Abstract
Electrophysiological data suggest a dual role of Y2 receptors (Y2Rs) as autoreceptors regulating neuropeptide Y release and heteroceptors regulating gamma-aminobutyric acid release in the central amygdala (CeA). Here, we report that neither systemic (JNJ-31020028) nor intra-CeA (BIIE0246) Y2R antagonism altered operant alcohol responding by alcohol-dependent or non-dependent rats. Conversely, BIIE0246 in the CeA reduced anxiety-like behavior in alcohol-dependent and alcohol-naïve rats. The finding that Y2R antagonism reduces anxiety-like behavior but not alcohol drinking suggests that these two effects may occur via different functions of the Y2R (e.g. autoreceptor versus heteroceptor function).
Keywords: Alcohol dependence, anxiety, negative reinforcement, self-administration, vapor model, Y2 receptor
Neuropeptide Y (NPY) reduces anxiety-like behavior and alcohol consumption in rats via effects in the central nucleus of the amygdala (CeA; Heilig et al. 1993; Gilpin et al. 2011). Recent electrophysiological data show that NPY blocks the ability of acute alcohol to increase inhibitory transmission in CeA via actions at presynaptic Y2 autoreceptors and that NPY also reverses chronic alcohol effects on gamma-aminobutyric acid (GABA) release in CeA, likely via the same mechanism (Gilpin et al. 2011). Although prior studies have examined the effect of systemic and whole-brain Y2R antagonism on alcohol-drinking behaviors by alcohol-dependent rats (Rimondini, Thorsell & Heilig 2005; Cippitelli et al. 2011), the effect of Y2R antagonism in CeA remains to be investigated. Here, we examined the effects of systemic and intra-CeA Y2R antagonism on alcohol drinking and anxiety-like behavior in alcohol-dependent rats.
Adult male Wistar rats weighing 300–350 g (Charles River; Kingston, NY, USA) were group housed and kept under a 12 hours light/12 hours dark cycle (lights off: 8AM) with free access to food and water. Procedures were conducted in the dark cycle and met the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Rats were trained to respond for 10% (w/v) ethanol versus water on a fixed ratio-1 schedule (each response resulted in delivery of 0.1 ml of fluid) and divided into two groups: rats exposed to chronic intermittent (14 hours/day for 2-3 months) alcohol vapor (dependent) and air-exposed control rats (non-dependent). Operant tests occurred twice per week during acute withdrawal (6-8 hours after removal from vapor). Once responding stabilized, rats were injected with JNJ-31020028 (0, 3, 10, 30 mg/kg; 3 ml/kg; subcutaneously) (Shoblock et al. 2010), a Y2R antagonist provided by Janssen Research & Development, LLC. (San Diego, CA, USA), 60 minutes prior to testing. Vehicle consisted of 5/5% dimethylformamide/Alkamuls-620 (Rhodia, New Brunswick, NJ, USA) in saline. A separate group of rats was trained to respond for alcohol, exposed to alcohol vapor, or ambient air for ≥4 weeks, then implanted with bilateral guide cannulae aimed at the CeA (−2.6AP/±4.2ML/−6.6DV from skull) and bilaterally infused with the Y2R antagonist BIIE0246 (0, 5, 50, 500 ng) dissolved in artificial cerebrospinal fluid (0.5 μl) 30 minutes prior to operant tests during acute withdrawals in a within-subjects Latin-square design. Infusions occurred over a period of 2 minutes plus 1 minute for diffusion. A final group of rats was exposed to alcohol vapor or ambient air then injected in CeA with BIIE0246 (0 or 50 ng) and tested on the elevated plus-maze 30 minutes later, also during acute withdrawal. Data were analyzed using two-way analyses of variance with or without repeated measures. Statistical significance was set at P < 0.05.
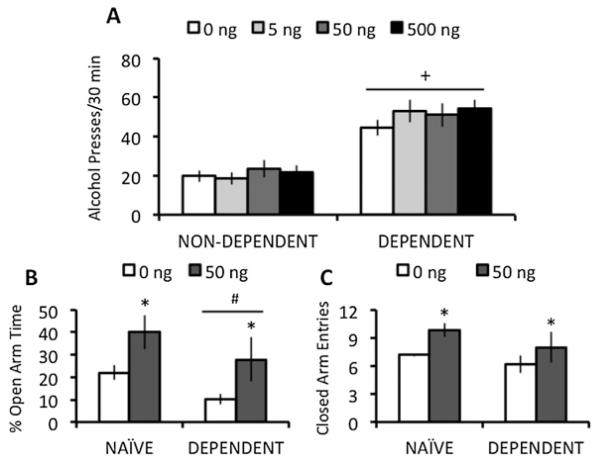
Dependent rats responded more for alcohol than non-dependent rats in the first (F(1,16) = 14.7, P < 0.005) and second (F(1,27) = 50.6, P < 0.0001) experiments. Neither systemic administration of JNJ-31020028 (Table 1) nor intra-CeA injections with BIIE0246 affected responding for alcohol (Fig. 1a) or water (data not shown).
Table 1.
Systemic Y2R antagonism with JNJ-31020028 does not alter alcohol self-administration in rats.
| Group | Dose (mg/kg) | Alcohol deliveries |
|---|---|---|
| Dependent | 0 | 42.4 ± 2.5 |
| 3 | 47.0 ± 9.3 | |
| 10 | 45.4 ± 6.2 | |
| 30 | 55.4 ± 10.2 | |
| Non-dependent | 0 | 25.0 ± 3.0 |
| 3 | 25.9 ± 5.3 | |
| 10 | 26.4 ± 4.5 | |
| 30 | 26.4 ± 5.1 |
Figure 1.
(a) Mean ± SEM lever presses for alcohol by alcohol-dependent and non-dependent rats, and (b) %open-arm time and (c) closed-arm entries in the elevated plus-maze by alcohol-dependent and alcohol-naïve rats following bilateral intra-CeA infusion (30 minutes prior to operant test) of BIIE0246. Rats (n = 14/group) in the self-administration experiment were infused with four doses (0, 5, 50 and 500 ng; 0.5 μL/side) of BIIE0246, and rats (n = 5-6/group) in the elevated plus-maze experiment were infused with one of two doses (0, 50 ng; 0.5 μL/side) of BIIE0246. *P < 0.05 relative to vehicle condition; +P < 0.05 relative to non-dependent controls; #P = 0.07 tendency toward significant difference form naïve controls
In the plus-maze, dependent rats tended to spend less % open-arm time than naïve rats (F(1,19) = 3.6, P = 0.07). BIIE0246 in CeA increased % open-arm time (F(1,19) = 8.1, P < 0.01; Fig. 1b) and closed-arm entries (F(1,19) = 8.0, P < 0.01; Fig. 1c) in both groups.
Antagonism of Y2R systemically (Cippitelli et al. 2011) or in CeA (present study) reduces anxiety-like behavior in alcohol-naïve rats and also during acute withdrawal, without affecting operant alcohol self-administration, suggesting that NPY in CeA modulates alcohol drinking and anxiety-like behavior via different actions of presynaptic Y2Rs. In this study, antagonism of Y2Rs in CeA reduced anxiety-like behavior in alcohol-naïve and alcohol-dependent rats, confirming prior results that withdrawal-induced anxiety-like behavior is reversed by systemic JNJ-31020028 (Cippitelli et al. 2011), ablation of theY2R gene in CeA produces a low-anxiety phenotype (Tasan et al. 2010), and pharmacological activation of Y2Rs in basolateral amygdala increases anxiety-like behavior in rats in the social interaction test (Sajdyk et al. 2002). The likely mechanism for this effect is that Y2R antagonists facilitate presynaptic release of NPY that activates postsynaptic Y1Rs to produce anxiolytic effects (Heilig et al. 1993). Intra-CeA BIIE0246 also increased closed-arm entries, but this pharmacologically effective dose did not produce specific or non-specific increases in alcohol or water self-administration. That said, the increase in elevated plus-maze closed-arm entries produced by intra-CeA BIIE0246 infusion is a potential confound of the observed anxiolytic-like effects. Also, the effects of the single BIIE0246 dose on open-arm time and closed-arm entries were similar in alcohol-dependent and alcohol-naïve rats, which suggest this dose is too high to detect dependence-induced changes in the number, availability or function of Y2Rs.
Neither intra-CeA (BIIE0246) nor systemic (JNJ-31020028) Y2R antagonism altered operant alcohol self-administration by dependent or non-dependent rats, suggesting that exogenously administered NPY produces extracellular NPY quantities that are not reproduced by Y2R antagonist-induced NPY release. Prior studies showed that intraventricular infusion of BIIE0246 suppresses operant self-administration of a sweetened-alcohol solution by rats with a history of alcohol dependence (Rimondini et al. 2005). Discrepancies between results across studies may be attributable to multiple design factors that include: testing during different phases of alcohol withdrawal (acute versus protracted), self-administration of sweetened versus unsweetened alcohol solution, dosing, and/or site of drug action (ventricles versus CeA).
One potential explanation for the differential effects of Y2R antagonism on anxiety-like behavior and alcohol self-administration is that Y2Rs have multiple actions at the CeA synapse. Brain Y2Rs function as autoreceptors that regulate NPY release (Chen et al. 1997) and as heteroceptors that regulate the release of other neurotransmitters (Greber, Schwarzer & Sperk 1994; Gilpin et al. 2011). NPY blocks acute alcohol-induced facilitation of GABA release in CeA of rats via heteroceptor function of Y2Rs and also normalizes alcohol dependence-induced increases in CeA GABA release, likely via the same mechanism (Gilpin et al. 2011). NPY effects mirror the facilitatory effects of prostress peptides [i.e. corticotropin-releasing factor (CRF)] on CeA GABA release (Gilpin & Roberto 2012). Collectively, these results suggest that during the transition to alcohol dependence, it may not be NPY function or receptor number that are recruited, but rather the ability of NPY to modulate GABAergic transmission in CeA. Conversely, it is unlikely thatY2R antagonism reduces anxiety-like behavior via changes in GABA release, as Y2R antagonism mimics the effects of CRF on GABA release in CeA. One possibility is thatY2Rs in CeA modulate anxiety-like behavior via auto receptor function (i.e. increased NPY release and activation of postsynaptic Y1Rs), but another possibility is that Y2Rs in CeA affect anxiety-like behavior via modulation of glutamate release.
In summary, Y2R antagonism in CeA reduces anxiety-like behavior but does not affect alcohol drinking in rats, regardless of alcohol dependence history. These data illustrate that antagonism of Y2Rs in CeA mimics the anxiolytic effects of NPY in CeA but does not mimic NPY effects on alcohol drinking. The hypothesis that Y2R antagonism reduces anxiety-like behavior via autoreceptor function (i.e. increased NPY release and postsynaptic action) and alcohol drinking via heteroceptor function (i.e. reduced GABA release) should be confirmed by pretreatment with Y1R antagonists and GABA receptor antagonists prior to Y2Rs manipulations in CeA.
Acknowledgements
The authors thank Molly Brenan, Hillary Cormier and Sharon Chaing for their technical assistance. This is manuscript #21290 from TSRI. This work was supported by AA016436, AA018400, AA008459, AA006420.
Footnotes
Authors Contribution
MK, LFV, CYC and OG conducted the experiments, analyzed data and helped in writing the manuscript. GFK oversaw experimental design and editing of manuscript. NWG was responsible for the study concept and design, conducted the experiment and drafted the manuscript. All authors approved the final version of the manuscript for publication.
References
- Chen X, DiMaggio DA, Han SP, Westfall TC. Autoreceptor-induced inhibition of neuropeptide Y release from PC-12 cells is mediated by Y2 receptors. Am J Physiol. 1997;273:H1737–H1744. doi: 10.1152/ajpheart.1997.273.4.H1737. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Rezvani AH, Robinson JE, Eisenberg L, Levin ED, Bonaventure P, Motley ST, Lovenberg TW, Heilig M, Thorsell A. The novel, selective, brain-penetrant neuropeptideY Y2 receptor antagonist, JNJ-31020028, tested in animal models of alcohol consumption, relapse, and anxiety. Alcohol. 2011;45:567–576. doi: 10.1016/j.alcohol.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Misra K, Herman MA, Cruz MT, Koob GF, Roberto M. Neuropeptide Y opposes alcohol effects on gamma-aminobutyric acid release in amygdala and blocks the transition to alcohol dependence. Biol Psychiatry. 2011;69:1091–1099. doi: 10.1016/j.biopsych.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Roberto M. Neuropeptide modulation of central amygdala neuroplasticity is a key mediator of alcohol dependence. Neurosci Biobehav Rev. 2012;36:873–888. doi: 10.1016/j.neubiorev.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greber S, Schwarzer C, Sperk G. Neuropeptide Y inhibits potassium-stimulated glutamate release throughY2 receptors in rat hippocampal slices in vitro. Br J Pharmacol. 1994;113:737–740. doi: 10.1111/j.1476-5381.1994.tb17055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, McLeod S, Brot M, Heinrichs SC, Menzaghi F, Koob GF, Britton KT. Anxiolytic-like action of neuropeptide Y: mediation by Y1 receptors in amygdala, and dissociation from food intake effects. Neuropsychopharmacology. 1993;8:357–363. doi: 10.1038/npp.1993.35. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Thorsell A, Heilig M. Suppression of ethanol self-administration by the neuropeptide Y (NPY) Y2 receptor antagonist BIIE0246: evidence for sensitization in rats with a history of dependence. Neurosci Lett. 2005;375:129–133. doi: 10.1016/j.neulet.2004.10.084. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Schober DA, Smiley DL, Gehlert DR. NeuropeptideY-Y2 receptors mediate anxiety in the amygdala. Pharmacol Biochem Behav. 2002;71:419–423. doi: 10.1016/s0091-3057(01)00679-7. [DOI] [PubMed] [Google Scholar]
- Shoblock JR, Welty N, Nepomuceno D, Lord B, Aluisio L, Fraser I, Motley ST, Sutton SW, Morton K, Galici R, Atack JR, Dvorak L, Swanson DM, Carruthers NI, Dvorak C, Lovenberg TW, Bonaventure P. In vitro and in vivo characterization of JNJ-31020028 (N-(4-{4-[2-(diethylamino)-2-oxo-1-phenylethyl]piperazin-1-yl}-3-fluorophenyl)-2-pyridin-3-ylbenzamide), a selective brain penetrant small molecule antagonist of the neuropeptide Y Y(2) receptor. Psychopharmacology. 2010;208:265–277. doi: 10.1007/s00213-009-1726-x. [DOI] [PubMed] [Google Scholar]
- Tasan RO, Nguyen NK, Weger S, Sartori SB, Singewald N, Heilbronn R, Herzog H, Sperk G. The central and basolateral amygdala are critical sites of neuropeptide Y/Y2 receptor-mediated regulation of anxiety and depression. J Neurosci. 2010;30:6282–6290. doi: 10.1523/JNEUROSCI.0430-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]