Abstract
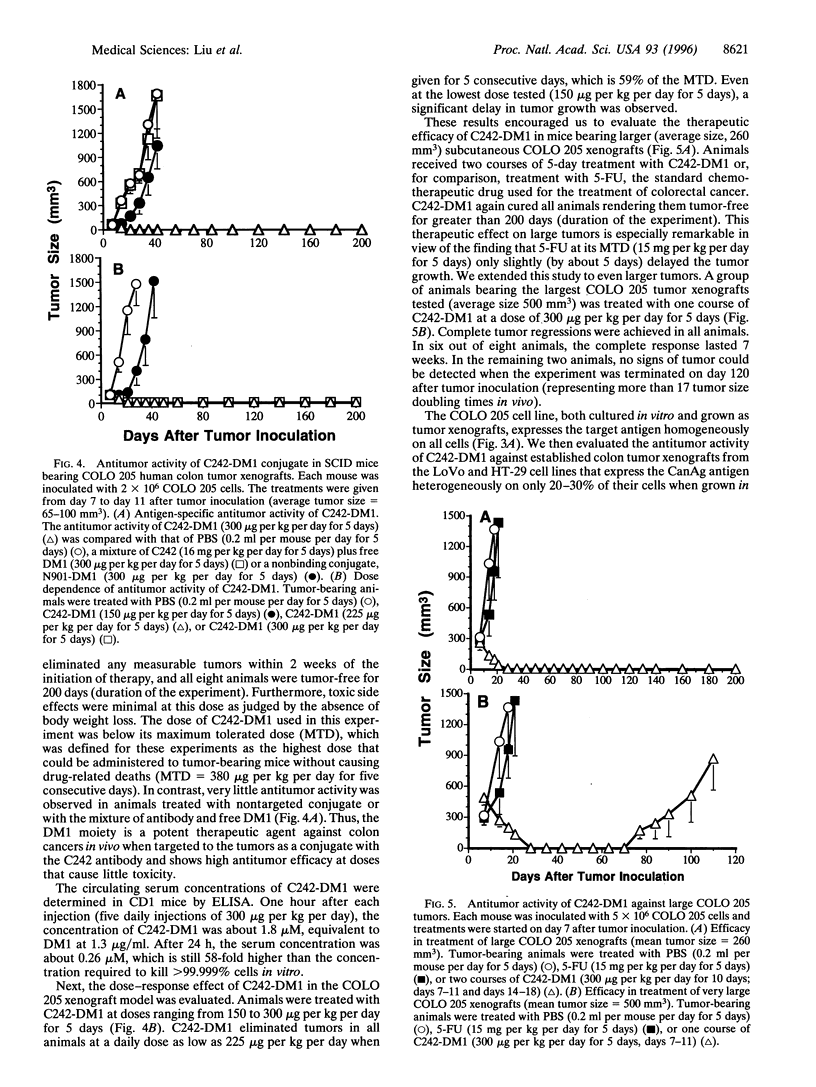
The maytansinoid drug DM1 is 100- to 1000-fold more cytotoxic than anticancer drugs that are currently in clinical use. The immunoconjugate C242-DM1 was prepared by conjugating DM1 to the monoclonal antibody C242, which recognizes a mucin-type glycoprotein expressed to various extents by human colorectal cancers. C242-DM1 was found to be highly cytotoxic toward cultured colon cancer cells in an antigen-specific manner and showed remarkable antitumor efficacy in vivo. C242-DM1 cured mice bearing subcutaneous COLO 205 human colon tumor xenografts (tumor size at time of treatment 65-130 mm3), at doses that showed very little toxicity and were well below the maximum tolerated dose. C242-DM1 could even effect complete regressions or cures in animals with large (260- to 500-mm3) COLO 205 tumor xenografts. Further, C242-DM1 induced complete regressions of subcutaneous LoVo and HT-29 colon tumor xenografts that express the target antigen in a heterogeneous manner. C242-DM1 represents a new generation of immunoconjugates that may yet fulfill the promise of effective cancer therapy through antibody targeting of cytotoxic agents.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeckström D., Hansson G. C., Nilsson O., Johansson C., Gendler S. J., Lindholm L. Purification and characterization of a membrane-bound and a secreted mucin-type glycoprotein carrying the carcinoma-associated sialyl-Lea epitope on distinct core proteins. J Biol Chem. 1991 Nov 15;266(32):21537–21547. [PubMed] [Google Scholar]
- Buyse M., Zeleniuch-Jacquotte A., Chalmers T. C. Adjuvant therapy of colorectal cancer. Why we still don't know. JAMA. 1988 Jun 24;259(24):3571–3578. [PubMed] [Google Scholar]
- Calvete J. A., Newell D. R., Wright A. F., Rose M. S. In vitro and in vivo antitumor activity of ZENECA ZD0490, a recombinant ricin A-chain immunotoxin for the treatment of colorectal cancer. Cancer Res. 1994 Sep 1;54(17):4684–4690. [PubMed] [Google Scholar]
- Chari R. V., Jackel K. A., Bourret L. A., Derr S. M., Tadayoni B. M., Mattocks K. M., Shah S. A., Liu C., Blättler W. A., Goldmacher V. S. Enhancement of the selectivity and antitumor efficacy of a CC-1065 analogue through immunoconjugate formation. Cancer Res. 1995 Sep 15;55(18):4079–4084. [PubMed] [Google Scholar]
- Chari R. V., Martell B. A., Gross J. L., Cook S. B., Shah S. A., Blättler W. A., McKenzie S. J., Goldmacher V. S. Immunoconjugates containing novel maytansinoids: promising anticancer drugs. Cancer Res. 1992 Jan 1;52(1):127–131. [PubMed] [Google Scholar]
- Debinski W., Karlsson B., Lindholm L., Siegall C. B., Willingham M. C., FitzGerald D., Pastan I. Monoclonal antibody C242-Pseudomonas exotoxin A. A specific and potent immunotoxin with antitumor activity on a human colon cancer xenograft in nude mice. J Clin Invest. 1992 Aug;90(2):405–411. doi: 10.1172/JCI115875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debinski W., Pastan I. Recombinant F(ab') C242-Pseudomonas exotoxin, but not the whole antibody-based immunotoxin, causes regression of a human colorectal tumor xenograft. Clin Cancer Res. 1995 Sep;1(9):1015–1022. [PubMed] [Google Scholar]
- Elias D. J., Kline L. E., Robbins B. A., Johnson H. C., Jr, Pekny K., Benz M., Robb J. A., Walker L. E., Kosty M., Dillman R. O. Monoclonal antibody KS1/4-methotrexate immunoconjugate studies in non-small cell lung carcinoma. Am J Respir Crit Care Med. 1994 Oct;150(4):1114–1122. doi: 10.1164/ajrccm.150.4.7921445. [DOI] [PubMed] [Google Scholar]
- Fuchs C. S., Mayer R. J. Adjuvant chemotherapy for colon and rectal cancer. Semin Oncol. 1995 Oct;22(5):472–487. [PubMed] [Google Scholar]
- Haglund C., Lindgren J., Roberts P. J., Kuusela P., Nordling S. Tissue expression of the tumour associated antigen CA242 in benign and malignant pancreatic lesions. A comparison with CA 50 and CA 19-9. Br J Cancer. 1989 Dec;60(6):845–851. doi: 10.1038/bjc.1989.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman L. M., Hamann P. R., Wallace R., Menendez A. T., Durr F. E., Upeslacis J. Preparation and characterization of monoclonal antibody conjugates of the calicheamicins: a novel and potent family of antitumor antibiotics. Cancer Res. 1993 Jul 15;53(14):3336–3342. [PubMed] [Google Scholar]
- Kanellos J., Pietersz G. A., McKenzie I. F. Studies of methotrexate-monoclonal antibody conjugates for immunotherapy. J Natl Cancer Inst. 1985 Aug;75(2):319–332. [PubMed] [Google Scholar]
- Klein E., Mantovani A. Action of natural killer cells and macrophages in cancer. Curr Opin Immunol. 1993 Oct;5(5):714–718. doi: 10.1016/0952-7915(93)90126-d. [DOI] [PubMed] [Google Scholar]
- Lindholm L., Holmgren J., Svennerholm L., Fredman P., Nilsson O., Persson B., Myrvold H., Lagergård T. Monoclonal antibodies against gastrointestinal tumour-associated antigens isolated as monosialogangliosides. Int Arch Allergy Appl Immunol. 1983;71(2):178–181. doi: 10.1159/000233384. [DOI] [PubMed] [Google Scholar]
- Medley Q. G., Kedersha N., O'Brien S., Tian Q., Schlossman S. F., Streuli M., Anderson P. Characterization of GMP-17, a granule membrane protein that moves to the plasma membrane of natural killer cells following target cell recognition. Proc Natl Acad Sci U S A. 1996 Jan 23;93(2):685–689. doi: 10.1073/pnas.93.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moertel C. G., Fleming T. R., Macdonald J. S., Haller D. G., Laurie J. A., Tangen C. M., Ungerleider J. S., Emerson W. A., Tormey D. C., Glick J. H. Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: a final report. Ann Intern Med. 1995 Mar 1;122(5):321–326. doi: 10.7326/0003-4819-122-5-199503010-00001. [DOI] [PubMed] [Google Scholar]
- Nilsson O., Johansson C., Glimelius B., Persson B., Nørgaard-Pedersen B., Andrén-Sandberg A., Lindholm L. Sensitivity and specificity of CA242 in gastro-intestinal cancer. A comparison with CEA, CA50 and CA 19-9. Br J Cancer. 1992 Feb;65(2):215–221. doi: 10.1038/bjc.1992.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K., Harada K., Ikeyama S., Iwasa S. Therapeutic effect of ansamitocin targeted to tumor by a bispecific monoclonal antibody. Jpn J Cancer Res. 1992 Jul;83(7):761–768. doi: 10.1111/j.1349-7006.1992.tb01977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice G. E., Munro J. M., Corless C., Bevilacqua M. P. Vascular and nonvascular expression of INCAM-110. A target for mononuclear leukocyte adhesion in normal and inflamed human tissues. Am J Pathol. 1991 Feb;138(2):385–393. [PMC free article] [PubMed] [Google Scholar]
- Roguska M. A., Pedersen J. T., Keddy C. A., Henry A. H., Searle S. J., Lambert J. M., Goldmacher V. S., Blättler W. A., Rees A. R., Guild B. C. Humanization of murine monoclonal antibodies through variable domain resurfacing. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):969–973. doi: 10.1073/pnas.91.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneck D., Butler F., Dugan W., Littrell D., Petersen B., Bowsher R., DeLong A., Dorrbecker S. Disposition of a murine monoclonal antibody vinca conjugate (KS1/4-DAVLB) in patients with adenocarcinomas. Clin Pharmacol Ther. 1990 Jan;47(1):36–41. doi: 10.1038/clpt.1990.5. [DOI] [PubMed] [Google Scholar]
- Starling J. J., Maciak R. S., Law K. L., Hinson N. A., Briggs S. L., Laguzza B. C., Johnson D. A. In vivo antitumor activity of a monoclonal antibody-Vinca alkaloid immunoconjugate directed against a solid tumor membrane antigen characterized by heterogeneous expression and noninternalization of antibody-antigen complexes. Cancer Res. 1991 Jun 1;51(11):2965–2972. [PubMed] [Google Scholar]
- Trail P. A., Willner D., Lasch S. J., Henderson A. J., Hofstead S., Casazza A. M., Firestone R. A., Hellström I., Hellström K. E. Cure of xenografted human carcinomas by BR96-doxorubicin immunoconjugates. Science. 1993 Jul 9;261(5118):212–215. doi: 10.1126/science.8327892. [DOI] [PubMed] [Google Scholar]
- Vater C. A., Reid K., Bartle L. M., Goldmacher V. S. Characterization of antibody binding to cell surface antigens using a plasma membrane-bound plate assay. Anal Biochem. 1995 Jan 1;224(1):39–50. doi: 10.1006/abio.1995.1006. [DOI] [PubMed] [Google Scholar]
- Wadler S. The role of immunotherapy in colorectal cancer. Semin Oncol. 1991 Feb;18(1 Suppl 1):27–38. [PubMed] [Google Scholar]




