Abstract
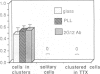
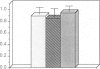
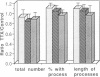
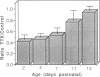
During the first 2 postnatal weeks, up to 50% of the ganglion cells in the mammalian retina normally die. Natural cell death may result from several factors, and electrical activity has been proposed as one critical element. Recent experiments in vivo using intraocular injection of tetrodotoxin (TTX) have suggested that competition for survival between ganglion cells from the two eyes is mediated by their degree of neuronal activity. In addition, the level of activity of afferents to the ganglion cells has been postulated to be an important variable in determining their survival. To investigate the mechanism of cell death engendered by altered activity, I studied the effect of electrical blockade with TTX (to block sodium channels and thus action potentials) or low Ca/high Mg (to block transmitter release and hence synaptic activity) on individual neurons in vitro. For this purpose, identified retinal ganglion cells (RGCs) from postnatal rats were maintained in culture. Unlike the previous in vivo experiments, this approach permitted the exact concentration of each agent to be controlled and the electrical activity of the RGCs to be recorded. In cultures from animals of postnatal day 2-10 (P2-10), 1 microM TTX or 0.2 mM Ca/20 mM Mg resulted in the death of about 50% of the RGCs, representing those cells that had displayed spontaneous electrical activity, but did not affect RGCs that lacked activity. However, the death of RGCs with spontaneous activity from P11-13 animals was not influenced by these drugs. These findings suggest that during a critical period of development neurons become dependent upon electrical activity, and the cessation of this activity can result in their death. In addition, conditioned medium, collected from cultures lacking TTX, rescued from death a large proportion of TTX-treated RGCs. Thus, the critical element for survival may represent modulation of a trophic factor related to the level of activity rather than electrical activity itself. Since, in vivo, natural cell death occurs in neurons of similar type and age, and in the same proportion as that induced by the artificial blockade of electrical activity in culture, these findings may be germane to the mechanism of natural cell death in the retina.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenneman D. E., Eiden L. E. Vasoactive intestinal peptide and electrical activity influence neuronal survival. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1159–1162. doi: 10.1073/pnas.83.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan W. M., Fawcett J. W., O'Leary D. D., Stanfield B. B. Regressive events in neurogenesis. Science. 1984 Sep 21;225(4668):1258–1265. doi: 10.1126/science.6474175. [DOI] [PubMed] [Google Scholar]
- Cunningham T. J. Naturally occurring neuron death and its regulation by developing neural pathways. Int Rev Cytol. 1982;74:163–186. doi: 10.1016/s0074-7696(08)61172-9. [DOI] [PubMed] [Google Scholar]
- Fawcett J. W., O'Leary D. D., Cowan W. M. Activity and the control of ganglion cell death in the rat retina. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5589–5593. doi: 10.1073/pnas.81.17.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. L., Wilson K. G., Schneider G. E. Anomalous ipsilateral retinotectal projections in Syrian hamsters with early lesions: topography and functional capacity. J Comp Neurol. 1979 Feb 15;183(4):721–740. doi: 10.1002/cne.901830404. [DOI] [PubMed] [Google Scholar]
- Forda S. R., Jessell T. M., Kelly J. S., Rand R. P. Use of the patch electrode for sensitive high resolution extracellular recording. Brain Res. 1982 Oct 14;249(2):371–378. doi: 10.1016/0006-8993(82)90071-3. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Insausti R., Blakemore C., Cowan W. M. Ganglion cell death during development of ipsilateral retino-collicular projection in golden hamster. Nature. 1984 Mar 22;308(5957):362–365. doi: 10.1038/308362a0. [DOI] [PubMed] [Google Scholar]
- Land P. W., Lund R. D. Development of the rat's uncrossed retinotectal pathway and its relation to plasticity studies. Science. 1979 Aug 17;205(4407):698–700. doi: 10.1126/science.462177. [DOI] [PubMed] [Google Scholar]
- Leifer D., Lipton S. A., Barnstable C. J., Masland R. H. Monoclonal antibody to Thy-1 enhances regeneration of processes by rat retinal ganglion cells in culture. Science. 1984 Apr 20;224(4646):303–306. doi: 10.1126/science.6143400. [DOI] [PubMed] [Google Scholar]
- Masland R. H., Ames A., 3rd Responses to acetylcholine of ganglion cells in an isolated mammalian retina. J Neurophysiol. 1976 Nov;39(6):1220–1235. doi: 10.1152/jn.1976.39.6.1220. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Henderson Z., Linden R. Postnatal changes in retinal ganglion cell and optic axon populations in the pigmented rat. J Comp Neurol. 1983 Sep 20;219(3):356–368. doi: 10.1002/cne.902190309. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Linden R. Evidence for dendritic competition in the developing retina. Nature. 1982 Jun 24;297(5868):683–685. doi: 10.1038/297683a0. [DOI] [PubMed] [Google Scholar]
- Rakic P., Riley K. P. Regulation of axon number in primate optic nerve by prenatal binocular competition. Nature. 1983 Sep 8;305(5930):135–137. doi: 10.1038/305135a0. [DOI] [PubMed] [Google Scholar]
- Thompson I. D. Changes in the uncrossed retinotectal projection after removal of the other eye at birth. Nature. 1979 May 3;279(5708):63–66. doi: 10.1038/279063a0. [DOI] [PubMed] [Google Scholar]