Abstract
In Drosophila, the monoamine octopamine, through mechanisms that are not completely understood, regulates both aggression and mating behavior. Interestingly, our study demonstrates that the Drosophila obesity-linked homologs Transcription factor AP-2 (TfAP-2; TFAP2B in humans) and Tiwaz (Twz; KCTD15 in humans) interact to modify male behavior by controlling the expression of Tyramine β-hydroxylase and Vesicular monanime transporter, genes necessary for octopamine production and secretion. Furthermore, we reveal that octopamine in turn regulates aggression through the Drosophila cholecystokinin satiation hormone homolog Drosulfakinin (Dsk). Finally, we establish that TfAP-2 is expressed in octopaminergic neurons known to control aggressive behavior and that TfAP-2 requires functional Twz for its activity. We conclude that genetically manipulating the obesity-linked homologs TfAP-2 and Twz is sufficient to affect octopamine signaling, which in turn modulates Drosophila male behavior through the regulation of the satiation hormone Dsk.
Keywords: obesity-linked genes, aggression, cholecystokinin, octopamine, noradrenaline
AGGRESSION is an important behavioral trait enabling animals to fight for food, shelter, and mates or over territories where these resources can be found. The behavioral decision to be aggressive is in part controlled by systems that also regulate metabolism, such as the monoamine system (Dierick and Greenspan 2007; Hoyer et al. 2008; Zhou et al. 2008; Alekseyenko et al. 2010). In the fruit fly Drosophila melanogaster, it was determined that a major regulator of male aggressive and mating behavior is the noradrenaline analog, octopamine (Baier et al. 2002; Hoyer et al. 2008; Zhou et al. 2008, 2012; Certel et al. 2010; Erion et al. 2012). In addition, the monoamines dopamine and serotonin have been linked to the regulation of aggressive behavior (Lucki 1998; Baier et al. 2002; Alekseyenko et al. 2010, 2013; Belsare et al. 2010). Yet the molecular mechanisms underlying aggression are still not fully understood.
The human genes Transcription factor AP-2 (TFAP2B) (encoding AP-2β) and KCTD15 have been identified as novel loci associated with obesity (Bauer et al. 2009; Renstrom et al. 2009; Willer et al. 2009; Zhao et al. 2011), although it is still not known how they regulate obesity at the molecular level. TFAP2B is a member of the AP-2 family of transcription factors, key regulators of various developmental processes (Eckert et al. 2005; Meng et al. 2010; Wenke and Bosserhoff 2010), and in mice it was demonstrated that TFAP2B is necessary for the proper development of peripheral and central nervous system noradrenergic neurons (Hong et al. 2008; Schmidt et al. 2011). KCTD15 belongs to a family of potassium-channel tetramerization domain-containing proteins. In zebrafish embryos, Kctd15 functions to inhibit the activity of AP-2α to restrict neural crest formation, although the exact mechanism of this inhibition is unknown (Dutta and Dawid 2010; Zarelli and Dawid 2013).
In Drosophila, TFAP2B is highly conserved, encoded by the gene TfAP-2. There is preliminary evidence from a yeast two-hybrid screen that TfAP-2 associates with the Drosophila KCTD15 homolog CG10440, which we have named Tiwaz (Twz; see Materials and Methods) (Giot et al. 2003). Furthermore, both genes are highly expressed in the central nervous system (Chintapalli et al. 2007). In mice, TFAP2B regulates the noradrenergic system, and we asked if TfAP-2 and Twz could be involved in regulating behavior through octopamine signaling, a central controller of aggression in Drosophila (Hoyer et al. 2008; Zhou et al. 2008). Octopaminergic neurons are known to innervate the insulin-producing cells located in the Par intercerebralis of the Drosophila brain (Crocker et al. 2010). Intriguingly, it was recently discovered that these insulin-producing cells also produce the Drosophila homolog of cholecystokinin (CCK), known as Drosulfakinin (Dsk) (Söderberg et al. 2012), and in rodents levels of the satiation hormone CCK are correlated with aggression (Zwanzger et al. 2012). Furthermore, it has been reported that in Drosophila both octopamine and Dsk are involved in regulating muscle contractions necessary for the control of locomotory behavior (Koon et al. 2011; Chen and Ganetzky 2012; Chen et al. 2012), although it is not known if they interact. From these previous studies, we hypothesized that octopamine signaling could be modulating aggressiveness by regulating the expression of Dsk.
In the current study, we show that TfAP-2 and Twz genetically interact in Tdc2 octopaminergic neurons to modulate male behavior. Furthermore, we demonstrate that TfAP-2 and Twz are required for the proper expression of two genes necessary for the production and secretion of octopamine. Finally, we have evidence that octopamine regulates aggression by controlling the expression of the Drosophila CCK homolog Dsk.
Materials and Methods
Fly stocks and maintenance
w*, P{w[+mW.hs]=GawB}elav[C155], w*; P{w[+mC]=Tdc2-GAL4.C}2, y1 w[*]; P{w[+mC]=UAS-AP-2.PB}a4-2 and w*, P{w[+mC]=UAS-GFP.S65T} were received from the Bloomington Stock Center. TfAP-2 (y1w3; P{KK109052}VIE-260B) and CG10440 (y1w3; P{KK107922}VIE-260B) RNA interference (RNAi) flies were obtained from the Vienna Drosophila RNAi Centre (Table 1). w*; Dilp2-GAL4 was a gift from Eric Rulifson (Wang et al. 2007), and w*, UAS-Dsk flies were a gift from Barry Ganetzky (Chen et al. 2012). All flies were maintained on enriched Jazz mix standard fly food (Fisher Scientific) and maintained at 25°, 60% humidity, on a 12:12 light:dark cycle. To inhibit the GAL4 driver, flies crossed to a GAL4 driver were kept at 18° and, once the progeny had eclosed, were shifted to 29° for at least 5–7 days before any assays were performed. Due to its involvement in male aggression, we have decided to name the Drosophila KCTD15 homolog CG10440 after the Nordic god of single-combat, Tiwaz (Twz).
Table 1. Information on the RNAi constructs from the Vienna Drosophila RNAi Center.
| Transformant ID | Construct ID | Library | Gene no. | Gene | On targets | Off targets | s19 | CAN repeats |
|---|---|---|---|---|---|---|---|---|
| 101552 | 109052 | KK | CG7807 | TfAP-2 | 1 | 0 | 1 | 2 |
| 110265 | 107922 | KK | CG10440 | Tiwaz | 1 | 3 | 0.99 | 3 |
KK = the phiC31 RNAi library, CAN repeats = defined as greater than or equal to 6 repeats of the sequence CAN (where N = any nucleotide), which can lead to off-target effects.
Aggression assay
Cylindrical behavioral chamber dimensions were 2 × 2.5 cm (height × diameter) and filled with 1% agarose to 1.5 cm in height to maintain proper humidity. Newly emerged male flies were collected and isolated for 5–7 days at 29°, 60% humidity, on a 12:12 light:dark cycle. Behavioral tests were carried out at room temperature with 60% humidity. Two male flies were anesthetized using an ice-water bath before being transferred to a behavioral chamber. After a recovery period of at least 3 min, a camera (Panasonic HDC-SD90), positioned above the chamber, was used to record activity for a minimum of 30 min. After the 3-min recovery period, the behavioral interactions between the males were scored for 20 min. Distinct stereotypic aggressive interactions were scored as described by Nilsen et al. (2004). Aggressive interactions were further scored as either low- or high-intensity engagements. Low-intensity fighting (LIF) was scored as side-by-side pushing with a leg (“shoving”) or quick wing flicking (“wing flick”); high-intensity fighting (HIF) was graded as lunging (“lunging”) or boxing face-to-face with the two front legs (“boxing”), holding the wings at a 30° angle (“wing threat”), as well as chasing one another (“chasing”). Courtship behavior was marked as one wing extended at a 90° angle (“singing”), circling to the posterior (“circling”), tapping the abdomen (“tapping”), licking the genitalia (“licking”), or bending the abdomen toward the other fly (“abdomen bending”). At least 10 replicates were conducted for each genotype.
Mating behavior assay
Newly eclosed males were collected and aged in isolation for 5–7 days at 29°, 60% humidity, on a 12:12 light:dark cycle. Individual males and 3- to 4-day-old virgin wild-type CSORC females were then transferred to a behavioral chamber, using ice-water anesthetization. After a recovery period of at least 3 min, a camera (Panasonic HDC-SD90), positioned above the chamber, was used to record activity for a minimum of 30 min. After the 3-min recovery period, the behavioral interactions of the males were scored for 20 min or until copulation occurred. CSORC is a lab wild-type strain created by crossing Canton-S and Oregon-R wild-type strains. Scoring of the courtship behaviors was performed as described by Becnel et al. (2011). Latency, courtship index, and the frequency of mating behaviors were measured. Latency was calculated by counting the time that it took a male to initiate mating, and courtship index was calculated as the percentage of time that a male spends actively courting a female over a 20-min period (seconds spent actively courting/(1200 sec − latency seconds). At least 10 replicates per genotype were conducted.
Activity assay
Cylindrical behavioral chamber dimensions were 2 × 2.5 cm (height × diameter) and filled with 1% agarose to 1.5 cm in height to maintain proper humidity. Newly emerged male flies were collected and isolated for 5–7 days at 29°, 50% humidity, on a 12:12 light:dark cycle. Behavioral tests were carried out at room temperature with 60% humidity. The male fly to be analyzed was anesthetized using an ice-water bath before being transferred to a behavioral chamber. After a recovery period of at least 3 min, a camera (Panasonic HDC-SD90), positioned above the chamber, was used to record activity for a minimum of 30 min. Activity was determined at the percentage of time that the male spent actively walking over the 30-min period; preening activity was ignored for this assay. Ctrax and Matlab were used to determine speed.
Antagonist assays
Newly eclosed TfAP-2 overexpressing males were collected and isolated on normal food for 3 days. They were then fed by the capillary feeding assay method for 2–3 days with 1, 3, and 5 mM of the octopamine antagonists phentolamine (Dudai 1982), Sigma-Aldrich) or epinastine (Stevenson et al. 2005; Unoki et al. 2005) or with 1 mM CCK antagonist (SR27897, Sigma-Aldrich) (Harper et al. 1999). Each fly was transferred to a transparent plastic cylindrical vial (9 × 2 cm) (height × diameter), containing 1% agarose (5 cm high) to provide moisture and humidity for the flies, and the opening of the vial was covered with paraffin film. A calibrated capillary glass tube (5 μl, VWR International) was filled with liquid food (5% sucrose and 5% yeast extract) containing the appropriate antagonist, and a mineral oil layer was used to prevent evaporation from the capillary tube. Feeding tubes were inserted through the paraffin film into the chambers. After 2–3 days of feeding, two male flies were transferred into a behavioral chamber, and activity was videotaped and scored as before.
Immunohistochemistry
Male flies were anesthetized and decapitated, and the proboscis was removed. Heads were placed into a staining glass bowl containing 4% paraformaldehyde in 0.1 M sodium phosphate buffer (PBS) and left to fixate in the dark for 2 hr on ice. After fixation, heads were placed in a petri dish containing 4% agarose, and brains were dissected under a light microscope using fine forceps. Brains were washed four times for 15 min each with 0.1 M PBS. Tissues were blocked in 10% normal goat serum (NGS) for 1 hr. The NGS was then discarded, and tissues were incubated with primary antibody (Abcam rabbit polyclonal AP-2γ), diluted 1:5000 in 0.01 M PBS containing 0.25% Triton X-100 (PBX) for 2 days at 4°. Bowls were sealed with parafilm and aluminum foil. Following 48 hr of incubation, brains were washed four times for 15 min each with 0.01 M PBX and incubated with secondary antibody (Alexa Fluor 594 goat anti-rabbit), diluted 1:1000 in 0.01 M PBX, overnight. Bowls were sealed with parafilm and aluminum foil. Tissues were washed once with 0.01 M PBX for 15 min and twice with 0.01 M PBS for 15 min. Samples were mounted with 60% glycerol containing 1.6% propyl gallate.
RNA extraction
The phenol–chloroform method was used for RNA extraction from tissue samples (Chomczynski and Sacchi 1987). Fifty fly heads were homogenized with 800 µl TRIzol (Invitrogen), 200 µl chloroform (Sigma-Aldrich) was added, and samples were centrifuged at 12,000 × g for 15 min at 4°. The aqueous layer, which contained RNA, was separated and 500 µl isopropanol (Solvaco AB) was added.The RNA was precipitated by storing the samples at −32° for 2 hr. Samples were centrifuged at 12,000 × g for 10 min at 4° to collect the RNA pellets, which were then washed with 75% ethanol (Solvaco AB) to remove the organic impurities. Samples were allowed to air dry to remove any traces of ethanol. Dried RNA pellets were dissolved in 21.4 µl of RNAse free water (Qiagen) and 2.6 µl of DNAse incubation buffer (Roche). The samples were incubated at 75° for 15 min to ensure complete dissolution of RNA pellets. Two microliters of DNAse I (10 U/µl, Roche) was added to each sample and incubated at 37° for 3 hr to remove DNA contamination. DNAse was deactivated by incubating the samples at 75° for 15 min. Removal of DNA was confirmed by PCR using Taq polymerase (5 U/µl, Biotools B & M Labs), followed by agarose gel electrophoresis. The RNA concentration was measured using a nanodrop ND 1000 spectrophotometer (Saveen Werner).
Complementary DNA synthesis
Complementary DNA (cDNA) was synthesized from RNA template by using dNTP 20 mM (Fermentas Life Science), random hexamer primers, and M-MLV Reverse Transcriptase (200 U/µl, Invitrogen) and by following manufacturer instructions. cDNA synthesis was confirmed by PCR followed by agarose gel electrophoresis.
Quantitative RT-PCR
Relative expression levels of three housekeeping genes (EF-1, Rp49, and RpL11) and of the genes of interest were determined with quantitative RT-PCR (qPCR). Each reaction, with a total volume of 20 µl, contained 20 mM Tris–HCl, pH 9.0, 50 mM KCl, 4 mM MgCl2, 0.2 mM dNTP, DMSO (1:20), and SYBR Green (1:50,000). Template concentration was 5 ng/µl, and the concentration of each primer was 2 pmol/µl. Primers were designed with Beacon Designer (Premier Biosoft) using the SYBR Green settings. All qPCR experiments were performed in duplicate; for each primer pair, a negative control with water and a positive control with 5 ng/µl of genomic DNA were included on each plate. Amplifications were performed with 0.02 µg/ml Taq DNA polymerase (Biotools) under the following conditions: initial denaturation at 95° for 3 min, 50 cycles of denaturing at 95° for 15 sec, annealing at 52.8°–60.1° for 15 sec and extension at 72° for 30 sec. Analysis of qPCR data was performed using MyIQ 1.0 software (Bio-Rad) as previously reported (Lindblom et al. 2006). Primer efficiencies were calculated using LinRegPCR (Ramakers et al. 2003), and samples were corrected for differences in primer efficiencies. The GeNorm protocol described by Vandesompele et al. (2002) was used to calculate normalization factors from the expression levels of the housekeeping genes. Grubbs’ test was performed to remove outliers. Differences in gene expression between groups were analyzed with ANOVA followed by Fisher’s protected least significant difference (PLSD) test where appropriate. P < 0.05 was used as the criterion of statistical significance. The following primers were used: EF-1 (forward—5′-GCGTGGGTTTGTGATCAGTT-3′; reverse—5′-GATCTTCTCCTTGCCCATCC-3′); Rp49 (forward—5′-CACACCAAATCTTACAAAATGTGTGA-3′; reverse—5′-AATCCGGCCTTGCACATG-3′); RpL11 (forward—5′-CCATCGGTATCTATGGTCTGGA-3′; reverse—5′-CATCGTATTTCTGCTGGAACCA-3′); TfAP-2 (forward—5′-CTAAGAGCAAGAACGGAG-3′; reverse—5′-AACCAAGGATGTCAGTAG-3′); Tiwaz (forward—5′-GCCACATTCTGAACTTTATG-3′; reverse—5′-GCCACTACCTCGTAATTG-3′); Mur89F (forward—5′-GGAGTCCAATTCGGGATCTA-3′; reverse—5′-GAACTTTGATTGCTGCCAGA-3′); Psi (forward—5′-AACTACGGCTATGGGTACGG-3′; reverse—5′-TGGTTGATCAGCTTGATGGT-3′); sens-2 (forward—5′-TGGAGAAAGTGTTCGAGTGC-3′; reverse—5′-CGCAGTAGTTGCAGGGATAA-3′); Tdc2 (forward—5′-GGCACTCCCAAGCTCTCAAT-3′; reverse—5′-ATGGTCGTACGTTGGTGTCC-3′); Tyramine β hydroxyase (Tbh) (forward—5′-TTATGCCAGTGATGCTGCTC-3′; reverse—5′-TGAAAGCATTCTGCAAGTGG-3′); and Vesicular momoamine transporter (Vmat) (forward—5′-CGTGACCTTCGGGACGATAG-3′; reverse—5′-ACTAGAGCGGGAAAACCAGC-3′).
Statistical analysis
Mean and standard error from all replicates of each experiment were calculated. All analysis was performed with GraphPad Prism 4, using ANOVA with appropriate post hoc analysis for multiple comparisons.
Results
TfAP-2 is expressed in octopaminergic neurons
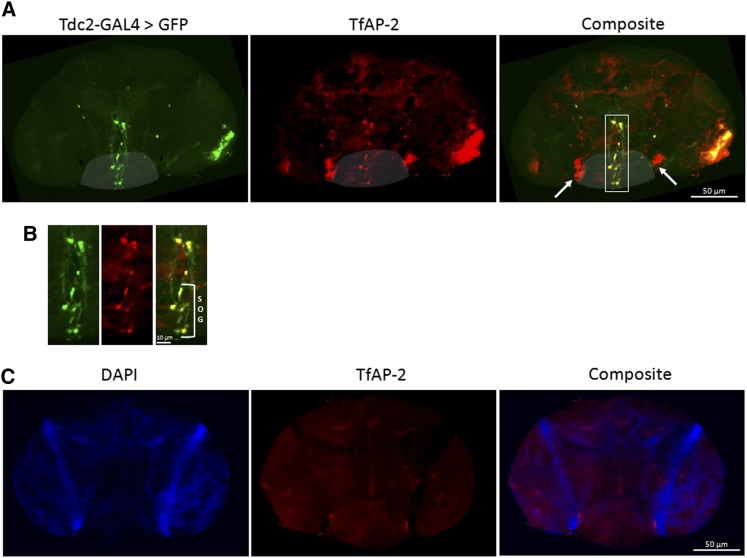
In mice the transcription factor TFAP2B is known to regulate noradrenaline signaling (Hong et al. 2008; Schmidt et al. 2011), and in Drosophila TFAP2B is encoded by the homolog TfAP-2 (Monge et al. 2001). Since octopamine, the Drosophila analog of noradrenaline, is known to control aggression and mating behavior in males, we first wanted to determine if TfAP-2 was expressed in octopaminergic neurons. TfAP-2 expression in octopaminergic neurons was verified by staining Drosophila adult male brains with human anti-AP-2γ (see Materials and Methods). To do this, Tdc2-GAL4 flies were crossed with UAS-GFP flies. Tyrosine decarboxylase 2 (Tdc2) is specifically expressed in octopaminergic neurons, where it is necessary to produce the monoamine tyramine from tyrosine (Cole et al. 2005). TfAP-2 was expressed in Tdc2-GFP-positive neurons found in the subesophageal ganglion (SOG) (Figure 1A, shaded region, and Figure 1B bracket marked “SOG”), an area of the brain known to regulate aggressive behavior (Zhou et al. 2008). TfAP-2 protein was also observed in Tdc2-GFP-negative neurons on either side of the brain near the SOG (Figure 1A, arrows). Staining was severely reduced in brains from flies expressing TfAP-2 RNAi in all neurons using the pan-neuronal Elav-GAL4 driver (Figure 1C) (Lin and Goodman 1994).
Figure 1.
TfAP-2 is expressed in octopaminergic neurons. (A) Immunofluorescence of whole Drosophila male brain to visualize TfAP-2 expression (red) in Tdc2 neurons (GFP, green). Shaded area indicates approximate region of the subesophageal ganglia. Box represents section of brain shown in B. Arrows indicate two neurons that are TfAP-2 positive but GFP negative. (B) Extensive TfAP-2 (red) expression in Tdc2 neurons (GFP, green) overlap appears as yellow in composite picture (SOG). (C) TfAP-2 RNAi was expressed in the entire CNS using the Elav-GAL4 driver, DAPI staining (blue), and TfAP-2 expression (red). The picture was overexposed to reveal any possible TfAP-2 staining, and some residual staining was observed in the two neurons that were TfAP-2 positive but GFP negative in A.
AP-2 and Twz regulate male behavior
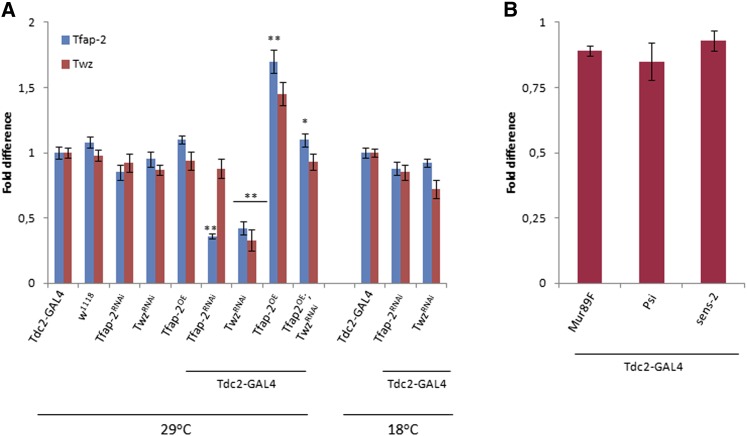
Before beginning the behavioral assays, to clarify if UAS-Tfap2RNAi (referred to as Tfap2RNAi), UAS-TwzRNAi (referred to as TwzRNAi), and UAS-Tfap2OE [yw; UAS-AP-2PB, referred to as TfAP-2OE (Monge et al. 2001)] were functioning properly, we first crossed these flies to the Tdc2-GAL4 driver and performed qPCR to measure the level of TfAP-2 and Twz transcript (Figure 2). These flies were raised at 18° until they eclosed, at which point the newly eclosed flies were collected and kept at 29° for 5–7 days. The flies were put at 29° to get maximal expression from the GAL4/UAS system (Brand and Perrimon 1993). Since raising the flies at 18° inhibits, but does not completely block, GAL4 activity, to make sure that the effects that we observed were not due to a developmental phenotype, we also collected and kept newly eclosed flies at 18° for 5–7 days before preparing them for qPCR analysis. Tdc2-GAL4, UAS-Tfap2RNAi, UAS-TwzRNAi, and UAS-TfAP-2OE were all crossed to the white (w) allele w1118, and the heterozygous progeny were used as controls. The w1118 allele was used because we always set up our experimental crosses such that the F1 males will be in a w mutant background. The level of TfAP-2 and Twz expression in Tdc2-GAL4 heterozygous controls were set as 100%, represented as 1 on the graph (Figure 2A). Compared with Tdc2-GAL4 heterozygous controls (SE ± 0.05), TfAP-2RNAi males kept at 29° had only 0.36-fold (SE ± 0.02, P < 0.005) of the normal TfAP-2 RNA expression levels, while males maintained at 18° had 0.88-fold (SE ± 0.05, P = 0.182) of normal TfAP-2 expression (Tdc2-GAL4 at 18°, SE ± 0.04). TfAP-2RNAi males raised at 29° had 0.88-fold (SE ± 0.07, P < 0.322) of the normal Twz RNA expression levels. On the other hand, expressing TwzRNAi with the Tdc2-GAL4 driver affected both TfAP-2 and Twz expression. TwzRNAi males maintained at 29° had only 0.33-fold (SE ± 0.08, P < 0.005) of the normal Twz RNA expression levels, while males at 18° had 0.85-fold (SE ± 0.06, P = 0.132) of normal expression (Tdc2-GAL4 at 18°, SE ± 0.03). Furthermore, TwzRNAi males maintained at 29° had only 0.42-fold (SE ± 0.05, P < 0.005) of normal TfAP-2 expression (Figure 2A). Due to this result, for the rest of the article when we refer to “Twz knockdown males” we actually mean “Twz and Tfap-2 double knockdowns,” whereas when we refer to “Tfap-2 knockdowns,” we mean “flies where only Tfap-2 transcript levels were lowered.” Overexpressing TfAP-2 in Tdc2 neurons induced a strong increase in TfAP-2 transcript levels (1.7-fold, SE ± 0.09, P < 0.005); this expression was decreased when AP-2OE was expressed in a TwzRNAi background (AP-2OE;TwzRNAi, 1.1-fold, SE ± 0.05, P = 0.433). Similar to TfAP-2, there was an increase in Twz transcript levels in AP-2OE males (1.45-fold, SE ± 0.09, P < 0.05) (Figure 2A).
Figure 2.
Relative transcript levels of TfAP-2 and Twz. (A) Relative level of TfAP-2 and Twz expression in octopaminergic neurons in males kept at either 29° or 18° to verify the efficiency of the various UAS constructs. (B) Relative expression of possible UAS-TwzRNAi line off-target genes Mur89F, Psi, and sens-2 (see Materials and Methods). (n = 10 qPCR runs; *P < 0.05 **P < 0.005 compared with controls, two-way ANOVA with Bonferroni post hoc test for multiple comparisons).
The RNAi line used to knock down Twz possibly affects the expression of three off-target genes, Mucin related 89F (Mur89F), P-element somatic inhibitor (Psi), and senseless-2 (sens-2) (Table 1). To make sure that the phenotypes we observe are actually due to the knocking down of Twz, we also performed qPCR to study Mur89F, Psi, and sens-2 expression in TwzRNAi males kept for 5–7 days at 29° (Figure 2B). Expressing TwzRNAi in octopaminergic neurons had no significant affect on the expression levels of Mur89F, Psi, and sens-2 (Figure 2B).
Since octopamine is known to control aggression in Drosophila males (Baier et al. 2002; Hoyer et al. 2008; Zhou et al. 2008, 2012; Certel et al. 2010; Erion et al. 2012), to establish if TfAP-2 and Twz were required for octopamine-regulated male behavior, we performed an aggression assay. To do this, Tdc2-GAL4, UAS-Tfap2RNAi, UAS-TwzRNAi, and UAS-Tfap2OE were all crossed to w1118, and the heterozygous progeny were used as controls. Since there is debate on how w influences behavior, we also used w1118 hemizygous males as controls (Zhang and Odenwald 1995; Anaka et al. 2008).
Aggression analysis experiments were executed by placing pairs of 5- to 7-day-old males, raised in isolation, in a behavioral assay chamber containing 1% agarose, and their interactions were monitored over a 20-min period. The total number of interactions for each fly was recorded, whether it involved aggressive or courtship behavior. The assayed male–male interactions consisted of eight distinct behaviors. Aggressive interactions were scored as either low- or high-intensity engagements. LIF was scored as side-by-side pushing with a leg (shoving), or quick wing flicking (wing flick); HIF was graded as lunging (lunging), boxing face-to-face with the two front legs (boxing), as well as holding the wings up at a 30–45° angle (wing threat). Courtship behavior was marked as one-wing extended at a 90° angle (singing), circling to the posterior (circling), or bending the abdomen toward the other fly (abdomen bending).
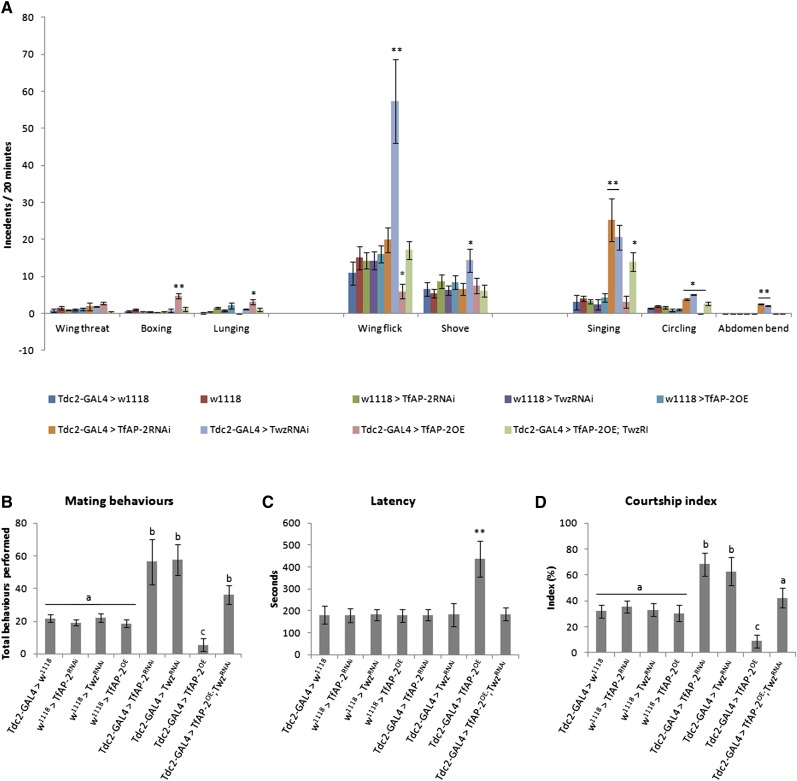
When HIF behaviors are considered, there was no significant difference between controls, TfAP-2, and Twz knockdown males. However, there was a significant difference between controls and TfAP-2OE males. Tdc2-GAL4+/− control males performed 0.6 (SE ± 0.3) boxing behaviors per bout and TfAP-2OE+/− control males performed 0.3 (SE ± 0.08), while TfAP-2-overexpressing males performed 4.8 (SE ± 0.7, P < 0.005) boxing behaviors per bout. Also, TfAP-2-overexpressing males performed significantly more lunges (3.2, SE ± 0.07, P < 0.05) than either Tdc2-GAL4+/− (0.2, SE ± 0.02) or TfAP-2OE+/− (2.1, SE ± 0.08) control males (Figure 3A). TfAP-2OE-induced HIF behaviors could be rescued by co-expressing TwzRNAi (Tdc2-GAL4;TfAP-2OE/TwzRNAi—boxing: 1.2, SE ± 0.5; lunges: 1.0, SE ± 0.5) (Figure 3A). When LIF behaviors were compared, TwzRNAi performed significantly more wing flicks (57.3, SE ± 11.4, P < 0.005) and shoves (14.4, SE ± 3.1, P < 0.05) over a 20-min fighting bout than either Tdc-GAL4+/− (wing flicks: 10.9, SE ± 3.2; shoves: 6.6, SE ± 1.8) or TwzRNAi+/− (wing flicks: 14.3, SE ± 2.5; shoves: 6.3, SE ± 1.3) control males. On the other hand, TfAP-2OE males performed significantly fewer wing flicks than controls (6.0, SE ± 2.0, P < 0.05). Finally, knocking down either TfAP-2 or Twz had a significant effect on all scored mating behaviors. Compared to Tdc2-GAL4+/− (3.1, SE ± 1.9), TfAP-2RNAi+/− (3.3, SE ± 0.6), and TwzRNAi+/− (2.4, SE ± 1.4) controls, TfAP-2RNAi (25.2, SE ± 5.8, P < 0.005) and TwzRNAi (20.6, SE ± 3.4, P < 0.005) knockdown males performed significantly more singing behaviors (Figure 3A). TfAP-2RNAi and TwzRNAi knockdown males also performed more circling maneuvers than controls, and unlike controls they performed abdomen bends toward other males (Figure 3A).
Figure 3.
Disrupting TfAP-2 or Twz expression in Tdc2-specific octopaminergic neurons affects male behavior. TfAP-2 and Twz RNAi were expressed specifically in octopaminergic neurons using the Tdc2-GAL4 driver. (A) Total interactions that were either high- or low-intensity aggression or courtship behavior for all genotypes were determined. All males were between 5 and 7 days old. The types of behaviors were distributed into three catagories—HIF, LIF, and courtship behavior—and the number of each type of behavior performed is represented. In all instances, the assay was repeated at least 10 times (n = 20 males/treatment; *P < 0.05, **P < 0.005 compared with controls, two-way ANOVA with Bonferroni post hoc test for multiple comparisons). (B–D) Mating behavior of 5- to 7-day-old males toward 3- to 4-day-old Csorc wild-type virgin female was recorded over a 10-min period or until copulation occurred. In all instances the assay was repeated at least 10 times. (B) Male courtship behavior toward a virgin female. (C) Total number of behaviors a male performed. (D) Amount of time, in seconds, before the first courtship behavior, or latency, was determined. Percentage of time a male spent actively courting (courtship index) was determined. In B and D, different letters indicate similar groups (i.e., “a” is significantly different than “b” or “c” and so on). n = 20 males per aggression or mating assay; *P<0.05 **P < 0.005 compared with controls, one-way ANOVA with Bonferroni post hoc test for multiple comparisons.
Next we wanted to determine if TfAP-2 and Twz also regulate male behavior toward virgin females. To do this, males were paired with wild-type CSORC virgin females, and three aspects of male–female courtship were measured: number of behaviors, latency, and courtship index (see Materials and Methods). When TfAP-2 (114.1 behaviors, SE ± 31.1, P < 0.05) or Twz (113.8 behaviors, SE ± 21.1, P < 0.05) were knocked down in octopaminergic neurons, males performed significantly more courtship behaviors than Tdc2-GAL4+/− (48.6 behaviors, SE ± 5.4), Tfap2RNAi+/− (43.0 behaviors, SE ± 4.0), TwzRNAi+/− (47.3 SE ± 5.8), or w1118 (46.5 behaviors, SE ± 4.0) controls (Figure 3B), and there was a substantial increase in the courtship index (CI) (Figure 3D). Tdc2-GAL4+/− males had a CI of 56.6% (SE ± 5.2), Tfap2RNAi+/−controls’ CI was 55.3 (SE ± 4.0), TwzRNAi+/− controls had a similar CI (48.6% SE ± 5.2), and w1118 males’ CI was 55.2% (SE ± 5.3), while TfAP-2RNAi males CI was significantly higher at 80.7% (SE ± 3.9, P < 0.005) and TwzRNAi males CI was 79.5% (SE ± 5.9, P < 0.005). TfAP-2OE males had a significant decrease in the number of courtship behaviors that they performed (10.9 behaviors, SE ± 7.2, P < 0.005) (Figure 3B), as well as a substantial increase in latency (422.3 sec, SE ± 78.2, P < 0.005) compared to Tdc2-GAL4+/− (125.3 sec, SE ± 20.3), TfAP-2OE+/− (112.3 sec, SE ± 18.7), or w1118 (157.2 sec, SE ± 33.5) controls (Figure 3C). TfAP-2OE males had little interest in mating (CI = 12.3%, SE ± 6.2, P < 0.005) (Figure 3D).
TfAP-2 regulates activity levels via octopamine signaling
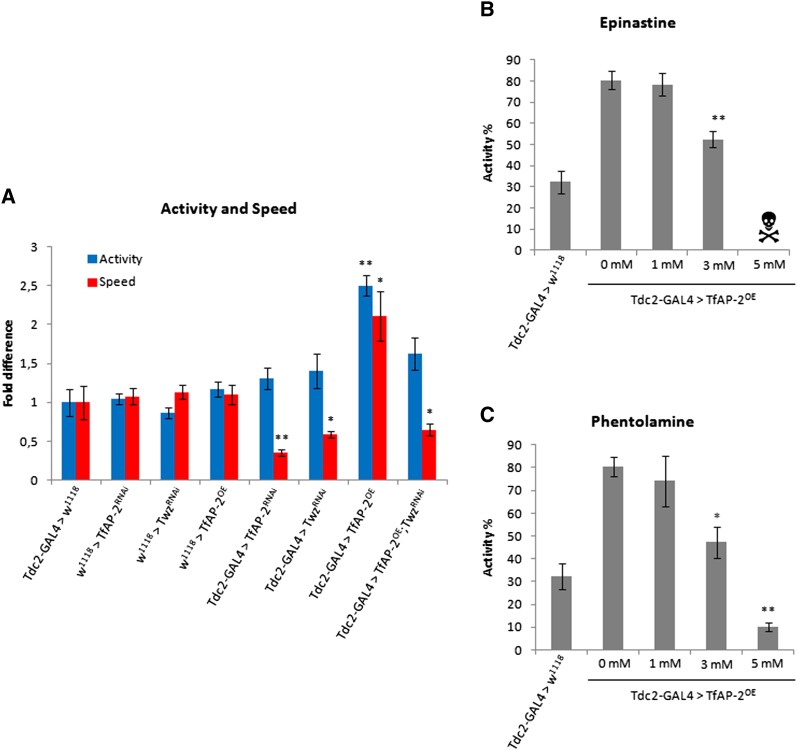
One possibility for a change in behavior could be an overall change in activity. To ascertain the general movement of flies where TfAP-2 or Twz expression is disrupted, the activity and speed of the various genotypes were measured. Tdc2-Gal4+/− control males were used as a reference, represented as 1 (activity: SE ± 0.17; speed: SE ± 0.21) on the graph (Figure 4A). No change in overall activity was observed when TfAP-2 or Twz were knocked down specifically in octopaminergic neurons. Although overall activity was not affected, compared to controls, TFAP-2RNAi and TwzRNAi males walked at a significantly slower pace—0.36-fold (SE ± 0.04, P < 0.005) and 0.59-fold (SE ± 0.04, P < 0.05), respectively. Overexpression of TfAP-2 in octopaminergic neurons induced a hyperactive phenotype, whereas TfAP-2OE males were both significantly more active (2.5-fold, SE ± 0.13, P < 0.005) and walked considerably faster (2.1-fold, SE ± 0.32, P < 0.005) than controls (Figure 4A). Finally, TfAP-2OE;TwzRi males were significantly less active than TFAP-2OE males (1.63-fold compared to controls, SE ± 0.21, P < 0.05). Furthermore, TfAP-2OE;TwzRNAi males also walked significantly slower than TfAP-2OE males (P < 0.005), but not significantly slower than controls (0.65-fold, SE ± 0.08, P = 0.082).
Figure 4.
Inhibiting octopamine signaling affects TfAP-2 induced hyperactivity. (A) Ctrax and Matlab were used to measure both activity and speed of walking of 5- to 7-day-old males for each genotype. Males were put individually into a behavioral assay chamber and monitored for 30 min (n = 20 males; *P < 0.05 **P < 0.005 compared with controls, one-way ANOVA with Bonferroni post hoc test for multiple comparisons). (B-C) Five- to 7-day-old Tdc2-GAL4; TfAP-2OE males were fed varying concentrations of the octopamine antagonists phentolamine or epinastine for 24 hr before an activity test was performed. (B) Epinastine (C) Phentolamine (n = 20 males per concentration; *P < 0.05 **P < 0.005 compared with controls, one-way ANOVA with Bonferroni post hoc test for multiple comparisons).
To confirm that TfAP-2 and Twz were actually regulating octopamine signaling, varying concentrations of two different octopamine antagonists, epinastine and phentolamine (Dudai 1982; Stevenson et al. 2005; Unoki et al. 2005), were fed to 3-day-old TfAP-2OE males, after which an activity assay was performed. All manipulations were compared to TfAP-2OE flies not fed antagonist (0 mM). Compared to TfAP-2OE controls (80.4%, SE ± 4.3) a significant reduction in activity was observed when TfAP-2OE males were fed 3 mM of either epinastine (54%, SE ± 3.6, P < 0.005) (Figure 4B) or phentolamine (47.2%, SE ± 6.7, P < 0.005) (Figure 4C). Feeding TfAP-2OE males 5 mM of epinastine was lethal (Figure 4B), while 5 mM of phentolamine reduced activity even further (10%, SE ± 4.2, P < 0.005) (Figure 4C).
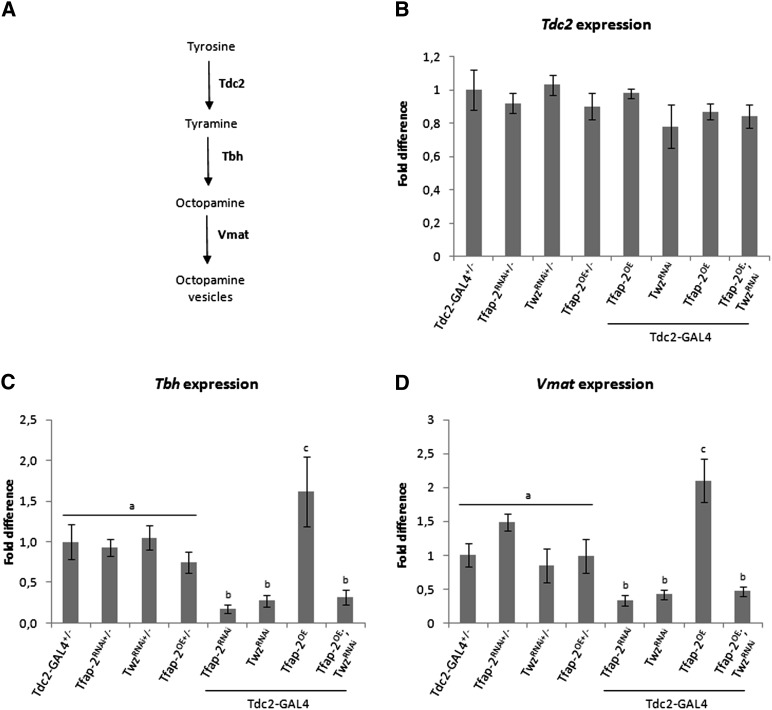
TfAP-2 and Twz regulate genes involved in octopamine synthesis
The transcription levels of a select number of genes—Tdc2, Tbh, and Vmat—known to influence octopamine production or release were examined (Figure 5A). Transcription levels of the Tdc2-GAL4 heterozygous controls were set at 100%, shown as 1 on the various graphs (Figure 5, B–D). The transcript level of Tdc2, necessary to convert tyrosine to tyramine, was not affected in TfAP-2RNAi (0.98-fold, SE ± 0.03, P = 0.98), TwzRNAi (0.78-fold, SE ± 0.13, P = 0.76), or TfAP-2OE males (0.87-fold, SE ± 0.05, P = 0.75), compared to controls (Figure 5B). Tbh, necessary to convert tyramine to octopamine, was decreased significantly in TfAP-2RNAi and TwzRNAi males, 0.17-fold (SE ± 0.05, P < 0.005) and 0.28-fold (SE ± 0.07, P < 0.005), compared to controls, while in TfAP-2OE males Tbh expression was significantly increased (1.61-fold, SE ± 0.43, P < 0.05). TfAP-2OE;TwzRNAi males had significantly lower levels of Tbh expression (0.31-fold, SE ± 0.09, P < 0.005) (Figure 5C). Finally, the transcript level of Vmat, involved in the transportation of monoamines such as octopamine into the synaptic vesicles, was also significantly reduced in TfAP-2RNAi (0.33-fold, SE ± 0.08, P < 0,005) and TwzRNAi (0.42-fold, SE ± 0.07, P < 0,005) males, compared to controls. In TfAP-2OE males, Vmat expression was significantly increased (2.1-fold, SE ± 0.32, P < 0.005) (Figure 5D). TfAP-2OE;TwzRNAi males had significantly increased Vmat expression levels, which is more similar to TwzRNAi males (0.47-fold, SE ± 0.07, P < 0.005) (Figure 5D).
Figure 5.
TfAP-2 and Twz regulate genes involved in octopamine signaling. (A) Simplified schematic diagram of the pathway involved in octopamine production and secretion. (B–D) The transcript levels of genes (A) Tdc2, (B) Tbh, and (C) Vmat. RNA was collected from the heads of 5- to 7-day-old males for each genotype. qPCR was repeated at least seven times for each transcript. Tbh and Vmat transcript levels are significantly lower in flies where TfAP-2 or Twz are knocked down in octopaminergic neurons (one-way ANOVA, P < 0.005), while their transcript levels are significantly increased when TfAP-2 is overexpressed in these same neurons (n = 7 qPCR runs; *P < 0.05 **P < 0.005 compared with controls, one-way ANOVA with Bonferroni post hoc test for multiple comparisons). In C and D different letters indicate similar groups (i.e., ‘a’ is significantly different than ‘b’ or ‘c’ and so on).
CCK homolog Dsk regulates aggression downstream of octopamine
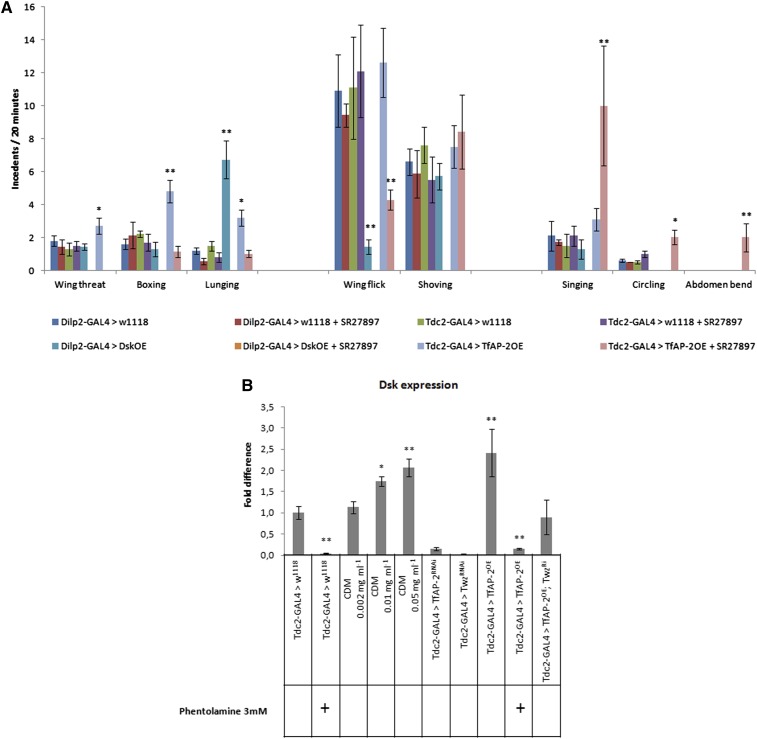
So far we have determined that TfAP-2 and Twz genetically interact to regulate male behavior. Furthermore, we show that TfAP-2 and Twz regulate the expression of genes involved in controlling octopamine production and signaling. Yet we still have not determined how TfAP-2 and Twz control of octopamine production may regulate behavior. Although it is well known that octopamine regulates aggressive behavior in Drosophila (Certel et al. 2007, 2010; Hoyer et al. 2008; Zhou et al. 2008), it is not known how octopamine exerts is affects. In rodents, levels of the satiation hormone CCK are correlated with aggressiveness (Zwanzger et al. 2012), and the Drosophila homolog of CCK is Dsk (Chen and Ganetzky 2012; Chen et al. 2012; Söderberg et al. 2012). Previously, it was shown that octopaminergic neurons innervate the insulin-producing cells (IPCs) and that the IPCs produce Dsk (Crocker et al. 2010; Söderberg et al. 2012). To begin to determine if Dsk could be regulating male behavior, we performed an aggression assay. Initially, we determined that 1 mM of the CCK antagonist SR27897 was insufficient to cause a phenotype in control heterozygous males (Figure 6A). Since Dsk is expressed by IPCs in the brain (Söderberg et al. 2012), the IPC-specific driver Dilp2-GAL4 was used to overexpress UAS-Dsk (referred to as DskOE), and an aggression assay was performed on these males or on DskOE males fed CCK antagonist. Males overexpressing Dsk in the IPCs performed significantly more lunges (6.7, SE ± 1.1, P < 0.005) than controls. Intriguingly, feeding DskOE males 1 mM SR27897 induced severe intermittent jump behaviors, and no interactions were observed. Similar to what was observed before, overexpressing TfAP-2 in Tdc2 neurons significantly increased the number of HIF behaviors (Figure 6A). Unlike DskOE males, feeding TfAP-2OE males 1 mM SR27897 did not induce a tick-like phenotype, but did inhibit the number of HIF behaviors that flies performed (Figure 6A). Feeding 1 mM SR27897 to TfAP-2OE males significantly induced more courtship behavior (Figure 6A). Whereas TfAP-2OE males performed 3.1 (SE ± 0.7) singing behaviors, TfAP-2OE males fed 1 mM SR27897 performed 10.0 (SE ± 3.6, P < 0.005). Furthermore, while TfAP-2OE males never performed circling or abdomen-bending behaviors, feeding 1 mM SR27897 to TfAP-2OE males induced significant circling (2, SE ± 0.4, P < 0.05) and abdomen bending (2, SE ± 0.8, P < 0.005).
Figure 6.
Disrupting Dsk signaling affects male behavior. (A) Total interactions that were either high- or low-intensity aggression or courtship behavior for all genotypes were determined. All males were between 5 and 7 days old. The types of behaviors were distributed into three catagories—HIF, LIF, and courtship behavior—and the number of times that each type of behavior was performed is represented. In all instances the assay was repeated at least 10 times (n = 20 males/treatment; *P < 0.05, **P < 0.005 compared with controls, two-way ANOVA with Bonferroni post hoc test for multiple comparisons). (B) Relative levels of Dsk transcript in flies where TfAP-2 and Twz expression has been disrupted in octopaminergic neurons. RNA was collected from the heads of 5- to 7-day-old males for each genotype (n = 7 qPCR runs; *P < 0.05 **P < 0.005 compared with controls, two-way ANOVA with Bonferroni post hoc test for multiple comparisons).
Analysis by qPCR demonstrated that Dsk transcript decreased significantly in the brain when control flies were fed 3mM of the octopamine antagonist phentolamine (0.05-fold, SE ± 0.004, P < 0.005), (Figure 6B) (Evans and Robb 1993). Next, flies were fed varying concentrations (0.002, 0.01, and 0.05 mg⋅ml−1) of chlordimeform (CDM), an octopamine agonist (Stevenson et al. 2005). The lowest concentration of CDM did not induce Dsk expression, but feeding flies 0.01 mg⋅ml−1 (1.75-fold, SE ± 0.11, P < 0.05) or 0.05 mg⋅ml−1 (2.06-fold, SE ± 0,21, P < 0.005) CDM significantly induced Dsk expression. Interestingly, Dsk transcript levels decreased significantly in TfAP-2RNAi (0.15-fold, SE ± 0.04, P < 0,005) and TwzRNAi (0.02-fold, SE ± 0.01, P < 0.005) males and significantly increased in TfAP-2OE males (2.42-fold, SE ± 0.56, P < 0.005) (Figure 6B). Finally, feeding TfAP-2OE males the octopamine antagonist phentolamine blocked Dsk induction (0.15-fold, SE ± 0.02, P < 0.005), and TfAP-2OE;TwzRNAi males had wild-type levels of Dsk expression (0.90-fold, SE ± 0.41, P = 0.82) (Dudai 1982; Evans and Robb 1993; Stevenson et al. 2005).
Dsk regulates adult male activity
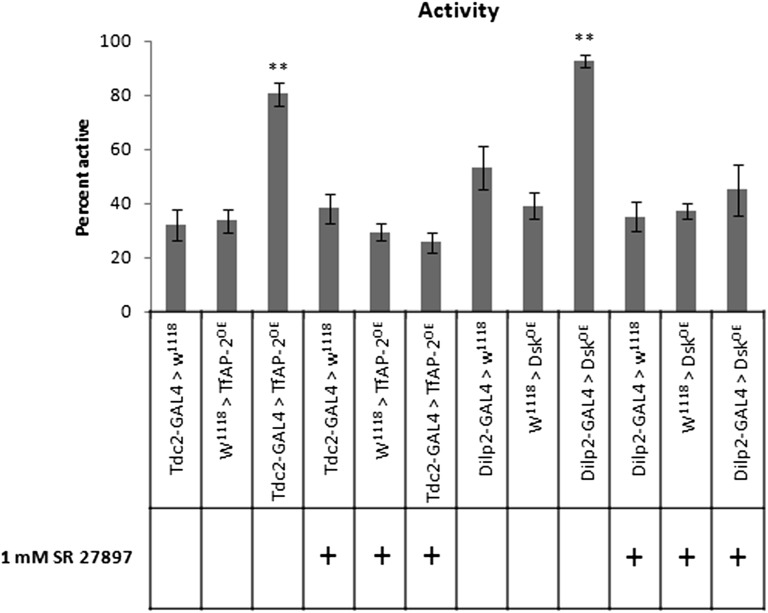
Interestingly, similar to TfAP-2, overexpressing Dsk in IPCs induced a hyperactive phenotype in adult males (Figure 7). While control males (Dilp2-Gal4) were active on average 53% of the time (SE ± 7.9), males overexpressing Dsk in the IPCs were active 92% of the time (SE ± 2.3, P < 0.005). Feeding males 1mM of the CCK antagonist SR27897, where Dsk was overexpressed in the IPCs, reduced their activity to 45% (SE ± 9.2, P = 0.51 compared to controls). As observed before, TfAP-2OE males were much more active than controls (Tdc2-Gal4+/−)—80.4% (SE ± 4.3, P < 0.005) for TfAP-2OE compared to 32.2% (SE ± 5.6) for controls. Feeding TfAP-2OE males the CCK antagonist reduced their activity to control levels (25.6%, SE ± 3.7, P < 0.34) (Figure 7).
Figure 7.
Overexpressing Dsk makes males hyperactive. (A) Ctrax and Matlab were used to measure both activity and speed of walking of 5- to 7-day-old males for each genotype. Males were put individually into a behavioral assay chamber and monitored for 30 min. n = 20 males per concentration; *P < 0.05 **P < 0.005 compared with controls, one-way ANOVA with Bonferroni post hoc test for multiple comparisons.
Discussion
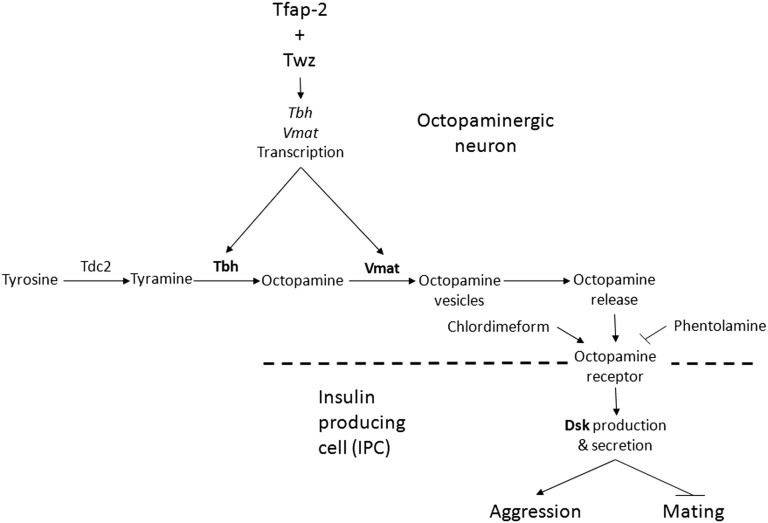
Our data reveal that the Drosophila homologs for TFAP2B and KCTD15, TfAP-2 and Twaz, respectively regulate at least two genes, Tbh and Vmat, known to be involved in the production and secretion of octopamine in Tdc2 octopaminergic neurons. Furthermore, we demonstrate that octopamine regulates aggression and mating in Drosophila by controlling the expression in IPCs of the CCK homolog Dsk, a neuropeptide known to influence feeding behavior (Söderberg et al. 2012) (Figure 8). Of notable interest, the CCK inhibitor SR27897 was able to rescue TfAP-2-induced hyperactivity, indicating that TfAP-2 is signaling through a CCK-like pathway.
Figure 8.
Model for possible modulation of aggressive behavior in Drosophila. Twz and TfAP-2 interact in octopaminergic neurons, possibly to regulate TfAP-2 activity. TfAP-2 induces the expression of Tbh and Vmat, which in turn regulate octopamine production and release from octopaminergic neurons. Octopamine signals to the IPCs to induce Dsk. Dsk signals to induce male aggressive behavior, while inhibiting mating behavior in males.
Our initial question was to determine if the Drosophila obesity-linked homologs could be linked to a pathway(s) known to regulate behavior. In mice, Tfap2b regulates noradrenaline signaling (Hong et al. 2008, 2011), and thus we addressed the octopaminergic system, the Drosophila equivalent (Roeder 2005). When Drosophila males are first introduced, they perform aggressive behaviors to establish a dominance hierarchy (Vrontou et al. 2006). Similar to what was observed when the enzyme Tbh, necessary to convert tyramine into octopamine, was mutated to inhibit its function, TfAP-2 and Twz knockdown males display reduced stereotypical high-intensity male aggressive behaviors (Zhou et al. 2008). Interestingly, we observed that Tbh expression was regulated by TfAP-2 and Twz (see Figure 5C). Also, overexpression in octopaminergic neurons of NaCHBac, a bacterially derived voltage-sensitive sodium channel used to lower the activation threshold, induces male aggression (Zhou et al. 2008), and overexpression of TfAP-2 in these same neurons induces aggressive behavior. All of these phenotypes lead us to suggest that TfAP-2 and Twz regulate octopaminergic neuronal signaling, perhaps similar to what was observed for AP-2β in the mouse noradrenergic system (Hoyer et al. 2008; Zhou et al. 2008).
CCK, a gastrointestinal hormone secreted by the gut when nutrients enter the lumen in mammals, binds to the cholecystokinin A receptor (CCKAR) located on vagal sensory terminals, which in turn delivers satiation signals to the nucleus of the solitary tract (NTS) (Wank et al. 1994; Mönnikes et al. 1997). CCK signaling within the brain to the cholecystokinin B receptor (CCKBR) induces hyperactivity and aggression in rodents (Bellier et al. 2004; Li et al. 2007). TfAP-2 overexpression in octopaminergic neurons induces the expression of the Drosophila CCK homolog, Dsk. This induction could be blocked by feeding TfAP-2-overexpressing males an octopamine antagonist and induced by feeding wild-type flies the octopamine agonist chlordimeform, revealing that TfAP-2 induces Dsk expression via octopamine signaling (Figure 6). Dsk is expressed in the Drosophila IPCs within a structure homologous to the hypothalamus, known as the Pars intercerebralis (Söderberg et al. 2012). We observed that overexpressing Dsk in the IPCs, similar to CCK signaling in mice, induced hyperactivity and aggressive behavior. The Drosophila genome encodes two different Dsk receptors, CCK-like receptor at 17D1 (CCKLR-17D1) and CCK-like receptor at 17D3 (CCKLR-17D3), with CCKLR-17D1 with slightly higher amino acid identity with CCKBR. Interestingly, in larvae, CCKLR-17D1 signaling is necessary to promote larval body-wall muscle contractions involved in stress-induced locomotory escape behavior (Chen et al. 2012). It was also shown that Dsk and CCKLR-17DI are required for proper neuromuscular junction formation in the developing Drosophila (Chen and Ganetzky 2012). Intriguingly, we could rescue aggressive behavior induced by TfAP-2 overexpression with the CCK antagonist SR27897, meaning that octopamine-induced aggression, downstream of TfAP-2 and Twz, is probably due to an increase in Dsk signaling.
Another intriguing result was that feeding Dsk-overexpressing flies the CCK receptor antagonist SR27897 induced severe intermittent jump behavior. Previously, it was observed that feeding flies the GABAB agonist 3-aminopropyl-(methyl)phosphinic acid (3-APMPA) induced intermittent jumps, a phenotype similar to what we observed (Dzitoyeva et al. 2003). In mammals, there are two CCK receptors, CCKAR and CCKBR. CCK activation of the CCKAR was shown to inhibit GABA release, while CCKBR activation induced GABA release (Ferraro et al. 1996; Lee and Soltesz 2011). In Drosophila, there are also two Dsk receptors, CCKLR-17D1 and CCKLR-17D3. The CCK receptor antagonist SR27897 is a much more potent antagonist for CCKAR than CCKBR (Gully et al. 1993; Poncelet et al. 1993). It could be that the Dsk pathway in Drosophila has a similar effect on GABA signaling. Overepxression of Dsk could activate both receptors, having no overall effect on GABA signaling. Furthermore, feeding the flies 1 mM SR27897 may have increased GABA release, but not over a threshold level necessary to cause the intermittent phenotype. Finally, overexpressing Dsk in adult males, while feeding them SR27897, may have induced the release of enough GABA to cause the intermittent jump phenotype that we observed. This is very speculative and will need to be tested in the future.
An intriguing task for the future will be to understand how neuropeptides such as CCK and Dsk, known to modify feeding behavior (Ritter et al. 1999; Söderberg et al. 2012), regulate disparate behaviors, including aggression. Of notable interest is the fact that anorexic patients, who have a higher propensity to be aggressive, also have higher levels of circulating CCK levels (Sturm et al. 2003; Zalar et al. 2011).
Acknowledgments
The authors thank Barry Ganetzky for his kind gift of UAS-Dsk flies and Eric Rulifson for his kind gift of Dilp2-GAL4 flies. This study was supported by the Swedish Research Council, the Åhléns Foundation, the Swedish Brain Research Foundation, the National Research Fund of Luxembourg, the Novo Nordisk Foundation, Carl Tryggers Stiftelse, Stiftelsen Olle Engkvist Byggmästare, and Stiftelsen Lars Hiertas Minne.
Footnotes
Communicating editor: T. Schüpbach
Literature Cited
- Alekseyenko O. V., Lee C., Kravitz E. A., 2010. Targeted manipulation of serotonergic neurotransmission affects the escalation of aggression in adult male Drosophila melanogaster. PLoS ONE 5: e10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alekseyenko O. V., Chan Y. B., Li R., Kravitz E. A., 2013. Single dopaminergic neurons that modulate aggression in Drosophila. Proc. Natl. Acad. Sci. USA 110: 6151–6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anaka M., MacDonald C. D., Barkova E., Simon K., Rostom R., et al. , 2008. The white gene of Drosophila melanogaster encodes a protein with a role in courtship behavior. J. Neurogenet. 22: 243–276. [DOI] [PubMed] [Google Scholar]
- Baier A., Wittek B., Brembs B., 2002. Drosophila as a new model organism for the neurobiology of aggression? J. Exp. Biol. 205: 1233–1240. [DOI] [PubMed] [Google Scholar]
- Bauer F., Elbers C. C., Adan R. A., Loos R. J., Onland-Moret N. C., et al. , 2009. Obesity genes identified in genome-wide association studies are associated with adiposity measures and potentially with nutrient-specific food preference. Am. J. Clin. Nutr. 90: 951–959. [DOI] [PubMed] [Google Scholar]
- Becnel J., Johnson O., Luo J., Nassel D. R., Nichols C. D., 2011. The serotonin 5–HT7Dro receptor is expressed in the brain of Drosophila, and is essential for normal courtship and mating. PLoS ONE 6: e20800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellier B., Crété D., Million M.-E., Beslot F., Bado A., et al. , 2004. New CCK2 agonists confirming the heterogeneity of CCK2 receptors: characterisation of BBL454. Naunyn Schmiedebergs Arch. Pharmacol. 370: 404–413. [DOI] [PubMed] [Google Scholar]
- Belsare P. V., Watve M. G., Ghaskadbi S. S., Bhat D. S., Yajnik C. S., et al. , 2010. Metabolic syndrome: aggression control mechanisms gone out of control. Med. Hypotheses 74: 578–589. [DOI] [PubMed] [Google Scholar]
- Brand A. H., Perrimon N., 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415. [DOI] [PubMed] [Google Scholar]
- Certel S. J., Savella M. G., Schlegel D. C. F., Kravitz E. A., 2007. Modulation of Drosophila male behavioral choice. Proc. Natl. Acad. Sci. USA 104: 4706–4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Certel S., Leung A., Lin C.-Y., Perez P., Chiang A.-S., et al. , 2010. Octopamine neuromodulatory effects on a social behavior decision-making network in Drosophila males. PLoS ONE 5: e13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Ganetzky B., 2012. A neuropeptide signaling pathway regulates synaptic growth in Drosophila. J. Cell Biol. 196: 529–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Peterson J., Nachman R., Ganetzky B., 2012. Drosulfakinin activates CCKLR-17D1 and promotes larval locomotion and escape response in Drosophila. Fly (Austin) 6: 290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintapalli V. R., Wang J., Dow J. A., 2007. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 39: 715–720. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N., 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162: 156–159. [DOI] [PubMed] [Google Scholar]
- Cole S., Carney G., McClung C., Willard S., Taylor B., et al. , 2005. Two functional but noncomplementing Drosophila tyrosine decarboxylase genes: distinct roles for neural tyramine and octopamine in female fertility. J. Biol. Chem. 280: 14948–15003. [DOI] [PubMed] [Google Scholar]
- Crocker A., Shahidullah M., Levitan I. B., Sehgal A., 2010. Identification of a neural circuit that underlies the effects of octopamine on sleep:wake behavior. Neuron 65: 670–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierick H. A., Greenspan R. J., 2007. Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nat. Genet. 39: 678–682. [DOI] [PubMed] [Google Scholar]
- Dudai Y., 1982. High-affinity octopamine receptors revealed in Drosophila by binding or [3H]octopamine. Neurosci. Lett. 28: 163–170. [DOI] [PubMed] [Google Scholar]
- Dutta S., Dawid I. B., 2010. Kctd15 inhibits neural crest formation by attenuating Wnt/β-catenin signaling output. Development 137: 3013–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzitoyeva S., Dimitrijevic N., Manev H., 2003. Gamma-aminobutyric acid B receptor 1 mediates behavior-impairing actions of alcohol in Drosophila: adult RNA interference and pharmacological evidence. Proc. Natl. Acad. Sci. USA 100: 5485–5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert D., Buhl S., Weber S., Jager R., Schorle H., 2005. The AP-2 family of transcription factors. Genome Biol. 6: 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erion R., Diangelo J., Crocker A., Sehgal A., 2012. Interaction between sleep and metabolism in Drosophila with altered octopamine signaling. J. Biol. Chem. 287: 32406–32414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P., Robb S., 1993. Octopamine receptor subtypes and their modes of action. Neurochem. Res. 18: 869–943. [DOI] [PubMed] [Google Scholar]
- Ferraro L., O’Connor W. T., Li X. M., Rimondini R., Beani L., et al. , 1996. Evidence for a differential cholecystokinin-B and -A receptor regulation of GABA release in the rat nucleus accumbens mediated via dopaminergic and cholinergic mechanisms. Neuroscience 73: 941–950. [DOI] [PubMed] [Google Scholar]
- Giot L., Bader J. S., Brouwer C., Chaudhuri A., Kuang B., et al. , 2003. A protein interaction map of Drosophila melanogaster. Science 302: 1727–1736. [DOI] [PubMed] [Google Scholar]
- Gully D., Frehel D., Marcy C., Spinazze A., Lespy L., et al. , 1993. Peripheral biological activity of SR 27897: a new potent non-peptide antagonist of CCKA receptors. Eur. J. Pharmacol. 232: 13–19. [DOI] [PubMed] [Google Scholar]
- Harper E. A., Griffin E. P., Shankley N. P., Black J. W., 1999. Analysis of the behaviour of selected CCKB/gastrin receptor antagonists in radioligand binding assays performed in mouse and rat cerebral cortex. Br. J. Pharmacol. 126: 1496–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S., Lardaro T., Oh M., Huh Y., Ding Y., et al. , 2008. Regulation of the noradrenaline neurotransmitter phenotype by the transcription factor AP-2beta. J. Biol. Chem. 283: 16860–16867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S. J., Huh Y. H., Leung A., Choi H. J., Ding Y., et al. , 2011. Transcription factor AP-2beta regulates the neurotransmitter phenotype and maturation of chromaffin cells. Mol. Cell. Neurosci. 46: 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer S. C., Eckart A., Herrel A., Zars T., Fischer S. A., et al. , 2008. Octopamine in male aggression of Drosophila. Curr. Biol. 18: 159–167. [DOI] [PubMed] [Google Scholar]
- Koon A. C., Ashley J., Barria R., DasGupta S., Brain R., et al. , 2011. Autoregulatory and paracrine control of synaptic and behavioral plasticity by octopaminergic signaling. Nat. Neurosci. 14: 190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. H., Soltesz I., 2011. Requirement for CB1 but not GABAB receptors in the cholecystokinin mediated inhibition of GABA release from cholecystokinin expressing basket cells. J. Physiol. 589: 891–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Deng X., Singh P., 2007. Significant increase in the aggressive behavior of transgenic mice overexpressing peripheral progastrin peptides: associated changes in CCK2 and serotonin receptors in the CNS. Neuropsychopharmacology 32: 1813–1821. [DOI] [PubMed] [Google Scholar]
- Lin D. M., Goodman C. S., 1994. Ectopic and increased expression of Fasciclin II alters motoneuron growth cone guidance. Neuron 13: 507–523. [DOI] [PubMed] [Google Scholar]
- Lindblom J., Johansson A., Holmgren A., Grandin E., Nedergård C., et al. , 2006. Increased mRNA levels of tyrosine hydroxylase and dopamine transporter in the VTA of male rats after chronic food restriction. Eur. J. Neurosci. 23: 180–186. [DOI] [PubMed] [Google Scholar]
- Lucki I., 1998. The spectrum of behaviors influenced by serotonin. Biol. Psychiatry 44: 151–162. [DOI] [PubMed] [Google Scholar]
- Meng X., Kondo M., Morino K., Fuke T., Obata T., et al. , 2010. Transcription factor AP-2β: a negative regulator of IRS-1 gene expression. Biochem. Biophys. Res. Commun. 392: 526–532. [DOI] [PubMed] [Google Scholar]
- Monge I., Krishnamurthy R., Sims D., Hirth F., Spengler M., et al. , 2001. Drosophila transcription factor AP-2 in proboscis, leg and brain central complex development. Development 128: 1239–1252. [DOI] [PubMed] [Google Scholar]
- Mönnikes H., Lauer G., Arnold R., 1997. Peripheral administration of cholecystokinin activates c-fos expression in the locus coeruleus/subcoeruleus nucleus, dorsal vagal complex and paraventricular nucleus via capsaicin-sensitive vagal afferents and CCK-A receptors in the rat. Brain Res. 770: 277–288. [DOI] [PubMed] [Google Scholar]
- Nilsen S. P., Chan Y.-B., Huber R., Kravitz E. A., 2004. Gender-selective patterns of aggressive behavior in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 101: 12342–12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncelet M., Arnone M., Heaulme M., Gonalons N., Gueudet C., et al. , 1993. Neurobehavioural effects of SR 27897, a selective cholecystokinin type A (CCK-A) receptor antagonist. Naunyn Schmiedebergs Arch. Pharmacol. 348: 102–107. [DOI] [PubMed] [Google Scholar]
- Ramakers C., Ruijter J., Deprez R., Moorman A., 2003. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 339: 62–66. [DOI] [PubMed] [Google Scholar]
- Renstrom F., Payne F., Nordstrom A., Brito E. C., Rolandsson O., et al. , 2009. Replication and extension of genome-wide association study results for obesity in 4923 adults from northern Sweden. Hum. Mol. Genet. 18: 1489–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter R., Covasa M., Matson C., 1999. Cholecystokinin: proofs and prospects for involvement in control of food intake and body weight. Neuropeptides 33: 387–399. [DOI] [PubMed] [Google Scholar]
- Roeder T., 2005. Tyramine and octapamine: ruling behavior and metabolism. Annu. Rev. Entomol. 50: 447–477. [DOI] [PubMed] [Google Scholar]
- Schmidt M., Huber L., Majdazari A., Schutz G., Williams T., et al. , 2011. The transcription factors AP-2beta and AP-2alpha are required for survival of sympathetic progenitors and differentiated sympathetic neurons. Dev. Biol. 355: 89–100. [DOI] [PubMed] [Google Scholar]
- Söderberg J., Carlsson M., Nässel D., 2012. Insulin-producing cells in the Drosophila brain also express satiety-inducing cholecystokinin-like peptide, drosulfakinin. Front Endocrinol. 3: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson P. A., Dyakonova V., Rillich J., Schildberger K., 2005. Octopamine and experience-dependent modulation of aggression in crickets. J. Neurosci. 25: 1431–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm K., MacIntosh C., Parker B., Wishart J., Horowitz M., et al. , 2003. Appetite, food intake, and plasma concentrations of cholecystokinin, ghrelin, and other gastrointestinal hormones in undernourished older women and well-nourished young and older women. J. Clin. Endocrinol. Metab. 88: 3747–3755. [DOI] [PubMed] [Google Scholar]
- Unoki S., Matsumoto Y., Mizunami M., 2005. Participation of octopaminergic reward system and dopaminergic punishment system in insect olfactory learning revealed by pharmacological study. Eur. J. Neurosci. 22: 1409–1425. [DOI] [PubMed] [Google Scholar]
- Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., et al. , 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: RESEARCH 0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Tulina N., Carlin D. L., Rulifson E. J., 2007. The origin of islet-like cells in Drosophila identifies parallels to the vertebrate endocrine axis. Proc. Natl. Acad. Sci. USA 104: 19873–19878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wank S., Pisegna J., de Weerth A., 1994. Cholecystokinin receptor family: molecular cloning, structure, and functional expression in rat, guinea pig, and human. Ann. N. Y. Acad. Sci. 713: 49–66. [DOI] [PubMed] [Google Scholar]
- Wenke A. K., Bosserhoff A. K., 2010. Roles of AP-2 transcription factors in the regulation of cartilage and skeletal development. FEBS J. 277: 894–902. [DOI] [PubMed] [Google Scholar]
- Willer C. J., Speliotes E. K., Loos R. J., Li S., Lindgren C. M., et al. , 2009. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat. Genet. 41: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrontou E., Nilsen S. P., Demir E., Kravitz E. A., Dickson B. J., 2006. fruitless regulates aggression and dominance in Drosophila. Nat. Neurosci. 9: 1469–1471. [DOI] [PubMed] [Google Scholar]
- Zalar B., Weber U., Sernec K., 2011. Aggression and impulsivity with impulsive behaviours in patients with purgative anorexia and bulimia nervosa. Psychiatr. Danub. 23: 27–60. [PubMed] [Google Scholar]
- Zarelli V. E., Dawid I. B., 2013. Inhibition of neural crest formation by Kctd15 involves regulation of transcription factor AP-2. Proc. Natl. Acad. Sci. USA 110: 2870–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S. D., Odenwald W. F., 1995. Misexpression of the white (w) gene triggers male-male courtship in Drosophila. Proc. Natl. Acad. Sci. USA 92: 5525–5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Bradfield J. P., Zhang H., Sleiman P. M., Kim C. E., et al. , 2011. Role of BMI-associated loci identified in GWAS meta-analyses in the context of common childhood obesity in European Americans. Obesity (Silver Spring) 19: 2436–2439. [DOI] [PubMed] [Google Scholar]
- Zhou C., Rao Y., Rao Y., 2008. A subset of octopaminergic neurons are important for Drosophila aggression. Nat. Neurosci. 11: 1059–1126. [DOI] [PubMed] [Google Scholar]
- Zhou C., Huang H., Kim S. M., Lin H., Meng X., et al. , 2012. Molecular genetic analysis of sexual rejection: roles of octopamine and its receptor OAMB in Drosophila courtship conditioning. J. Neurosci. 32: 14281–14287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwanzger P., Domschke K., Bradwejn J., 2012. Neuronal network of panic disorder: the role of the neuropeptide cholecystokinin. Depress. Anxiety 29: 762–774. [DOI] [PubMed] [Google Scholar]