Abstract
Background
Alcohol use occurs across the lifespan beginning in adolescence and continuing through adulthood. Ethanol-induced pathology varies with age and includes changes in neurogenesis, neurodegeneration, and glial cell activation. Ethanol-induced changes in glial activation and immune activity are believed to contribute to ethanol-induced neuropathology. Recent studies indicate an emerging role of glial-derived neuroimmune molecules in alcohol abuse and addiction.
Methods
Adolescent and adult C57BL/6 mice were treated via gavage with 6 g/kg ethanol for 10 days and tissue was harvested one day post-treatment. We compared the effects of ethanol on chemokine and cytokine expression and astrocyte GFAP immunostaining and morphology in the hippocampus, cerebellum, and cerebral cortex.
Results
Ethanol increased mRNA levels of the chemokine CCL2/MCP-1 in all three regions of adult mice relative to controls. The cytokine IL-6 was selectively increased only in the adult cerebellum. Ethanol did not affect mRNA levels of the cytokine TNF-α in any of these brain regions in adult animals. Interestingly, CCL2, IL-6, and TNF-α mRNA levels were not increased in the hippocampus, cerebellum, or cortex of adolescent mice. Ethanol treatment of adult and adolescent mice resulted in increased GFAP immunostaining.
Conclusions
Collectively, these data indicate an age- and region-specific susceptibility to ethanol regulation of neuroinflammatory and addiction-related molecules as well as astrocyte phenotype. These studies may have important implications concerning differential alcohol-induced neuropathology and alcohol addiction across the lifespan.
Keywords: Adolescent, Adult, Neuroinflammation, Astrocyte, CCL2
INTRODUCTION
Alcohol has profound effects on the central nervous system (CNS) of adolescents and adults. However, the specific effects of alcohol at these distinct life stages have not been fully characterized. A high percentage of adolescents experiment with alcohol and alcohol consumption by this population is characterized by heavy or binge drinking (SAMHSA, 2012). These phenomena are of particular concern because neuronal plasticity, synaptic remodeling, neurotransmission, and neurogenesis are highly active, alcohol-sensitive processes in the adolescent brain as demonstrated in animal models (Carpenter-Hyland and Chandler, 2007; Crews and Nixon, 2009; Pascual et al., 2009; Sabeti and Gruol, 2008). Alcohol disruption of these processes likely underlies the fact that adolescent drinking leads to increased incidence of alcohol abuse and addiction in adulthood (Alaux-Cantin et al., 2012; Hingson et al., 2006; Maldonado-Devincci et al., 2010). Further, alcohol produces less aversive effects in adolescents including less sedation, lower motor impairment, and reduced withdrawal symptoms paired with greater rewarding effects (Moy et al., 1998; Silveri and Spear, 1998; Van Skike et al., 2010; White et al., 2002). These altered consequences in adolescents may underlie the increased alcohol consumption and binge drinking behaviors seen in this population (Nixon and McClain, 2010; Spear, 2011). Although chronic alcohol use, alcohol abuse, and alcoholism occur at concerning levels in adults (SAMHSA, 2012) and are associated with marked alcohol neuropathology (Harper, 2009), the mature adult brain is a different environment than the adolescent brain and, thus, may be vulnerable to alcohol pathogenesis in unique ways compared to adolescents. Because of the fundamental differences in adolescent and adult brain, it is important to identify the neuropathological, cellular, and molecular consequences of drinking that differ at these distinct life stages.
Alcohol abuse results in increased immune activity in the CNS, which has been suggested to contribute to neurodegeneration and impaired neurological function associated with excessive alcohol consumption. Seminal studies demonstrated that alcohol increases the production of pro-inflammatory molecules including cytokines, chemokines, nitric oxide, and cyclooxygenase-2 in the CNS resulting in neuroinflammation and oxidative stress, which may lead to neuron cell death (Crews et al., 2006; Davis and Syapin, 2004; Knapp and Crews, 1999). More recently, pro-inflammatory molecules including cytokines and chemokines have been shown to affect alcohol consumption and addiction, resulting in a paradigm shift concerning the role of immune molecules in alcohol use disorders (Cui et al., 2011).
Astrocytes play a critical role in maintaining the health of the CNS by maintaining ionic homeostasis, regulating energy metabolism and synaptic transmission, removing neurotoxic molecules, and producing neurotrophic factors. However, astrocytes also function as resident immune cells in the brain and mediate innate immune responses in the CNS parenchyma. Upon activation, astrocytes produce cytokines, chemokines, nitric oxide, and other reactive oxygen species, all of which can induce an inflammatory response and damage neurons (Ransohoff and Brown, 2012). Importantly, alcohol induction of neuroinflammation is believed to contribute to alcohol-induced neuropathology. In addition, proinflammatory molecules including CCL2, which is highly expressed by astrocytes, have been demonstrated to alter addiction to alcohol (Blednov et al., 2005). Thus, alcohol- induced neuropathology may result, in part, from alcohol effects on astrocyte function.
This study was designed to examine the response of cytokines and chemokines and changes in astrocyte phenotype in different regions of the adolescent and adult mouse brain following alcohol exposure. Gene expression and astrocyte GFAP immunohistochemistry were analyzed in the hippocampus, cerebellum, and cerebral cortex, which are brain regions known to be highly susceptible to the behavioral and pathological effects of alcohol. Collectively, these brain regions play critical roles in synaptic plasticity, memory and cognition, motor function, emotion, and addiction, all of which are altered by excessive alcohol consumption (Alfonso-Loeches and Guerri, 2011; Nixon and McClain, 2010; Zahr et al., 2010b). In order to discern distinctions between ages or between brain regions, we employed a model of alcohol exposure that produces less than maximal changes in the measured endpoints. Our studies indicate that ethanol differentially alters immune activity and astrocyte phenotype in an age- and region-specific manner in vivo in the mouse brain. These findings may have important implications concerning alcohol induced neuropathology and addiction.
MATERIALS AND METHODS
Animal treatment
C57BL/6 mice were obtained from Jackson Laboratories and maintained as a breeding colony in the Division of Laboratory Animal Medicine at the University of Arkansas for Medical Sciences. Animal protocols were approved by the Institutional Animal Care and Use Committee. Weaned mice were used as either adolescents 35–46 days of age or adults 84–120 days of age. Mice were treated with water (vehicle control) or ethanol (15% w/v diluted from 95% v/v ethanol) by gavage for 10 days. Ethanol was administered as 6 g/kg/day in two doses separated by 7h. Tissue was harvested 1 day after the final ethanol treatment. Blood ethanol concentrations were evaluated on the first and last day of treatment on a separate set of adolescent (Day 1: n = 8, 3 female and 5 male; Day 10: n = 9, 5 female and 4 male) and adult (Day 1: n = 12, 5 female and 7 male; Day 10: n = 11, 4 female and 7 male) animals 1h following the second daily administration of ethanol using an Analox AM1 Alcohol Analyzer as described by the manufacturer (Analox, Huntington Beach, CA).
RNA isolation and cDNA synthesis
Animals (n = 37, 10 female and 27 male) were anesthetized with isoflurane and perfused transcardially with heparinized phosphate buffered saline to remove blood from the vasculature within the brain. Fresh brain tissue was harvested, and the hippocampus, cerebellum, and cerebral cortex were dissected and flash frozen in liquid nitrogen. Tissue was homogenized with a BBX24B Bullet Blender Blue homogenizer using 0.5 mm RNAse-free beads (Next Advance, Averill Park, NY). RNA was isolated using an RNeasy Lipid Tissue Mini Kit (Qiagen, Valencia, CA). RNA concentration and integrity was evaluated using an Agilent 2100 bioanalyzer and associated RNA 6000 Nano kit (Agilent Technologies, Santa Clara, CA) and RIN values were greater than 8. RNA samples were treated with DNAseI (Invitrogen, Grand Island, NY), and cDNA was prepared using an iScriptTM cDNA synthesis kit as described by the manufacturer (Bio-Rad, Hercules, CA).
Real-time polymerase chain reaction
CCL2, IL-6, and TNF-α mRNAs were quantified by real time polymerase chain reaction (rtPCR) using a CFX96 Real-time PCR Detection System (Bio-Rad, Hercules, CA). TaqMan primers were synthesized by Applied Biosystems (Foster City, CA). PCR was performed in duplicate 20 µl reactions containing SosFast™ probes supermix (Bio-Rad). Data were calculated as the mean ∆Ct relative to the gene β-actin. The ΔΔCt method was used to generate fold expression variance of ethanol versus vehicle groups. Variance in gene expression between age groups was analyzed by multivariate ANOVA. In addition, a priori planned comparisons were performed with Student’s t-tests between treatment groups in each region within each age group.
Tissue preparation and immunostaining
Three animals per treatment per age group (n = 4 female and 8 male) were anesthetized with isoflurane and transcardially perfused with heparinized phosphate buffered saline followed by phosphate buffered 4% paraformaldehyde fixative. The brains were removed and stored in fixative until sectioning. Serial 25 µm parasagittal sections were cut using a Leica VT1200S vibrating blade microtome and collected in phosphate buffer containing 0.05% sodium azide. Alternate sections were treated with a 1:1 methanol: 0.3% H2O2 solution for 17 min to block endogenous peroxidase. The sections were rinsed and placed in phosphate buffered saline (PBS) containing 9% normal goat serum (Vector Laboratories, Burlingame, CA) and 0.3% Triton X solution for 30 minutes. Sections were incubated with rabbit anti-cow GFAP primary antibody (Dako, Carpinteria, CA) prepared in PBS containing 3% normal goat serum and 0.3% Triton-X solution at a 1:20,000 dilution for 23h at room temperature. The sections were rinsed and incubated with sheep anti-rabbit secondary antibody (Antibodies, Inc., Davis, CA) at a 1:600 dilution in PBS containing 3% normal goat serum and 0.3% Triton-X for 1 h at room temperature, rinsed and incubated in rabbit peroxidase anti-peroxidase (MP Biomedicals, Santa Ana, CA) at a 1:1000 dilution in a PBS containing 3% normal goat serum and 0.3% Triton-X solution for 1h at room temperature. Sections were processed for 20 minutes with diaminobenzidine (0.5 mg/ml) for peroxidase detection.
Photomicrographs of hippocampus, cerebellum, and cerebral cortex were obtained using either a CoolSNAPcf or CoolSNAPES digital camera attached to an Olympus BX51 microscope and Metamorph® imaging software (Molecular Devices, Sunnyvale, CA). Quantitative analyses were performed using the same software with measurement of staining intensity in the hippocampus, hippocampal CA1 region, vermal lobule X in the cerebellum, and GFAP-positive cell clusters in the cerebral cortex. Single cell staining intensity, cell soma size, and the territory occupied by single cells as defined by a perimeter of the ends of the cell processes was quantified in the CA1 region and in cortical cell clusters; the cytoarchitecture of the cerebellum did not permit meaningful single-cell level measurements. A priori planned statistical comparison was performed in each region with Student’s t-tests of ethanol- and vehicle-treated animals at each age.
RESULTS
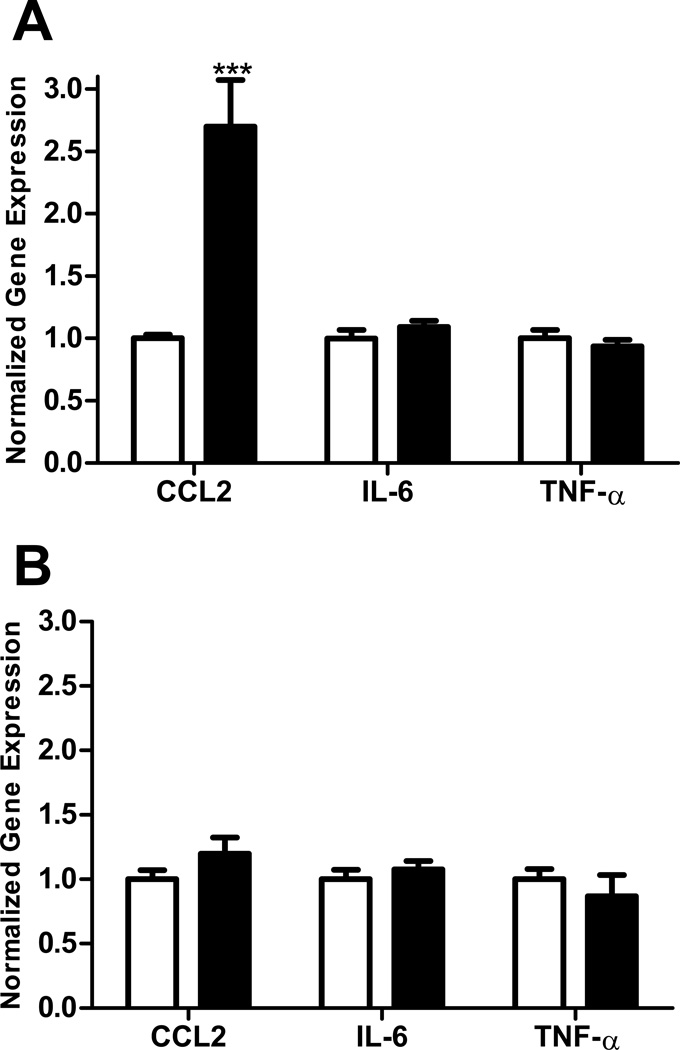
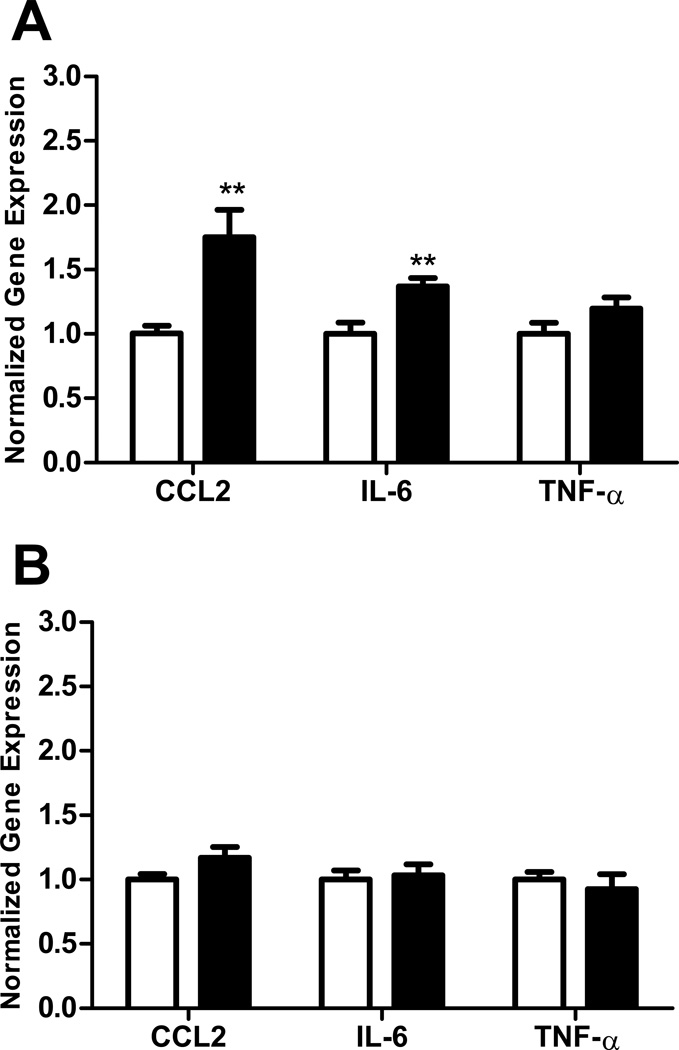
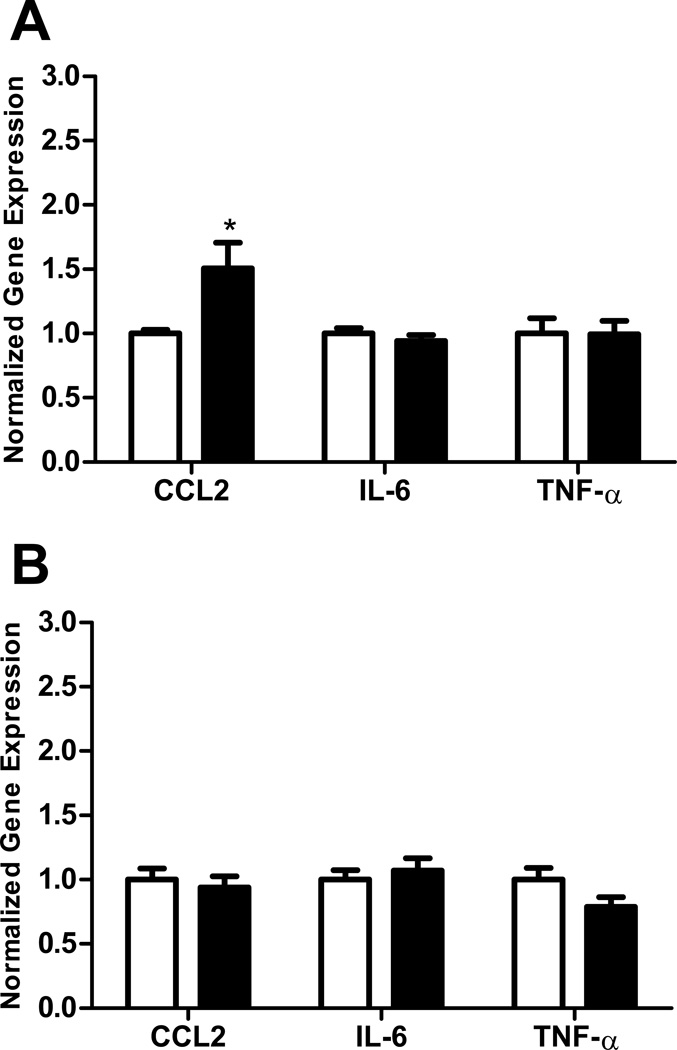
In order to compare the effects of ethanol on immune response in adolescent versus adult brain regions, we employed a model in which the different aged mice were exposed to the same dose of ethanol for the same period of time. The peak blood ethanol concentration was 221 +/− 12 mg/dl (mean +/− s.e.m) in adolescent and 244 +/− 11 mg/dl in adult animals on the first day of treatment and 292 +/− 14 mg/dl in adolescent and 290 +/− 16 mg/dl in adult animals on the last day of treatment. There was not a significant difference in the blood ethanol concentration between adult and adolescent animals on either day of treatment. This model resulted in a moderate immune response in the CNS. In these studies, ethanol increased the expression of the chemokine CCL2 in the hippocampus of adult (p<0.001, Figure 1A) but not adolescent (Figure 1B) mice. However, ethanol did not significantly increase the expression of mRNAs encoding the cytokines IL-6 or TNF-α in the hippocampus of adult or adolescent mice. In the adult cerebellum, ethanol increased the expression of CCL2 and IL-6 (both p<0.01), but not TNF-α, mRNA (Figure 2A). In contrast, ethanol did not alter the expression of CCL2, IL-6, or TNF-α mRNA in the adolescent cerebellum (Figure 2B). In the cerebral cortex, ethanol increased CCL2 expression in adult mice (p<0.05, Figure 3A), but not adolescent mice (Figure 3B). Ethanol did not increase IL-6 or TNF-α expression in adult or adolescent cortex. In addition to the a priori comparisons within age and region, multivariate ANOVA analyses without regard to age indicated that expression of CCL2 and IL-6 were significantly different in the cerebellum {[F(1,34)=7.36, p=0.01] and [F(1,34)=6.01, p=0.02] respectively} as a result of ethanol treatment. Expression of CCL2 was also significantly different in the hippocampus [F(1,32)=10.47, p=0.003] as a result of ethanol treatment.
Figure 1.
Effect of ethanol on CCL2, IL-6, and TNF-α expression in adult and adolescent mouse hippocampus. Animals were given 6.0 g/kg/day ethanol split into two doses and administered 7 h apart. Ethanol was administered for 10 days and mice were sacrificed 24h after the final dose of ethanol. Water was administered to vehicle mice. The hippocampus was isolated, RNA prepared, cDNA synthesized, and mRNA levels were determined by real-time quantitative RT-PCR. Results are expressed as fold changes relative to vehicle treated mice, and all values are normalized against β-actin. Values are mean +/− SEM from 10 vehicle and 13 ethanol treated adult mice (A) or from 8 vehicle and 6 ethanol treated adolescent mice (B). Duplicate PCR reactions were performed on each sample. Open bars represent vehicle-treated mice. Solid bars represent ethanol-treated mice. ***, P<0.001 versus vehicle treated mice.
Figure 2.
Effect of ethanol on CCL2, IL-6, and TNF-α expression in adult and adolescent mouse cerebellum. Animals were treated and samples analyzed as described in Figure 1. Adult animals (A). Adolescent animals (B). Open bars represent vehicle-treated animals. Solid bars represent ethanol-treated animals. Results are expressed as fold changes relative to vehicle treated mice, and all values are normalized against β-actin. Values are mean +/− SEM from 10 vehicle and 13 ethanol treated adult mice (A) or from 8 vehicle and 6 ethanol treated adolescent mice (B). Duplicate PCR reactions were performed on each sample. **, P<0.01, versus vehicle treated mice.
Figure 3.
Effect of ethanol on CCL2, IL-6, and TNF-α expression in adult and adolescent mouse cerebral cortex. Animals were treated and samples analyzed as described in Figure 1. Adult animals (A). Adolescent animals (B). Open bars represent vehicle-treated animals. Solid bars represent ethanol-treated animals. Results are expressed as fold changes relative to vehicle treated mice, and all values are normalized against β-actin. Values are mean +/− SEM from 10 vehicle and 13 ethanol treated adult mice (A) or from 8 vehicle and 6 ethanol treated adolescent mice (B). Duplicate PCR reactions were performed on each sample. *, P<0.05, versus vehicle treated mice.
Collectively, analysis of gene expression indicated that ethanol differentially altered the production of pro-inflammatory cytokines and chemokines in an age dependent manner. Ethanol selectively increased the production of these pro-inflammatory molecules in adult but not adolescent mice in this experimental model. In addition, ethanol differentially increased the expression of these pro-inflammatory molecules in distinct brain regions of adult mice, with CCL2 increased in the hippocampus, cerebellum, and cerebral cortex, but IL-6 increased only in the cerebellum. Furthermore, the expression of a given inflammatory molecule varied between the brain regions investigated, with stronger ethanol induction of CCL2 in the hippocampus, followed by the cerebellum, and then the cerebral cortex.
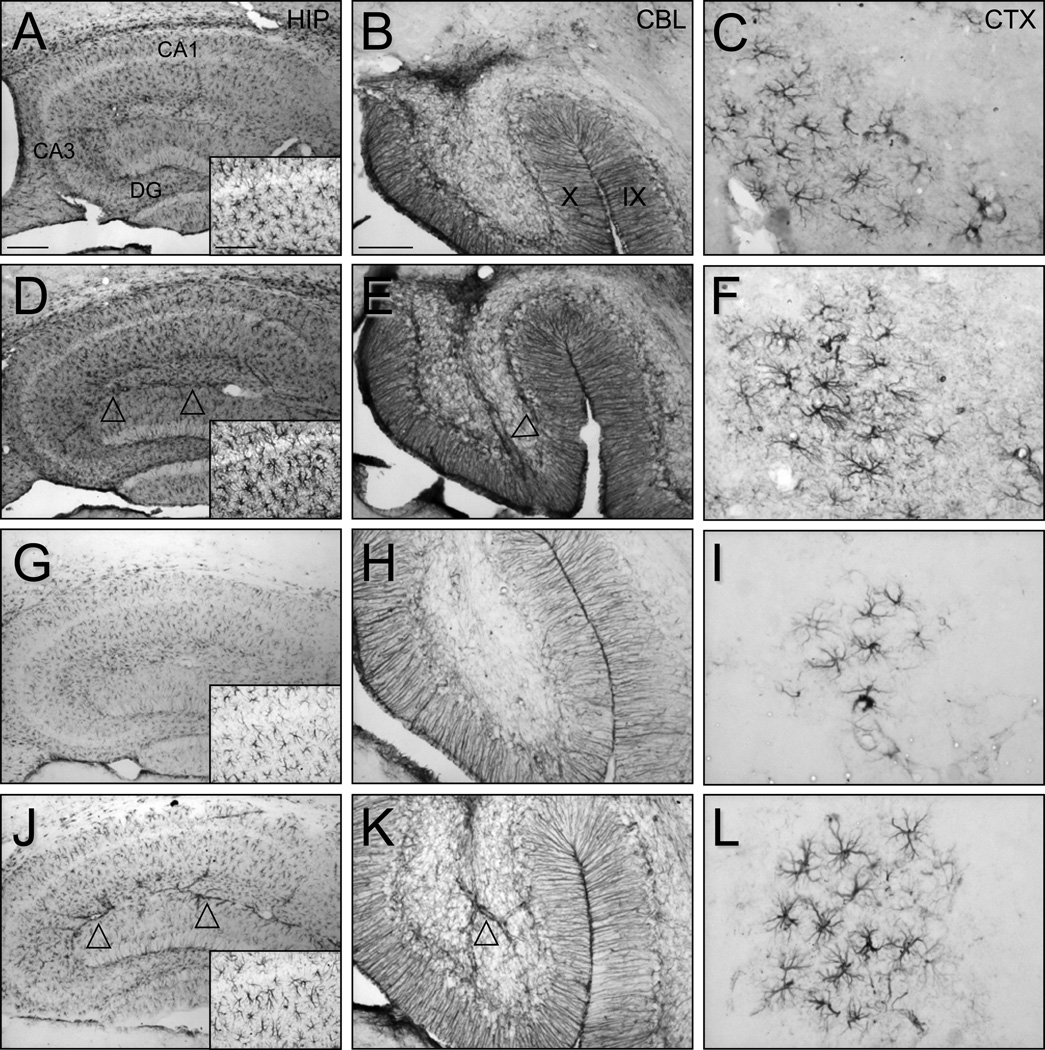
Our studies indicate that the adult brain is more sensitive to immune activation by ethanol than the adolescent brain. For example, CCL2 and IL-6 were increased in response to ethanol in adult, but not adolescent mice. Since these molecules are highly expressed in astrocytes, we investigated the effects of ethanol on astrocytes in different brain regions of adult and adolescent mice. In these studies, astrocytes in ethanol-treated adult animals demonstrated an apparent increase in the intensity of GFAP immunostaining in the hippocampus, cerebellum, and cerebral cortex relative to vehicle-treated control animals (Figure 4). With an increase in GFAP staining, the size of the astrocyte cell soma and the width and length of the stained processes appeared to be greater in ethanol-treated adult animals. Quantitative analyses of the animals illustrated in Figure 4 demonstrated that the intensity of staining in the whole hippocampus (109.7 ± 7.2% of vehicle, mean ± SD, p<0.01) as well as the CA1 region (108.9 ± 8.2%, p<0.01) was greater as a result of ethanol treatment. The intensity of single cells (126.3 ± 4.0%), the size of the cell body (139.2 ± 40.0%), and the territory of single cells and their processes (117.4 ± 27.2%) was increased (all p<0.001) in the hippocampal CA1 region following ethanol treatment. Analysis of the cell clusters of GFAP stained astrocytes in the cerebral cortex revealed an increase in the intensity of cell cluster staining (112.2 ± 11.1%, p<0.05) and the intensity of single cells (106.1 ± 5.8%, p<0.001). In the cerebellum, the intensity of GFAP staining was qualitatively greater in the vermis (Figure 4) and deep cerebellar nuclei (data not shown) following ethanol treatment but quantification did not reveal a statistically significant difference.
Figure 4.
Effect of ethanol on GFAP immunostaining and astrocyte morphology in adult and adolescent mice. Photomicrographs illustrate the appearance of GFAP stained astrocytes in vehicle (A-C, G-I) and ethanol (D-F, J-L) treated adult (A-F) and adolescent (G-L) mice. Arrowheads denote ethanol induced increases in GFAP staining of astrocytes associated with larger vascular elements. Inset: astrocytes in the hippocampal CA1 region. Animals were treated as described in Figure 1. Scale bars in A represent 200 um and 100 um (inset) and apply to all hippocampal images. Scale bar in B represents 100 um in B and E and 50 um in H and K and all cerebral cortex images. Hippocampus (HIP), hippocampal field CA1 (CA1), hippocampal field CA3 (CA3), dentate gyrus (DG), cerebellum (CBL), cerebral cortex (CTX).
Parallel analysis of GFAP immunostaining was performed in adolescent animals treated with vehicle or ethanol. Overall, astrocyte GFAP staining was less in adolescent animals compared to adults, including less staining in adolescent than adult vehicle-treated controls. However, similar to adults, ethanol treatment of adolescent animals generated an apparent increase in the intensity of GFAP staining relative to vehicle-treated adolescents. This ethanol-induced increase in staining intensity in adolescent animals illustrated in Figure 4 was observed in all three brain regions investigated (whole hippocampus: 106.2 ± 4.1% of vehicle, mean ± SD, p<0.01; hippocampal CA1: 105.9 ± 1.5%, p<0.001; cerebellum: 117.2 ± 8.8%, p<0.001). An increase in the intensity of single cells was observed in CA1 (103.7 ± 6.1%, p<0.001), as in adults. In adolescent animals, the number of cerebral cell clusters was too low to compare intensity quantitatively but qualitative analysis of the clusters indicated increased astrocyte staining following ethanol treatment. An increase in the size of the cell soma and the territory of single cells and their processes as observed in the adult animals was not evident in adolescent animals in any region investigated. One of the most striking observations in adolescent animals compared to adults was an increase in GFAP immunostaining and morphological alterations of astrocytes associated with and resident near blood vessels (Figure 4, arrowheads). Thus, in both adult and adolescent animals, GFAP immunostaining was increased by ethanol in each of the brain regions investigated. However, ethanol induced changes in astrocyte morphology in the hippocampal CA1 region in adult but not adolescent animals. The increased GFAP expression evidenced by increased immunostaining coupled with the nature of the alterations in astrocyte morphology suggested that ethanol partially activated astrocytes in this experimental paradigm.
DISCUSSION
Alcohol is known to have profound effects on the immune system. For example, human alcoholics exhibit a compromised immune system and are prone to infections. Interestingly, in spite of being immunocompromised, alcoholics have been shown to have increased serum levels of pro-inflammatory cytokines (Achur et al., 2010), likely due to ethanol-induced damage to and inflammation of the liver (Goral et al., 2008). These pro-inflammatory cytokines increase craving for alcohol, which contributes to the vicious cycle of alcohol addiction (Kiefer et al., 2002). While the effects of alcohol on immune response in peripheral tissues are well established, relatively little is known concerning alcohol effects on the CNS (reviewed in Blanco and Guerri, 2007; Crews et al., 2006; Crews and Nixon, 2009). Alcoholics commonly exhibit impaired neurologic function and neurodegeneration, and CNS inflammation has been suggested to contribute to these pathologies. However, it should be noted that CNS inflammation has not definitively been shown to contribute to ethanol-induced neurodegeneration. The effects of ethanol on the CNS are complex and appear to vary depending on the peak blood ethanol concentration, treatment paradigm (including length of treatment and the presence or absence of a withdrawal period following ethanol administration), and the age of the animal (reviewed in Drew and Kane, 2013). A short term 4-day binge ethanol treatment did not result in CNS inflammation in rats, for example (Zahr et al., 2010a). In contrast, and although it varied from study to study, long-term acute or chronic ethanol exposure resulted in glial activation and production of inflammatory molecules such as nitric oxide, cyclooxygenase-2, cytokines including TNF-α and IL-1β, and chemokines including CCL2, MIP-1α/CCL3, and MIP-1β/CCL4 (Alfonso-Loeches et al., 2010; He and Crews, 2008; Pascual et al., 2009; Qin et al., 2008).
The present study demonstrated that ethanol increased the expression of CCL2 and IL-6 in the adult but not the adolescent CNS. These molecules are commonly expressed by activated astrocytes. Ethanol also increased the expression of GFAP in the hippocampus, cerebellum, and cerebral cortex of adult and adolescent mice with alterations in astrocyte morphology in the hippocampus of adult but not adolescent mice. Previous studies indicated that ethanol administered to 70 day-old adult rats at 7–13g/kg/day for 4 consecutive days in a binge model increased expression of the radial glia marker vimentin at 4 and 7 days post ethanol exposure. Vimentin-positive cells were also positive for GFAP (Kelso et al., 2011), which is consistent with the current observations of GFAP immunostained astrocytes. In composite, our molecular and immunohistochemical studies and those of others indicate that ethanol can activate astrocytes, either partially or fully, depending on the treatment paradigm as seen by morphological changes and increase in pro-inflammatory molecules.
Recent studies have demonstrated a link between the expression of pro-inflammatory molecules in the CNS and addiction to alcohol. This leads to the suggestion that immunosuppressive agents could be effective in the treatment of alcohol addiction (Cui et al., 2011). Our current studies demonstrated that ethanol increased CCL2 chemokine expression in the adult mouse hippocampus, cerebellum, and cerebral cortex and increased IL-6 cytokine expression in the adult cerebellum. In human studies, CCL2 was demonstrated to be elevated in the hippocampus, amygdala, substantia nigra, and ventral tegmental regions of the brains of alcoholics relative to controls (He and Crews, 2008). Treatment of adult mice with ethanol was shown to increase CCL2 expression in whole brain (Qin et al., 2008). Studies utilizing array technologies have indicated that alcohol preferring strains of mice exhibited increased expression of a variety of immune related molecules in the CNS (Mulligan et al., 2006). Recent studies have begun to evaluate drinking behavior in animals in which genes encoding immune molecules have been selectively deleted. These studies support a role of immune molecules including CCL2 and IL-6 in contributing to alcohol consumption (Blednov et al., 2005; 2012). In addition, immune molecules are likely to trigger additional alcohol consumption by increasing anxiety during alcohol withdrawal. Collectively, these studies suggest that ethanol induces neuroinflammation and that inflammatory molecules including CCL2 and IL-6 play a role in alcohol consumption and addiction. Alternatively, it is possible that these chemokines and cytokines may influence or control drinking and addiction independent of their role as neuroinflammatory factors.
One of the major purposes of the current study was to compare the effects of ethanol on immune activity in the CNS of adolescent versus adult mice, which has not been previously evaluated in a comparative study. In order to address this question, mice were treated with the same amount of ethanol using the same experimental treatment paradigm, which resulted in a moderate increase in pro-inflammatory CCL2 and IL6, allowing direct comparison between adolescent and adult animals. Comparison of alcohol effects on the CNS in adolescents and adults is important because adolescents exhibit decreased sedation and motor impairment relative to adults in response to ethanol (Silveri and Spear, 1998; Van Skike et al., 2010). Alcohol abuse in adolescents has lasting effects on the brain and behavior (Maldonado-Devincci et al., 2010; Nixon and McClain, 2010). Thus it is critical to understand the effects of ethanol on the adolescent brain which is undergoing developmental processes including higher levels of neurogenesis than adults. That being said, our data indicate that adult mice were more susceptible to ethanol induced increases in CCL2 and IL-6 in the CNS than adolescent mice. In addition, although astrocyte GFAP immunostaining increased in both adult and adolescent mice, changes in astrocyte morphology were only significant in the adult. Thus, there is a distinction in adolescent animals between ethanol effects on expression of CCL2 and IL-6 and astrocyte morphology. This may be important in interpretation of the current data. Further, it indicates that the non-immune impact of changes in CCL2 expression, IL-6 expression, GFAP immunostaining, and morphological astrocyte responses must be considered. Studies investigating the effects of ethanol on immune activation in the adolescent CNS are limited but include the observation that ethanol partially activates microglial cells in the adolescent rat (McClain et al., 2011). The effects of ethanol on immune activation in the adult brain have been more extensively studied and the results vary significantly, likely due to differences in the experimental paradigms employed. For example, a short term acute model of ethanol exposure did not result in production of immune molecules in the adult CNS (Zahr et al., 2010a), while longer term acute or chronic ethanol exposure resulted in the CNS production of pro-inflammatory molecules (Alfonso-Loeches et al., 2010; Pascual et al., 2009; Qin et al., 2008). Interestingly, our studies demonstrating an absence of CCL2 induction by ethanol in the adolescent brain may contribute to increased vulnerability of the adolescent CNS to ethanol neuropathology, behavioral dysfunction, and risk of addiction as CCL2 has been shown to be protective against ethanol effects in some models (Bray et al., 2013).
Another major comparison in our study was to determine whether there were region-specific changes in the expression of pro-inflammatory molecules and astrocyte activation. To this end, we examined expression of CCL2, IL-6, and TNF-α, astrocyte GFAP immunostaining, and astrocyte morphology in the hippocampus, cerebellum, and cerebral cortex. These tissues were selected because of their established sensitivity to ethanol neuropathology, their role in mediating the neurocognitive, neuropsychological, and motor deficits caused by ethanol, and their role in the neurobiology of alcohol abuse and addiction. It is well documented that acute or chronic ethanol consumption or ethanol withdrawal causes structural and functional damage in these three regions (Alfonso-Loeches and Guerri, 2011). Different regions of the brain respond differently to chronic alcohol consumption in humans based on proteomic analysis of gene expression profiles (Matsumoto, 2009). Limbic regions including the hippocampus are involved in ethanol induced deficits in learning, memory, motor performance, and alcohol seeking behavior (Janak and Chaudhri, 2010). Hippocampal neurogenesis is linked to susceptibility to alcohol use disorders (Nixon et al., 2010). The cerebral cortex and cerebellum mediate the ataxic, sedative, and hypnotic effects of ethanol, impacting ethanol intoxication and preference. Ethanol disrupts balance, posture, motor coordination, and cognition which are mediated by the cerebellum. As observed in this study, increases in CCL2 and IL-6 as well as changes in GFAP immunostained astrocytes in all three of these regions may reflect a role for these molecules as well as astrocyte phenotype in particular, or perhaps neuroinflammatory processes more broadly, in ethanol neuropathology, deficits in cognition and behavior, and alcohol addiction.
The current studies evaluated the effects of ethanol on immune activation in the CNS of adolescent and adult mice. The studies demonstrated that adult mice were more susceptible to immune activation by ethanol than adolescent mice. The studies further demonstrated that ethanol differentially increased the production of pro-inflammatory molecules in the hippocampus, cerebellum, and cerebral cortex of adult mice, increased the intensity of astrocyte GFAP immunostaining, and altered astrocyte morphology in these tissues suggesting that ethanol partially activated astrocytes. These studies provide important insights concerning the potential role of ethanol induced neuroinflammation in modulating neurodegeneration and addiction to alcohol across the lifespan.
ACKNOWLEDGMENTS
This work was supported by NIH grants AA18834, AA18839, and AA19108.
REFERENCES
- Achur RN, Freeman WM, Vrana KE. Circulating cytokines as biomarkers of alcohol abuse and alcoholism. J Neuroimmune Pharmacol. 2010;5:83–91. doi: 10.1007/s11481-009-9185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaux-Cantin S, Warnault V, Legastelois R, Botia B, Pierrefiche O, Vilpoux C, Naassila M. Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology. 2012;67C:521–531. doi: 10.1016/j.neuropharm.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Guerri C. Molecular and behavioral aspects of the actions of alcohol on the adult and developing brain. Crit Rev Clin Lab Sci. 2011;48:19–47. doi: 10.3109/10408363.2011.580567. [DOI] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J Neurosci. 2010;30:8285–8295. doi: 10.1523/JNEUROSCI.0976-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco AM, Guerri C. Ethanol intake enhances inflammatory mediators in brain: role of glial cells and TLR4/IL-1RI receptors. Front Biosci. 2007;12:2616–2630. doi: 10.2741/2259. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Bergeson SE, Walker D, Ferreira VM, Kuziel WA, Harris RA. Perturbation of chemokine networks by gene deletion alters the reinforcing actions of ethanol. Behav Brain Res. 2005;165:110–125. doi: 10.1016/j.bbr.2005.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Ponomarev I, Geil C, Bergeson S, Koob GF, Harris RA. Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies. Addict Biol. 2012;17:108–120. doi: 10.1111/j.1369-1600.2010.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray JG, Reyes KC, Roberts AJ, Ransohoff RM, Gruol DL. Synaptic plasticity in the hippocampus shows resistance to acute ethanol exposure in transgenic mice with astrocyte-targeted enhanced CCL2 expression. Neuropharmacology. 2013;67:115–125. doi: 10.1016/j.neuropharm.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Chandler LJ. Adaptive plasticity of NMDA receptors and dendritic spines: implications for enhanced vulnerability of the adolescent brain to alcohol addiction. Pharmacol Biochem Behav. 2007;86:200–208. doi: 10.1016/j.pbb.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Bechara R, Brown LA, Guidot DM, Mandrekar P, Oak S, Qin L, Szabo G, Wheeler M, Zou J. Cytokines and alcohol. Alcohol Clin Exp Res. 2006;30:720–730. doi: 10.1111/j.1530-0277.2006.00084.x. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C, Grandison L, Noronha A. Neuroimmune mechanisms of brain function and alcohol related disorders. Brain Behav Immun. 2011;25(Suppl 1):S1–S3. doi: 10.1016/j.bbi.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Davis RL, Syapin PJ. Ethanol increases nuclear factor-kappa B activity in human astroglial cells. Neurosci Lett. 2004;371:128–132. doi: 10.1016/j.neulet.2004.08.051. [DOI] [PubMed] [Google Scholar]
- Drew PD, Kane CJM. Neuroimmune Mechanisms of Glia and Their Interplay with Alcohol Exposure Across the Lifespan. In: Cui CEA, editor. Neural-Immune Interactions in Brain Function and Alcohol Related Disorders. New York: Springer Science + Business Media; 2013. [Google Scholar]
- Goral J, Karavitis J, Kovacs EJ. Exposure-dependent effects of ethanol on the innate immune system. Alcohol. 2008;42:237–247. doi: 10.1016/j.alcohol.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C. The neuropathology of alcohol-related brain damage. Alcohol Alcohol. 2009;44:136–140. doi: 10.1093/alcalc/agn102. [DOI] [PubMed] [Google Scholar]
- He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol. 2008;210:349–358. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Winter MR. Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Arch Pediatr Adolesc Med. 2006;160:739–746. doi: 10.1001/archpedi.160.7.739. [DOI] [PubMed] [Google Scholar]
- Janak PH, Chaudhri N. The Potent Effect of Environmental Context on Relapse to Alcohol-Seeking After Extinction. Open Addict J. 2010;3:76–87. doi: 10.2174/1874941001003010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso ML, Liput DJ, Eaves DW, Nixon K. Upregulated vimentin suggests new areas of neurodegeneration in a model of an alcohol use disorder. Neuroscience. 2011;197:381–393. doi: 10.1016/j.neuroscience.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer F, Jahn H, Schick M, Wiedemann K. Alcohol intake, tumour necrosis factor-alpha, leptin and craving: factors of a possibly vicious circle? Alcohol Alcohol. 2002;37:401–404. doi: 10.1093/alcalc/37.4.401. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Crews FT. Induction of cyclooxygenase-2 in brain during acute and chronic ethanol treatment and ethanol withdrawal. Alcohol Clin Exp Res. 1999;23:633–643. [PubMed] [Google Scholar]
- Maldonado-Devincci AM, Alipour KK, Michael LA, Kirstein CL. Repeated binge ethanol administration during adolescence enhances voluntary sweetened ethanol intake in young adulthood in male and female rats. Pharmacol Biochem Behav. 2010;96:476–487. doi: 10.1016/j.pbb.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto I. Proteomics approach in the study of the pathophysiology of alcohol-related brain damage. Alcohol Alcohol. 2009;44:171–176. doi: 10.1093/alcalc/agn104. [DOI] [PubMed] [Google Scholar]
- McClain JA, Morris SA, Deeny MA, Marshall SA, Hayes DM, Kiser ZM, Nixon K. Adolescent binge alcohol exposure induces long-lasting partial activation of microglia. Brain Behav Immun. 2011;25(Suppl 1):S120–S128. doi: 10.1016/j.bbi.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Duncan GE, Knapp DJ, Breese GR. Sensitivity to ethanol across development in rats: comparison to [3H]zolpidem binding. Alcohol Clin Exp Res. 1998;22:1485–1492. [PubMed] [Google Scholar]
- Mulligan MK, Ponomarev I, Hitzemann RJ, Belknap JK, Tabakoff B, Harris RA, Crabbe JC, Blednov YA, Grahame NJ, Phillips TJ, Finn DA, Hoffman PL, Iyer VR, Koob GF, Bergeson SE. Toward understanding the genetics of alcohol drinking through transcriptome meta-analysis. Proc Natl Acad Sci U S A. 2006;103:6368–6373. doi: 10.1073/pnas.0510188103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K, Mcclain JA. Adolescence as a critical window for developing an alcohol use disorder: current findings in neuroscience. Curr Opin Psychiatry. 2010;23:227–232. doi: 10.1097/YCO.0b013e32833864fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K, Morris SA, Liput DJ, Kelso ML. Roles of neural stem cells and adult neurogenesis in adolescent alcohol use disorders. Alcohol. 2010;44:39–56. doi: 10.1016/j.alcohol.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem. 2009;108:920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Qin L, He J, Hanes RN, Pluzarev O, Hong JS, Crews FT. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflammation. 2008;5:10. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Brown MA. Innate immunity in the central nervous system. J Clin Invest. 2012;122:1164–1171. doi: 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeti J, Gruol DL. Emergence of NMDAR-independent long-term potentiation at hippocampal CA1 synapses following early adolescent exposure to chronic intermittent ethanol: role for sigma-receptors. Hippocampus. 2008;18:148–168. doi: 10.1002/hipo.20379. [DOI] [PubMed] [Google Scholar]
- SAMHSA. Substance Abuse and Mental Health Services Administration. Publication No. (SMA) 12-4725 ed. Rockville, MD: 2012. Results from the 2011 National Survey on Drug Use and Health: Mental Health Findings. [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Spear LP. Adolescent neurobehavioral characteristics, alcohol sensitivities, and intake: Setting the stage for alcohol use disorders? Child Dev Perspect. 2011;5:231–238. doi: 10.1111/j.1750-8606.2011.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Skike CE, Botta P, Chin VS, Tokunaga S, Mcdaniel JM, Venard J, Diaz-Granados JL, Valenzuela CF, Matthews DB. Behavioral effects of ethanol in cerebellum are age dependent: potential system and molecular mechanisms. Alcohol Clin Exp Res. 2010;34:2070–2080. doi: 10.1111/j.1530-0277.2010.01303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, Truesdale MC, Bae JG, Ahmad S, Wilson WA, Best PJ, Swartzwelder HS. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacol Biochem Behav. 2002;73:673–677. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]
- Zahr NM, Luong R, Sullivan EV, Pfefferbaum A. Measurement of serum, liver, and brain cytokine induction, thiamine levels, and hepatopathology in rats exposed to a 4-day alcohol binge protocol. Alcohol Clin Exp Res. 2010a;34:1858–1870. doi: 10.1111/j.1530-0277.2010.01274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Pitel AL, Chanraud S, Sullivan EV. Contributions of studies on alcohol use disorders to understanding cerebellar function. Neuropsychol Rev. 2010b;20:280–289. doi: 10.1007/s11065-010-9141-y. [DOI] [PMC free article] [PubMed] [Google Scholar]