nat Neurosci. 2003;6:1079–1085. doi: 10.1038/nn1125. [DOI] [PubMed] [Google Scholar]
- Lagraize SC, Guo W, Yang K, Wei F, Ren K, Dubner R. Spinal cord mechanisms mediating behavioral hyperalgesia induced by neurokinin-1 tachykinin receptor activation in the rostral ventromedial medulla. Neuroscience. 2010;171:1341–1356. doi: 10.1016/j.neuroscience.2010.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q, Wu J, Willis WD. Dorsal root reflexes and cutaneous neurogenic inflammation after intradermal injection of capsaicin in rats. J Neurophysiol. 1999;82:2602–2611. doi: 10.1152/jn.1999.82.5.2602. [DOI] [PubMed] [Google Scholar]
- Lu SG, Zhang X, Gold MS. Intracellular calcium regulation among subpopulations of rat dorsal root ganglion neurons. J Physiol. 2006;577:169–190. doi: 10.1113/jphysiol.2006.116418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SG, Zhang XL, Luo ZD, Gold MS. Persistent inflammation alters the density and distribution of voltage-activated calcium channels in subpopulations of rat cutaneous DRG neurons. Pain. 2010;151:633–643. doi: 10.1016/j.pain.2010.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. A calcium-activated chloride current generates the after-depolarization of rat sensory neurons in culture. Journal of Physiology. 1985;364:217–239. doi: 10.1113/jphysiol.1985.sp015740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J, Zeilhofer HU, Fritschy JM. Selective distribution of GABA(A) receptor subtypes in mouse spinal dorsal horn neurons and primary afferents. J Comp Neurol. 2012;520:3895–3911. doi: 10.1002/cne.23129. [DOI] [PubMed] [Google Scholar]
- Pitcher MH, Price TJ, Entrena JM, Cervero F. Spinal NKCC1 blockade inhibits TRPV1-dependent referred allodynia. Mol Pain. 2007;3:17. doi: 10.1186/1744-8069-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott SA, Ratte S, De Koninck Y, Sejnowski TJ. Nonlinear interaction between shunting and adaptation controls a switch between integration and coincidence detection in pyramidal neurons. J Neurosci. 2006;26:9084–9097. doi: 10.1523/JNEUROSCI.1388-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, Cervero F, Gold MS, Hammond DL, Prescott SA. Chloride regulation in the pain pathway. Brain Res Rev. 2009;60:149–170. doi: 10.1016/j.brainresrev.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees H, Sluka KA, Westlund KN, Willis WD. The role of glutamate and GABA receptors in the generation of dorsal root reflexes by acute arthritis in the anaesthetized rat. J Physiol. 1995;484(Pt 2):437–445. doi: 10.1113/jphysiol.1995.sp020676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha-Gonzalez HI, Mao S, Alvarez-Leefmans FJ. Na+,K+,2Cl− Cotransport and Intracellular Chloride Regulation in Rat Primary Sensory Neurons: Thermodynamic and Kinetic Aspects. J Neurophysiol. 2008;100:169–184. doi: 10.1152/jn.01007.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung KW, Kirby M, McDonald MP, Lovinger DM, Delpire E. Abnormal GABAA receptor-mediated currents in dorsal root ganglion neurons isolated from Na-K-2Cl cotransporter null mice. J Neurosci. 2000;20:7531–7538. doi: 10.1523/JNEUROSCI.20-20-07531.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White G. GABAA-receptor-activated current in dorsal root ganglion neurons freshly isolated from adult rats. J Neurophysiol. 1990;64:57–63. doi: 10.1152/jn.1990.64.1.57. [DOI] [PubMed] [Google Scholar]
- Yaksh TL. Behavioral and autonomic correlates of the tactile evoked allodynia produced by spinal glycine inhibition: effects of modulatory receptor systems and excitatory amino acid antagonists. Pain. 1989;37:111–123. doi: 10.1016/0304-3959(89)90160-7. [DOI] [PubMed] [Google Scholar]
- Yuan WX, Chen SR, Chen H, Pan HL. Stimulation of alpha(1)-adrenoceptors reduces glutamatergic synaptic input from primary afferents through GABA(A) receptors and T-type Ca(2+) channels. Neuroscience. 2009;158:1616–1624. doi: 10.1016/j.neuroscience.2008.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X-L, Mok L, Charbonnet M, Lee K-Y, Gold MS. Inflammation-induced changes in BKCa currents in cutaneous dorsal root ganglion neurons from the adult rat. Mol Pain. 2012;8 doi: 10.1186/1744-8069-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Dua S, Gold MS. Inflammation-induced shift in spinal GABAA signaling is associated with a tyrosine-kinase dependent increase in GABAA current density in nociceptive afferents. J Neurophysiol. 2012a;108:2581–2593. doi: 10.1152/jn.00590.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Lu SG, Gold MS. Persistent inflammation increases GABA-induced depolarization of rat cutaneous dorsal root ganglion neurons in vitro. Neuroscience. 2012b;220:330–340. doi: 10.1016/j.neuroscience.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]