Abstract
Infantile spasms are seizures manifesting in infantile epileptic encephalopathies that are associated with poor epilepsy and cognitive outcomes. The current therapies are not always effective or are associated with serious side effects. Early cessation of spasms has been proposed to improve long-term outcomes. To identify new therapies for infantile spasms with rapid suppression of spasms, we are using the multiple-hit rat model of infantile spasms, which is a model of refractory infantile spasms. Here, we are testing the efficacy and tolerability of a single dose of the galanin receptor 1 preferring analog, NAX 5055, in the multiple-hit model of spasms. To induce the model, postnatal day 3 (PN3) male Sprague-Dawley rats underwent right intracerebral infusions of doxorubicin and lipopolysaccharide; p-chlorophenylalanine was then injected intraperitoneally (i.p.) at PN5. After the onset of spasms at PN4, 11–14 rats/group were injected i.p. with either NAX 5055 (0.5, 1, 2, or 4 mg/kg) or vehicle. Video monitoring for spasms included a 1hour pre-injection period, followed by 5 hours of recording post-injection, and two 2 hour sessions on PN5. The study was conducted in a randomized, blinded manner. Neurodevelopmental reflexes were assessed daily as well as at 2 hours after injection. Respiratory function, heart rate, pulse distension, oximetry and blood glucose were measured 4 hours after injection. The relative expression of GalR1 and GalR2 mRNA over β-actin in the cerebral cortex and hippocampus was determined with real time reverse transcription polymerase chain reaction. There was no acute effect of NAX 5055 on spasm frequency after the single dose of NAX 5055 (n=11–13 rats/group, following exclusions). Neurodevelopmental reflexes, vital signs, blood glucose measured 4 hours post-injection, and survival were not affected. A reduction in pulse and breath distention of unclear clinical significance was observed with the 7mg/kg NAX 5055 dose. GalR1 mRNA was present in the cerebral cortex and hippocampus of PN4 and adult rats. The hippocampal –but not the cortical- GalR1 mRNA expression was significantly lower in PN4 pups than in adults. GalR1 mRNA was also at least 20 times less abundant in the PN4 cortex than GalR2 mRNA. In conclusion, a single dose of NAX 5055 has no acute efficacy on spasms or toxicity in the multiple hit rat model of medically refractory infantile spasms. Our findings cannot exclude the possibility that repetitive NAX 5055 administration may show efficacy on spasms. The higher expression of GalR2 in the PN4 cortex suggests that GalR2-preferring analogs may be of interest to test for efficacy on spasms.
Keywords: Antiepileptic, Galanin receptor, glucose, antibody, neurodevelopmental reflexes, cerebral cortex
Introduction
Infantile spasms (IS) occur as clusters of epileptic spasms in the context of epileptic encephalopathies of infancy. IS are often associated with poor outcomes, including intractable epilepsies and cognitive deficits (Pellock et al., 2010; Riikonen, 2001b). IS do not typically respond to the usual antiseizure drugs. The current treatments for IS include adrenocorticotropic hormone (ACTH) and vigabatrin, which are not always effective on spasms, may have serious side effects and may not improve significantly the associated cognitive decline (Darke et al., 2010; Lux et al., 2004; Riikonen, 2001a; 2001b). Unfortunately, only 54 - 87% of patients in various small cohorts of IS patients are free of spasms 14 days after introduction of appropriate treatments (Lux et al., 2004; Mackay et al., 2004; Pellock et al., 2010). IS due to structural/metabolic etiologies are more often drug-resistant (Riikonen, 2010), and cognitive outcome is usually worse than IS of unknown etiology (O’Callaghan et al., 2011). Early cessation of the spasms has been proposed to improve outcome, at least in patients with IS of unknown etiology (Darke et al., 2010; Kivity et al., 2004; Lombroso, 1983; O’Callaghan et al., 2011). New therapies are needed to provide faster and complete control of spasms, with the ultimate goal being the improvement of short and long-term epilepsy and cognitive outcomes.
Several acute and chronic rodent models of IS have been developed recently to elucidate the pathophysiology and facilitate the identification of new, improved therapies for IS (Chudomelova et al., 2010; Galanopoulou, 2013). Here, we are using the multiple-hit chronic rat model of IS which models IS due to a structural underlying lesion (Scantlebury et al., 2010). The multiple-hit model of IS reproduces an early life epileptic encephalopathy with age-specific expression of clusters of epileptic spasms (postnatal day (PN) 4–13), later appearance of other seizure types, and poor neurodevelopmental outcomes. In the multiple-hit model, spasms are refractory to chronic administration of ACTH and transiently sensitive to vigabatrin, which render this a model of drug-resistant IS (Scantlebury et al., 2010). The multiple-hit model of IS has been successfully used for the identification of new rapid-onset (carisbamate, rapamycin) and disease-modifying (pulse rapamycin) therapies (Ono et al., 2011; Raffo et al., 2011).
Galanin is an inhibitory neuropeptide that regulates feeding, reproduction, growth, pain and neuronal development via activation of the galanin receptors GalR1 and GalR2 (Abbott and Pilowsky, 2009; Melander et al., 1986), and inhibits glutamate release in the hippocampus (Mazarati et al., 2001; Mazarati et al., 2000). GalR agonists (Bartfai et al., 2004) or allosteric modulators (Lu et al., 2010), or virus-mediated galanin expression (Haberman et al., 2003) decrease seizure susceptibility and can be neuroprotective. Galanin analogs have demonstrated promising antiseizure properties in preclinical studies (Bulaj et al., 2008; White et al., 2009). NAX 5055 is a galanin analog with higher affinity for GalR1 than GalR2 (Bulaj et al., 2008; White et al., 2009) which showed promising antiseizure effects in models of seizures in adults: the Frings audiogenic seizure-susceptible mouse, the mouse corneal kindling model of partial epilepsy, and the 6 Hz corneal stimulation model, a model of drug-resistant epilepsy (White et al., 2009).
In this study, we test whether a single intraperitoneal (i.p.) injection of NAX 5055 given after the onset of spasms decreases acutely the frequency of spasms in the multiple-hit rat model of drug-resistant IS, using dose and time-response experiments. In parallel, we also assess the tolerability of NAX 5055 in developing rats, in regards to respiratory function, survival, daily weights, and glucose regulation, due to the reported effects of galanin on insulin secretion (Ruczynski et al., 2002; Verchere et al., 1992).
Materials and Methods
Animals
This study was done in litters of 10 male offspring of timed pregnant Sprague-Dawley rats (Taconic farms, Inc., Hudson, NY, USA). The day of birth was considered as PN0. Rat pups were kept with their dam in our animal facility at constant temperature (21–23°C) and humidity (40–60%), in a 12 hours dark/12 hours light cycle with access to water and food ad libitum, according to the guidelines of the American Association for the Accreditation of Laboratory Animal Care. All procedures and experiments were in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the Albert Einstein College of Medicine.
The multiple-hit model of IS
Induction of the multiple-hit model of IS requires stereotactic infusions of doxorubicin (5μg/2.5μl, right intracerebroventricular injection) and lipopolysaccharide (3μg/1.5μl, right intraparietal injection) at PN3 under isoflurane anesthesia, following the previously used coordinates (Raffo et al., 2011; Scantlebury et al., 2010). Doxorubicin: 2.68mm anterior to lambda; 1.1 mm lateral to sagittal suture; 3.3 mm deep; lipopolysaccharide: 2.55 mm anterior to lambda; 1 mm lateral to sagittal suture; 1.7 mm deep. The skin was closed with Vetbond (3 M, St Paul, MN) and protected with dental acrylic. P-chlorophenylalanine (PCPA) was injected intraperitoneally (i.p.) at PN5 in the morning.
Galanin Analog administration
NAX 5055 (Robertson et al., 2010; White et al., 2009) was kept at −20°C, light protected until use. 500μg of NAX 5055 were initially diluted in 10μl of dimethylsulfoxide, and subsequently was diluted at the working concentrations with the addition of 1% Tween 20 in normal saline, then left for 30min, light-protected at room temperature, prior to injections. Group assignment to five dose groups was done in a randomized fashion, by an investigator not involved in the animal handling, seizure scoring or outcome assessment. NAX 5055 and its vehicle were administered i.p. as single injections in the afternoon of PN4, one hour after starting the afternoon video monitoring session. We chose to administer NAX 5055 on PN4, so that it is given after the onset of spasms, similar to clinical practice. Each litter was randomized to include pups treated with vehicle as well as pups given each of the 3 different doses of NAX 5055. We used the following abbreviations for the groups of multiple-hit pups given NAX 5055 or its vehicle: NAX-0.5: 0.5 mg/kg; NAX-1: 1mg/kg, NAX-2: 2 mg/kg, NAX-4: 4 mg/kg and VEH: vehicle-injected group. Code names were used for the different treatment groups and the investigator injecting the drug or vehicle, scoring the spasms, and assessing outcomes was blinded to the treatment group. In addition, a dose of 7mg/kg NAX 5055 i.p. (NAX-7 dose group, n=6 rats) was tested for tolerability only in PN4 male pups treated according to the multiple-hit model.
Inclusion/exclusion criteria
Exclusion criteria were set a priori to exclude rats that were either (a) neglected by the dam, (b) had lesions extending to bilateral hemispheres, (c) did not express spasms prior to the time of drug or vehicle injection, or (d) died as a result of accident (i.e. mechanical injury from the injection or surgery-related trauma or death). Exclusions were made by an investigator blinded to group assignment, prior to the unblinding stage of the study.
Monitoring
At PN4, pups were separated for video monitoring as described in our previous studies (Ono et al., 2011; Raffo et al., 2011; Scantlebury et al., 2010). The single-injection monitoring session at PN4 consisted of one pre-injection and 5 post-injection hours, i.e., six hours total. At PN5, two 2-hour sessions were performed (morning and afternoon). Assessment of pre-injection spasm frequency was conducted during the one hour pre-injection monitoring. “Behavioral spasms” were considered the sudden, synchronous and high amplitude movements of all four limbs and body presenting as flexion, or extension or mixed flexion/extension events. Events that were associated with flexion or extension in an attempt to change position, or with sudden but asynchronous limb movements were not scored.
Weights and neurodevelopmental reflexes were recorded each morning at PN3 to PN5. At PN4 neurodevelopmental reflexes were repeated 2 hours after the galanin analog injection to test for sedation. The battery of reflexes included: (a) open field activity (OFA), i.e. time to escape from a 12.5 cm diameter circular field; (b) negative geotaxis (NG), i.e. time to turn 90° after placed head downwards on a 45° inclined surface, and start climbing up; (c) surface righting time (SRT), i.e. time to turn from the supine position to the prone with the pup standing on four limbs. Maximal observation period for each of these tests was set at 60sec and hence a score of 60sec indicated failure to perform this test within the allotted period of time.
Respiration, heart rates and blood oxygenation by pulse oximetry were assessed using the Mouse Ox system (STARR Life Sciences Corp, USA), whereas blood glucose level was measured using tail blood glucose measurements (Optium kit, Abbott, IL, USA) at 4 hours post-injection.
Nissl staining
The animals were euthanized on PN5, after the end of the afternoon session with pentobarbital (100mg/kg i.p.). The brains were fast frozen in dry ice / 2-isomethylbutane and stored at −80°C. Coronal 40μm sections were stained with thionin staining for histology.
Total RNA purification and qRT-PCR for GalR1, GalR2, and β-actin
To determine the relative expression of GalR1 and GalR2 mRNA in the cortex, we used qRT-PCR. Relative expression was determined over the constitutive gene β-actin. Three male PN4 control rats, 3 PN4 rats treated according to the multiple hit model, and 3 adult male rats (180–200g) were euthanized with 100mg/kg i.p. pentobarbital. Cerebro-cortical samples were collected under sterile conditions at the frontal cortex and retrosplenial agranular cortex and through the full depth of the cortex [Figure 166 in (Paxinos et al., 1994)]. These were taken from the right cerebral cortex, spanning 1mm radius from the needle track as well as from the contralateral homotypic cortex. In addition, the anterior dorsal hippocampus was dissected from each hemisphere at the same level as the cortical samples. Samples were collected in RLT buffer (Qiagen Inc, Valencia, CA, USA) with β-mercaptoethanol (10μl/ml), underwent rotor-stator homogenization and total RNA was purified according to the RNeasy Mini kit suggested protocol for total RNA purification from animal tissues with DNAse digestion to remove genomic DNA (Qiagen Inc, Valencia, CA, USA). RNA quantitation was done with a Nanodrop spectrophotometer (Thermo Scientific, Wilmington, DE, USA). All samples had A260/A280 absorbance between 1.93 and 2.06. Reverse transcription polymerase chain reaction (RT-PCR) was done in a Hybaid PCR Sprint machine (Thermo Scientific, Wilmington, DE, USA). The RT-PCR reaction was done using either 100ng of total RNA (for GalR2 qPCR) or 60–300ng of total RNA (for GalR1 qPCR), because of the significantly lower expression of GalR1 versus GalR2 found by our preliminary experiments. We used the TaqMan RT reagents (Life Technologies, Grand Island, NY, USA). The RT-PCR protocol included incubation at 25°C for 10min, followed by 30 min at 48°C, and inactivation at 95°C for 5 min. The qPCR reaction was done using the TaqMan PCR Core reagents, according the manufacturer’s protocol (Life Technologies, Grand Island, NY, USA) in a StepOne Real Time PCR System (Life Technologies, Grand Island, NY, USA). The following gene expression assays were used from Life Technologies, which had amplification efficiency of 1 (Grand Island, NY, USA):
Rat GalR1 the Rn02132426_s1 assay, which amplifies a 115bp within a single exon (exon boundary 1–1, assay location 765). The assay includes a 6-FAM™ dye-labeled TaqMan® MGB probe.
Rat GalR2 the Rn01773918_m1 assay, which amplifies a 116bp fragment that spans exons (exon boundary 1–2, assay location 386). The assay includes a 6-FAM™ dye-labeled TaqMan® MGB probe.
Rat β-actin the Rn00667869_m1 assay, which amplifies a 91bp segment spanning exons (exon boundary 4–5, assay location 884). The assay includes a 6-VIC™ dye-labeled TaqMan® MGB probe.
Parallel quantitation of GalR1 and β-actin or GalR2 and β-actin was done, using triplicates of the right and left cerebral cortical or hippocampal samples from each rat. Quantitation was done according to the comparative CT (ΔΔCT) method as relative expression of GalR1 or GalR2 over β-actin.
Statistics
Based on our prior studies, preliminary power analysis determined that we would need at least 12 rats per group to reach 84% power to detect a difference in the spasms frequencies of 0.22 standard deviations on the log scale between treatments. Data analyses were performed of raw frequencies of spasms, frequencies normalized over the pre-injection frequencies of the same rat, [normalized frequency = (post-injection frequency/ pre-injection frequency) * 100] and log-transformed frequencies. The results based on the log-transformed data did not differ materially from those based on the raw or normalized values and therefore are not included. To assess the effects of the drug or vehicle on spasms and neurodevelopmental reflexes, a linear mixed effects modeling approach was used to account for the correlation in repeated measures from the same animal. Fixed effects in the model included time, treatment, and time * treatment interaction terms. Time was modeled as either a categorical or continuous variable, depending on whether the time trends were linear. In the analysis of the non-normalized spasm frequencies, pre-injection levels were included as an additional covariate in the model to adjust for baseline levels. The treatment effects on spasms, neurodevelopmental reflexes, weight and vital signs at specific time points were compared with ANOVA (SAS software, SAS Institute, Cary NC, USA). A two-sided p-value less than 0.05 was considered statistically significant.
Results
Exclusions, sample sizes, and histology
We have excluded from the analysis four pups treated according to the multiple-hit protocol. Two animals died post-operatively on PN3; one animal died from hemoperitoneum caused by mechanical injury from the i.p. injection of NAX-0.5 on PN4. One pup (NAX-2 group) was excluded on PN4 because it was neglected by the dam and was found consistently apart from the litter. No rats were excluded based on lesion severity assessed by Nissl staining. Therefore, following these exclusions, from an initial sample size of 11–14 rats/group, we included in this study 13 VEH pups, 12 NAX-0.5 pups, 11 NAX-1 pups, 13 NAX-2 pups, and 11 NAX-4 pups. To determine the separation from a maximally tolerated dose, an additional litter (4 VEH and 6 NAX-7 pups) was included only in the assessment of weights, neurodevelopmental reflexes, cardiorespiratory parameters, and blood glucose.
The typical lesion observed on Nissl staining at the injection site is localized at the peri-infusional region of the right parietal cortex and the adjacent periventricular regions (right anterior-dorsal hippocampus and corpus callosum) (Supplemental Figure 1). This pattern of lesion was found in all pups included in this study with the following exceptions. Three pups (one each from the following groups: NAX-0.5, NAX-2, NAX-4) showed predominant right parietal lesion with no visible injury of the right hippocampus. One pup (NAX-0.5 group) showed predominant right hippocampal lesion with small right parietal lesion. These four pups manifested spasms within the range of frequencies seen in the other rats.
Effects on spasms
Overall, there was no evidence of a treatment effect on the raw or normalized frequencies of spasms when data from all time points were combined in a mixed effects analysis. Time was analyzed as a categorical variable in this analysis since the time trends were not linear over the entire post-injection period. Neither the treatment nor the treatment * time interaction were significant (Table 1). However, pre-injection spasms frequencies were significantly predictive of raw frequencies at post-injection time points, and the time effect was significant for raw and normalized frequencies (Table 1).
Table 1.
Linear mixed model analysis of frequencies of spasms, weights and neurodevelopmental reflexes
| P-values | ||||
|---|---|---|---|---|
| Treatment | Time | Baseline frequencies of spasms | Treatment * Time interaction | |
| Raw frequencies of spasms | 0.44 | <0.001 | 0.02 | 0.25 |
| Normalized frequencies of spasms | 0.48 | <0.001 | 0.74 | |
| Weights | 0.77 | <0.001 | 0.67 | |
| OFA | 0.52 | <0.001 | 0.58 | |
| SRT | 0.07 | <0.001 | 0.43 | |
| NG | 0.69 | <0.001 | 0.50 | |
Only pups monitored for spasms are included here.
OFA: open field activity; SRT: Surface righting time; NG: Negative geotaxis.
When raw frequencies of spasms were compared between treatment groups at specific time points, a significant difference was observed only at 4 hours post-injection (P=0.009, ANOVA) (Figure 1). Pairwise comparisons of the mean raw frequencies of spasms among the different doses showed less frequent spasms in the NAX-4 group compared to each of the other NAX 5055 doses, but not compared to the VEH. Similar results were obtained when the 4-hour values were adjusted for the pre-injection values using analysis of covariance. The rates of change (i.e. slope) in spasm frequencies over the first 4 post-injection hours were also compared between groups (Table 2). The slope in the NAX-4 group was significantly lower compared to the other NAX 5055 treated groups but not compared to the VEH group. In addition, the slope in the VEH group was significantly lower compared with the NAX-0.5 group.
Figure 1. A single dose of NAX 5055 given after the onset of spasms does not suppress spasms in the multiple-hit model (PN4 and PN5).
Panel A. Hourly raw frequencies of spasms after a single injection of NAX 5055 or its vehicle at PN4 in pups treated according to the multiple-hit protocol. NAX-0.5 group had more frequent spasms compared with the VEH group at the 4th post-injection hour. NAX-4 group had less spasms compared to the other NAX groups but not compared to the VEH, at the 4th hour post-injection.
Panel B. Normalized frequencies of spasms (% of pre-injection frequencies of the same rat) after a single injection of NAX 5055 or its vehicle in pups treated according to the multiple-hit protocol. No significant differences were found among treatment groups.
Please see also Tables 1 and 2 for the statistics. Bars represent standard deviation. NAX 5055 dose groups are as follows: NAX-0.5 = 0.5 mg/kg, NAX-1 = 1mg/kg, NAX-2 = 2mg/kg, NAX-4 = 4 mg/kg, VEH = vehicle.
Panel C. The table depicts P values derived from ANOVA comparisons of raw and normalized frequencies of spasms among the different treatment groups at each time point. Only the P-value of the raw frequencies during the 4th post-injection hour is statistically significant. Pairwise comparisons of the mean raw frequencies of spasms among the different doses showed less frequent spasms in the NAX-4 group compared to each of the other NAX 5055 doses, but not compared to the VEH.
Table 2.
P-values for pairwise treatment comparisons of rate of change in raw and normalized frequencies of spasms over the first 4 post-injection hoursa.
| Treatment Group | Slope Estimate | P (vs. NAX-0.5) | P (vs. NAX-1) | P (vs. NAX-2) | P (vs. NAX-4) |
|---|---|---|---|---|---|
| Raw frequencies of spasms | |||||
| VEH | 0.32 | 0.015 | 0.205 | 0.187 | 0.139 |
| NAX-0.5 | 1.24 | 0.268 | 0.247 | < 0.001 | |
| NAX-1 | 0.81 | 0.997 | 0.009 | ||
| NAX-2 | 0.80 | 0.007 | |||
| NAX-4 | −0.24 | ||||
| Normalized frequencies of spasms | |||||
| VEH | 8.80 | 0.087 | 0.300 | 0.419 | 0.938 |
| NAX-0.5 | 36.8 | 0.531 | 0.356 | 0.118 | |
| NAX-1 | 26.13 | 0.792 | 0.356 | ||
| NAX-2 | 21.72 | 0.486 | |||
| NAX-4 | 10.1 | ||||
Adjusted for baseline levels (frequencies of spasms during the 1hr Pre-injection)
When normalized frequencies of spasms were compared, no significant differences were seen among groups at any time point (Table 2). Furthermore, no significant treatment-related differences in the slopes of normalized values up to the first 4 hours post-injection were observed. In combination, these suggest that NAX 5055 does not acutely suppress spasms compared to vehicle.
Effects on weights, neurodevelopmental reflexes, glucose homeostasis and vital signs
Treatment had no significant effect on neurodevelopmental reflexes (OFA, SRT, NG) obtained 2 hours post-injection (Table 1, Figure 2). Treatment also had no effect on heart rate, respiratory rate, pulse oximetry, and blood glucose obtained 4 hours post-injection (Table 3) or on daily weights (Table 1). NAX-7 pups showed reduced pulse and breath distention (Table 3) but no other alterations in the other cardiorespiratory parameters, neurodevelopmental reflexes, weights, blood glucose or survival.
Figure 2. NAX 5055 has no significant effect on neurodevelopmental reflexes.
Panels A–C. SRT (panel A), OFA (panel B), and NG (panel C) were tested daily in the morning (PN3, PN4AM, PN5) as well as at 2 hours following the NAX 5055 injection (PN4PM). Bars represent standard deviation.
Panel D. The table presents the P values derived from ANOVA comparisons of mean scores for SRT, OFA, and NG among the different treatment groups at each timepoint. No significant differences among groups were seen in these scores.
Table 3.
ANOVA analysis of vitals and blood glucose levels (PN4PM, 4 hours post-injection)
| Test | P | Mean (SD) | |||||
|---|---|---|---|---|---|---|---|
| VEH | NAX-0.5 | NAX-1 | NAX-2 | NAX-4 | NAX-7 | ||
| Heart Rate (beats/min) | 0.188 | 399 (55.5) | 385.41 (33.89) | 358.64 (51.99) | 393.09 (52.17) | 350.3 (19.12) | 383.7 (34.7) |
| Pulse Distention (μm) | 0.0155(*) | 208.9 (68.2) | 239.4 (65.9) | 183.7 (41.7) | 213.3 (74.3) | 231.3 (89.7) | 120.7 (40) |
| Pulse Oximetry (%) | 0.6432 | 98.7 (0.59) | 97.79 (2.41) | 97.79 (3.06) | 98.03 (1.45) | 86.84 (3.47) | 97.5 (2.6) |
| Respiratory rate (breaths/min) | 0.9964 | 73.6 (19.1) | 72.3 (9.37) | 72.87 (17.26) | 75.3 (9.6) | 74.8 (7.6) | 74.8 (11.8) |
| Breath Distention (μm) | 0.043(**) | 313.1 (157.6) | 376.6 (119.6) | 370.3 (125.5) | 319.9 (177.4) | 371 (124.6) | 151 (58.6) |
| Blood glucose (mg/dl) | 0.646 | 89.6 (20.4) | 99 (36.35) | 94 (35.28) | 107 (37.21) | 110 (33.18) | 102.8 (5.1) |
Significant differences are observed between the NAX-7 group and each of: NAX-05, NAX-2, NAX-4, VEH (Student’s t-tests, pairwise comparisons).
Significant differences are observed between the NAX-7 group and each of: NAX-05, NAX-1, NAX-2, NAX-4, VEH (Student’s t-tests, pairwise comparisons).
Expression of GalR1 and GalR2 in the cerebral cortex and hippocampus
To determine whether the lack of effect of NAX-5055 could be due to absence of GalR1, we quantified the relative expression of GalR1 mRNA in the sensorimotor cortex and hippocampus of PN4 (control and multiple-hit model) and adult male control rats, using qRT-PCR and β-actin as a constitutively expressed control gene. For comparison, GalR2 qRT-PCR was performed in cortical samples of PN4 male rats.
GalR1 mRNA was present in all age and treatment groups, at both regions (Figure 3). A developmental increase in GalR1 mRNA was observed in the hippocampus (more GalR1 in the adults) but not in the cortex. However, GalR1 mRNA was increased in the right hippocampus of the pups treated with the multiple-hit protocol compared to the right hippocampus of the controls.
Figure 3. Expression of GalR1 and GalR2 mRNA in PN4 rat cerebral cortex.
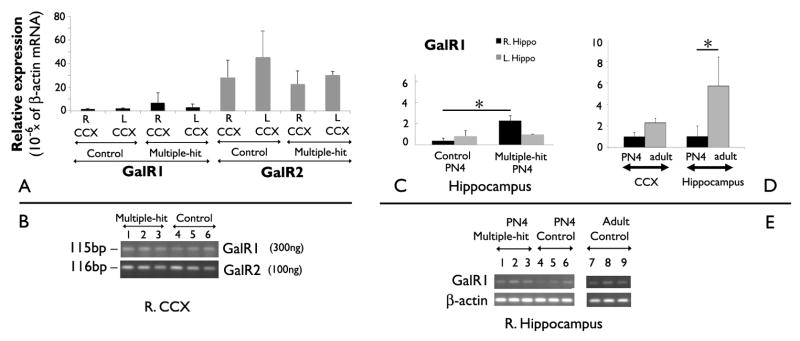
Panel A: Quantitative RT-PCR of total RNA extracts from cerebral cortex of male PN4 pups (controls or pups treated according to the multiple-hit protocol) revealed significantly more GalR2 mRNA compared to GalR1 mRNA (Gene effect: F(7, 23)= 40.45, P<0.0001; n=3 rats per group). There were no inter-hemispheric or treatment-related differences in GalR1 or GalR2 mRNA expression. Results are expressed as 10−6 * (β-actin mRNA).
Panel B: The DNA products of the qRT-PCR reactions for GalR1 (115 bp) and GalR2 (116 bp) from right cerebral cortical RNA samples were separated on a Tris-borate-EDTA/ ethidium bromide gel. RT-PCR was performed using 300ng of total RNA for the GalR1 assay and 100ng total RNA for the GalR2 assay.
Panel C: GalR1 mRNA was detected using qRT-PCR in the anterior dorsal hippocampus of both PN4 control rats and rats treated according to the multiple-hit model, but at low levels (n= 3 rats per group). The right hippocampi of the multiple-hit PN4 rats have higher GalR1 mRNA expression compared with the right hippocampi of the controls (Kruskall-Wallis, P<0.05). Black bars indicate right and grey bars the left hippocampal samples.
Panel D: Expression of GalR1 mRNA expression in the cortex and anterior dorsal hippocampus of PN4 and adult control rats, using qRT-PCR. No significant developmental differences are found in the sensorimotor cortex. In contrast, GalR1 mRNA expression increased in the hippocampus of adult control rats compared with PN4 rats (Kruskall-Wallis test, P<0.05; n=3 rats/group; 2 hippocampi per rat).
Panel E: Gel electrophoresis of the qRT-PCR products for GalR1 (115bp) and β-actin (91 bp) from the right hippocampus of nine PN4 and adult rats.
Bars indicate the standard deviations of the mean. The asterisks indicate statistical significance P<0.05. CCX: cerebral cortex; R: right; L: left; Hippo: hippocampus.
In PN4 pups, GalR1 mRNA expression in the cortex ranged between 1.5 × 10−6 to 6.7 × 10−6 times the expression of β-actin mRNA (Figure 3). In contrast, GalR2 mRNA ranged between 22.5 × 10−6 to 45 × 10−6 times the β-actin mRNA in the cortex, across groups (Figure 3) [GalR1 versus GalR2; F(7,23) = 40.45 (ANOVA, P<0.0001)]. Neither the treatment (control versus multiple-hit; F(7,23) = 0.6) nor the lateralization (right versus left cortex; F(7,23) = 1.49) had any effects on GalR2.
Discussion
We show that a single injection of the GalR1-preferring galanin analog NAX 5055 (0.5–4 mg/kg i.p.), given after the onset of spasms, has no significant acute effect on the frequency of spasms in the multiple hit rat model of IS. GalR1 mRNA was expressed, although at significantly lower doses than either GalR2 in PN4 rats or GalR1 in adult rats. The tolerability of the tested NAX 5055 doses was however good, neurodevelopmental reflexes were not affected, and no other side effects were observed. A significant reduction in pulse distention and breath distention was observed in the NAX-7 group, but without altering the other cardiorespiratory parameters.
We use a single-injection administration paradigm, because the aim of our screening strategy is to find treatments with rapid-onset control of spasms, based on the clinical observations that early cessation of spasms may improve outcomes. We also initiate treatment after the onset of spasms is documented to maintain a clinically-relevant treatment protocol. Currently, the appearance and onset of IS cannot be predicted in infants with IS of non-genetic etiology, which precludes the utilization of a pre-treatment approach.
GalR1 mRNA was present in both the cerebral cortex and anterior dorsal hippocampus in all studied age and treatment groups (Figure 3). GalR1 mRNA expression in PN4 pups was 5.7 times lower than the hippocampal expression of GalR1 in adults, an age when NAX 5055 was able to reduce seizures in adult rodents (White et al., 2009). This finding might imply that the lower GalR1 expression in the peri-infusional cortical and hippocampal regions of PN4 rats could explain the lack of NAX 5055 effect in our model. However, extrapolations across species, strains, ages, as well as among different seizure models need to be done cautiously. Further studies in other animal models of seizures in PN4 rats would be useful in testing whether the lack of NAX 5055 in our model is due to the low GalR1 expression in PN4 rats.
The lack of acute efficacy of NAX 5055 in this model of IS, as opposed to other seizure models (White et al., 2009), may also reflect the distinct pharmacosensitivity of spasms from other seizures. This is also reminiscent of our previous results showing no response of spasms to phenytoin (Ono et al., 2011), which is not among the treatments recommended for human IS (Mackay et al., 2004; Pellock et al., 2010). Another possibility is that the lack of observed acute effect of NAX 5055 over spasms may be attributed to the pharmacoresistant nature of spasms in the multiple-hit model (Scantlebury et al., 2010). Spasms in this model do not respond to ACTH and only transiently respond to vigabatrin, rendering the multiple-hit a model of drug-resistant IS (Scantlebury et al., 2010). On the other hand, the good therapeutic effect of the mTOR inhibitor rapamycin (Raffo et al., 2011) and carisbamate (Ono et al., 2011) in the multiple-hit model underlines the utility of this model as a screening tool for the identification of new candidate treatments for IS.
However, our findings cannot exclude the possibility that repetitive administration of NAX 5055 may be needed to suppress spasms in the multiple-hit model, as previously shown for low doses of rapamycin (Raffo et al., 2011). In the adult rodent brain, GalR1 inhibits presynaptic glutamate release by opening G-protein-mediated inward rectifier K+ channels (GIRK) or ATP sensitive K+ channels (Mazarati et al., 2006; Mazarati, 2004; Mazarati et al., 2000). In addition, GalR1 has been shown to inhibit the phosphorylation of cAMP response element binding protein (CREB), which can modulate neuronal and synaptic plasticity (Badie-Mahdavi et al., 2005). Further studies utilizing repeat administration of NAX 5055 may be useful to test the effect of prolonged treatment with NAX 5055 on spasms. Even though GalR1 is expressed in low levels in the PN4 cortex, it is possible that repetitive NAX 5055 administration could influence subcortical structures, in which GalR1 expression and signaling may be more potent (Burazin et al., 2000).
Our qRT-PCR results agree with in situ hybridization studies that showed lower GalR1 mRNA expression in the cortex and anterior dorsal hippocampus of PN4-70 rats than in other brain regions such as the ventral hippocampus, limbic regions, thalamus or brainstem (Burazin et al., 2000). Similar to our results, developmental increase in GalR1 mRNA between PN4 and adult rats has also been described in the dorsal dentate gyrus and ventral CA fields of Ammon’s horn, but not in the frontoparietal cortex (Burazin et al., 2000). Although GalR1 mRNA expression may not necessarily translate into functional protein, determination of GalR1 protein expression is hampered by the known lack of specificity of the available GalR1 antibodies when tested in knockout mice (Lu and Bartfai, 2009). Using a different anti-GalR1 antibody, we found staining in the cerebral cortex of control and multiple-hit PN4-5 male rats. However, nonspecific staining was also observed in GalR1 knockout mice, which prevented the utilization of these immunochemistry experiments in our manuscript. We report these findings as supplemental material, as a reference on the properties of the specific antibody we used (Supplemental Figure 2). Because of the lack of specificity of the available anti-GalR1 antibodies, we cannot therefore conclude whether differences in GalR mRNA expression correspond to protein expression differences.
Our study also demonstrates that GalR2 mRNA expression was at least 20 times more abundant than GalR1 mRNA in the cerebral cortex of PN4 pups, which is in agreement with a prior study (Burazin et al., 2000). It would therefore be very interesting, in future studies, to determine the effects of GalR2-preferring analogs in the treatment of IS.
We did not observe any clinically important adverse effects of NAX 5055 doses in PN4 rats, even though the tested NAX 5055 doses exceeded the median toxic dose for adult rats (Bialer et al., 2010). The reduction in pulse and breath distention seen in the NAX-7 group – usually indicative of reduced cardiac output and/or respiratory effort - is of unclear significance. High doses of galanin may lead to hypotension and tachycardia, decreased cardiac output or respiratory suppression through central mechanisms (Abbott et al., 2009; Abbott and Pilowsky, 2009; Diaz-Cabiale et al., 2005) or may have peripheral negative inotropic effects, as shown in guinea pig heart papillary muscle (Kocic, 1998). However, the lack of concomitant alterations in heart or respiratory rates or oxygenation in our study suggests that the observed reduction in pulse and breath distention is not clinically significant. However, a limitation of our experiments is that we only studied one timepoint, so that we do not disrupt our primary endpoint, the monitoring of the spasms. Future experiments should address the time course of the effects of very high doses of NAX 5055 on the cardiorespiratory system, in conjunction with the pharmacokinetics of NAX 5055. Even though our study did not permit the definition of a maximally tolerated dose for NAX 5055 in PN4 rats, further doses were not tested, because of the lack of effect on spasms and the low levels of GalR1 at this developmental age.
Galanin is known to have a regulatory effect on glucose metabolism and inhibits pancreatic insulin secretion in dogs and in rodents, in a stimulus-dependent manner (Gregersen et al., 1994; Hermansen, 1988; Lindskog et al., 1995; Ruczynski et al., 2002; Verchere et al., 1992) mediated through GalR1 (Mitsukawa et al 2008). However, we did not observe any definite hyperglycemic effects of NAX 5055 with any of the utilized doses, which could be due to either the young age of our pups or to missing the timepoint of the peak drug effect on the pancreas. Injection of galanin or its analogs can decrease body core temperature (Mitsukawa et al., 2009; Patel and Hutson, 1996). This possible side effect was not assessed in our study because pups need a controlled thermic environment when separated for monitoring, which makes the analysis of body temperature difficult.
To our knowledge, this is the first time that antiseizure properties of NAX 5055 are tested on a seizure model in immature rodents. We observed no effect on spasms, which could be due to (a) the low GalR1 mRNA expression in cortical and hippocampal regions of PN4 rats, or (b) to the drug-refractoriness of the multiple-hit model of IS or (c) the distinct pharmacosensitivity of IS, or (d) different pharmacokinetics of the drug in neonatal versus adult rats. Although we provide evidence for target expression in the brain of PN4 pups, the lack of effect did not allow us to prove target engagement by the drug. Future studies testing NAX 5055 in other models of seizures in PN4 rats would be useful in differentiating whether the lack of efficacy is model or age-related. Our study was not designed to test the effects of NAX 5055 on long-term epilepsy, cognitive and neurodevelopmental outcomes, which will be important to know as the therapeutic indications of this drug become better described (Crawley, 2008; Mazarati et al., 2006)
Supplementary Material
Nissl staining of a PN5 rat treated according to the multiple-hit protocol, demonstrates injury ipsilateral to the infusions at cortical and subcortical regions.
Panel A: Rostrally, the peri-infusional site (red 1) exhibits injury at the cerebral cortex (motor, cingulum) and corpus callosum.
Panel B: In addition to injury at the cortex (retrosplenial granular) and corpus callosum (red 1), there is also injury at the anterior dorsal hippocampus (red 2).
Panel C: At more caudal levels, the posterior and ventral hippocampus (red 3) appears without macroscopic injury.
The asterisk (red *) indicates a rostrocaudal cut done intentionally prior to cutting the brain, to mark the left hemisphere.
Panel A: sc-16216 antibody recognizes cortical neurons in both vehicle –injected PN5 pups treated according to the multiple-hit protocol (VEH), PN5 pups after sham surgery with only right intracerebral vehicle injections (SHAM). The negative control (no anti-GalR1 primary antibody used) does not show any immunoreactivity. The bar scale indicates a 100μm distance. Similar expression patterns were obtained from a total of five PN4–5 male rats/group (VEH versus SHAM).
Panel B: The sc-16216 antibody recognizes epitopes in the cerebral cortex (layer 5), the dentate gyrus, and CA1 (milder staining), but not in CA3 region of a 13 week old female GalR1 wild type mouse. Reduced but visible (see CA3 and CA1 regions) staining is seen in a GalR1 knockout same age female mouse (B6.129S-Galr1tm1Tpi/J, Jackson Laboratories, Bar Harbor Maine; provided by Dr Brian Klein), suggesting the presence of non-GalR1 related epitopes. This knockout mouse has been reported to have no GalR1 expression in the homozygous mutant mouse (Jacoby et al., 2002). The scale bar indicates 100μm distance.
Highlights.
NAX 5055 has no acute effect on spasms in the multiple-hit model.
NAX 5055 is well tolerated by immature rats.
The cerebral cortex of postnatal day 4 rats expresses more GalR2 than GalR1.
Hippocampal GalR1 mRNA expression increases between PN4 to adulthood.
Acknowledgments
This work was supported by NINDS/NICHD grant NS062947, NINDS grants R01 NS020253, R21 NS078333-01A1, R21 NS059669 and 1U01 NS066911-01A1, research grants from CURE and Autism Speaks, the Heffer Family Foundation, the Siegel family Foundation, grants from the Swiss National Science Foundation (PBLAP3-129421/1 and PBLAP3-137095) and from SICPA Foundation, Switzerland, the Clinical and Translational Science Award (1UL1RR025750-01), and the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (NIH) (Award Number P30HD071593). NAX 5055 and instructions on its solubility were provided by Drs Brian Klein and H. Steve White (Neuroadjuvants Inc and University of Utah, Salt Lake City UT respectively). We would like to acknowledge the excellent technical support by Qianyun Li, Wei Liu, and Hong Wang.
Abbreviations
- ACTH
adrenocorticotropic hormone
- GalR
galanin receptor
- i.p
intraperitoneal
- IS
infantile spasms
- NG
negative geotaxis
- OFA
open field activity
- PN
postnatal day
- SRT
surface righting time
Footnotes
Disclosures
MJG, MK, and ASG have no conflict of interest in the work reported in the present manuscript. HSW is a scientific cofounder and BDK is an employee of NeuroAdjuvants, Inc. Salt Lake City UT. The Albert Einstein College of Medicine holds a patent for the multiple-hit model (# 7863499). No royalties have been received or other financial interests exist on account of this patent. The work described in this article has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving animals http://ec.europa.eu/environment/chemicals/lab_animals/legislation_en.htm.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott SB, Burke PG, Pilowsky PM. Galanin microinjection into the PreBotzinger or the Botzinger Complex terminates central inspiratory activity and reduces responses to hypoxia and hypercapnia in rat. Respir Physiol Neurobiol. 2009;167(3):299–306. doi: 10.1016/j.resp.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Abbott SB, Pilowsky PM. Galanin microinjection into rostral ventrolateral medulla of the rat is hypotensive and attenuates sympathetic chemoreflex. Am J Physiol Regul Integr Comp Physiol. 2009;296(4):R1019–1026. doi: 10.1152/ajpregu.90885.2008. [DOI] [PubMed] [Google Scholar]
- Badie-Mahdavi H, Lu X, Behrens MM, Bartfai T. Role of galanin receptor 1 and galanin receptor 2 activation in synaptic plasticity associated with 3′,5′-cyclic AMP response element-binding protein phosphorylation in the dentate gyrus: studies with a galanin receptor 2 agonist and galanin receptor 1 knockout mice. Neuroscience. 2005;133(2):591–604. doi: 10.1016/j.neuroscience.2005.02.042. [DOI] [PubMed] [Google Scholar]
- Bartfai T, Lu X, Badie-Mahdavi H, Barr AM, Mazarati A, Hua XY, Yaksh T, Haberhauer G, Ceide SC, Trembleau L, Somogyi L, Krock L, Rebek JJ. Galmic, a nonpeptide galanin receptor agonist, affects behaviors in seizure, pain, and forced-swim tests. Proc Natl Acad Sci U S A. 2004;101(28):10470–10475. doi: 10.1073/pnas.0403802101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS. Progress report on new antiepileptic drugs: a summary of the Tenth Eilat Conference (EILAT X) Epilepsy Res. 2010;92(2–3):89–124. doi: 10.1016/j.eplepsyres.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Bulaj G, Green BR, Lee HK, Robertson CR, White K, Zhang L, Sochanska M, Flynn SP, Scholl EA, Pruess TH, Smith MD, White HS. Design, synthesis, and characterization of high-affinity, systemically-active galanin analogues with potent anticonvulsant activities. J Med Chem. 2008;51(24):8038–8047. doi: 10.1021/jm801088x. [DOI] [PubMed] [Google Scholar]
- Burazin TC, Larm JA, Ryan MC, Gundlach AL. Galanin-R1 and -R2 receptor mRNA expression during the development of rat brain suggests differential subtype involvement in synaptic transmission and plasticity. Eur J Neurosci. 2000;12(8):2901–2917. doi: 10.1046/j.1460-9568.2000.00184.x. [DOI] [PubMed] [Google Scholar]
- Chudomelova L, Scantlebury MH, Raffo E, Coppola A, Betancourth D, Galanopoulou AS. Modeling new therapies for infantile spasms. Epilepsia. 2010;51(Suppl 3):27–33. doi: 10.1111/j.1528-1167.2010.02605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. Galanin impairs cognitive abilities in rodents: relevance to Alzheimer’s disease. Cell Mol Life Sci. 2008;65(12):1836–1841. doi: 10.1007/s00018-008-8158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darke K, Edwards SW, Hancock E, Johnson AL, Kennedy CR, Lux AL, Newton RW, O’Callaghan FJ, Verity CM, Osborne JP. Developmental and epilepsy outcomes at age 4 years in the UKISS trial comparing hormonal treatments to vigabatrin for infantile spasms: a multi-centre randomised trial. Arch Dis Child. 2010;95(5):382–386. doi: 10.1136/adc.2009.160606. [DOI] [PubMed] [Google Scholar]
- Diaz-Cabiale Z, Parrado C, Vela C, Razani H, Covenas R, Fuxe K, Narvaez JA. Role of galanin and galanin(1–15) on central cardiovascular control. Neuropeptides. 2005;39(3):185–190. doi: 10.1016/j.npep.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Galanopoulou AS. Basic mechanisms of catastrophic epilepsy - Overview from animal models. Brain Dev. 2013 doi: 10.1016/j.braindev.2012.12.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregersen S, Langel U, Bartfai T, Ahren B. N-terminally elongated fragments of galanin(1–16) inhibit insulin secretion from isolated mouse islets. Regul Pept. 1994;53(1):31–37. doi: 10.1016/0167-0115(94)90156-2. [DOI] [PubMed] [Google Scholar]
- Haberman RP, Samulski RJ, McCown TJ. Attenuation of seizures and neuronal death by adeno-associated virus vector galanin expression and secretion. Nat Med. 2003;9(8):1076–1080. doi: 10.1038/nm901. [DOI] [PubMed] [Google Scholar]
- Hermansen K. Effects of galanin on the release of insulin, glucagon and somatostatin from the isolated, perfused dog pancreas. Acta Endocrinol (Copenh) 1988;119(1):91–98. doi: 10.1530/acta.0.1190091. [DOI] [PubMed] [Google Scholar]
- Jacoby AS, Hort YJ, Constantinescu G, Shine J, Iismaa TP. Critical role for GALR1 galanin receptor in galanin regulation of neuroendocrine function and seizure activity. Brain Res Mol Brain Res. 2002;107(2):195–200. doi: 10.1016/s0169-328x(02)00451-5. [DOI] [PubMed] [Google Scholar]
- Kivity S, Lerman P, Ariel R, Danziger Y, Mimouni M, Shinnar S. Long-term cognitive outcomes of a cohort of children with cryptogenic infantile spasms treated with high-dose adrenocorticotropic hormone. Epilepsia. 2004;45(3):255–262. doi: 10.1111/j.0013-9580.2004.30503.x. [DOI] [PubMed] [Google Scholar]
- Kocic I. The influence of the neuropeptide galanin on the contractility and the effective refractory period of guinea-pig heart papillary muscle under normoxic and hypoxic conditions. J Pharm Pharmacol. 1998;50(12):1361–1364. doi: 10.1111/j.2042-7158.1998.tb03360.x. [DOI] [PubMed] [Google Scholar]
- Lindskog S, Gregersen S, Hermansen K, Ahren B. Effects of galanin on proinsulin mRNA and insulin biosynthesis in normal islets. Regul Pept. 1995;58(3):135–139. doi: 10.1016/0167-0115(95)00061-f. [DOI] [PubMed] [Google Scholar]
- Lombroso CT. A prospective study of infantile spasms: clinical and therapeutic correlations. Epilepsia. 1983;24(2):135–158. doi: 10.1111/j.1528-1157.1983.tb04874.x. [DOI] [PubMed] [Google Scholar]
- Lu X, Bartfai T. Analyzing the validity of GalR1 and GalR2 antibodies using knockout mice. Naunyn Schmiedebergs Arch Pharmacol. 2009;379(4):417–420. doi: 10.1007/s00210-009-0394-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Roberts E, Xia F, Sanchez-Alavez M, Liu T, Baldwin R, Wu S, Chang J, Wasterlain CG, Bartfai T. GalR2-positive allosteric modulator exhibits anticonvulsant effects in animal models. Proc Natl Acad Sci U S A. 2010;107(34):15229–15234. doi: 10.1073/pnas.1008986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux AL, Edwards SW, Hancock E, Johnson AL, Kennedy CR, Newton RW, O’Callaghan FJ, Verity CM, Osborne JP. The United Kingdom Infantile Spasms Study comparing vigabatrin with prednisolone or tetracosactide at 14 days: a multicentre, randomised controlled trial. Lancet. 2004;364(9447):1773–1778. doi: 10.1016/S0140-6736(04)17400-X. [DOI] [PubMed] [Google Scholar]
- Mackay MT, Weiss SK, Adams-Webber T, Ashwal S, Stephens D, Ballaban-Gill K, Baram TZ, Duchowny M, Hirtz D, Pellock JM, Shields WD, Shinnar S, Wyllie E, Snead OCr. Practice parameter: medical treatment of infantile spasms: report of the American Academy of Neurology and the Child Neurology Society. Neurology. 2004;62(10):1668–1681. doi: 10.1212/01.wnl.0000127773.72699.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarati A, Langel U, Bartfai T. Galanin: an endogenous anticonvulsant? Neuroscientist. 2001;7(6):506–517. doi: 10.1177/107385840100700607. [DOI] [PubMed] [Google Scholar]
- Mazarati A, Lundstrom L, Sollenberg U, Shin D, Langel U, Sankar R. Regulation of kindling epileptogenesis by hippocampal galanin type 1 and type 2 receptors: The effects of subtype-selective agonists and the role of G-protein-mediated signaling. J Pharmacol Exp Ther. 2006;318(2):700–708. doi: 10.1124/jpet.106.104703. [DOI] [PubMed] [Google Scholar]
- Mazarati AM. Galanin and galanin receptors in epilepsy. Neuropeptides. 2004;38(6):331–343. doi: 10.1016/j.npep.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Mazarati AM, Hohmann JG, Bacon A, Liu H, Sankar R, Steiner RA, Wynick D, Wasterlain CG. Modulation of hippocampal excitability and seizures by galanin. J Neurosci. 2000;20(16):6276–6281. doi: 10.1523/JNEUROSCI.20-16-06276.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melander T, Hokfelt T, Rokaeus A, Cuello AC, Oertel WH, Verhofstad A, Goldstein M. Coexistence of galanin-like immunoreactivity with catecholamines, 5-hydroxytryptamine, GABA and neuropeptides in the rat CNS. J Neurosci. 1986;6(12):3640–3654. doi: 10.1523/JNEUROSCI.06-12-03640.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsukawa K, Lu X, Bartfai T. Bidirectional regulation of stress responses by galanin in mice: involvement of galanin receptor subtype 1. Neuroscience. 2009;160(4):837–846. doi: 10.1016/j.neuroscience.2009.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan FJ, Lux AL, Darke K, Edwards SW, Hancock E, Johnson AL, Kennedy CR, Newton RW, Verity CM, Osborne JP. The effect of lead time to treatment and of age of onset on developmental outcome at 4 years in infantile spasms: evidence from the United Kingdom Infantile Spasms Study. Epilepsia. 2011;52(7):1359–1364. doi: 10.1111/j.1528-1167.2011.03127.x. [DOI] [PubMed] [Google Scholar]
- Ono T, Moshe SL, Galanopoulou AS. Carisbamate acutely suppresses spasms in a rat model of symptomatic infantile spasms. Epilepsia. 2011;52(9):1678–1684. doi: 10.1111/j.1528-1167.2011.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Hutson PH. Effects of galanin on 8-OH-DPAT induced decrease in body temperature and brain 5-hydroxytryptamine metabolism in the mouse. Eur J Pharmacol. 1996;317(2–3):197–204. doi: 10.1016/s0014-2999(96)00716-9. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Ashwell KWS, Tork I. Atlas of the developing rat nervous system. San Diego, CA: Academic Press; 1994. [Google Scholar]
- Pellock JM, Hrachovy R, Shinnar S, Baram TZ, Bettis D, Dlugos DJ, Gaillard WD, Gibson PA, Holmes GL, Nordl DR, O’Dell C, Shields WD, Trevathan E, Wheless JW. Infantile spasms: a U.S. consensus report. Epilepsia. 2010;51(10):2175–2189. doi: 10.1111/j.1528-1167.2010.02657.x. [DOI] [PubMed] [Google Scholar]
- Raffo E, Coppola A, Ono T, Briggs SW, Galanopoulou AS. A pulse rapamycin therapy for infantile spasms and associated cognitive decline. Neurobiol Dis. 2011;43(2):322–329. doi: 10.1016/j.nbd.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riikonen R. ACTH therapy of West syndrome: Finnish views. Brain Dev. 2001a;23(7):642–646. doi: 10.1016/s0387-7604(01)00306-0. [DOI] [PubMed] [Google Scholar]
- Riikonen R. Long-term outcome of patients with West syndrome. Brain Dev. 2001b;23(7):683–687. doi: 10.1016/s0387-7604(01)00307-2. [DOI] [PubMed] [Google Scholar]
- Riikonen RS. Favourable prognostic factors with infantile spasms. Eur J Paediatr Neurol. 2010;14(1):13–18. doi: 10.1016/j.ejpn.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Robertson CR, Scholl EA, Pruess TH, Green BR, White HS, Bulaj G. Engineering galanin analogues that discriminate between GalR1 and GalR2 receptor subtypes and exhibit anticonvulsant activity following systemic delivery. J Med Chem. 2010;53(4):1871–1875. doi: 10.1021/jm9018349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruczynski J, Cybal M, Wojcikowski C, Rekowski P. Effects of porcine galanin, galanin(1–15)NH2 and its new analogues on glucose-induced insulin secretion. Pol J Pharmacol. 2002;54(2):133–141. [PubMed] [Google Scholar]
- Scantlebury MH, Galanopoulou AS, Chudomelova L, Raffo E, Betancourth D, Moshe SL. A model of symptomatic infantile spasms syndrome. Neurobiol Dis. 2010;37(3):604–612. doi: 10.1016/j.nbd.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verchere CB, Kwok YN, Brown JC. Stimulus-specific inhibition of insulin release from rat pancreas by both rat and porcine galanin. Life Sci. 1992;51(25):1945–1951. doi: 10.1016/0024-3205(92)90111-2. [DOI] [PubMed] [Google Scholar]
- White HS, Scholl EA, Klein BD, Flynn SP, Pruess TH, Green BR, Zhang L, Bulaj G. Developing novel antiepileptic drugs: characterization of NAX 5055, a systemically-active galanin analog, in epilepsy models. Neurotherapeutics. 2009;6(2):372–380. doi: 10.1016/j.nurt.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Nissl staining of a PN5 rat treated according to the multiple-hit protocol, demonstrates injury ipsilateral to the infusions at cortical and subcortical regions.
Panel A: Rostrally, the peri-infusional site (red 1) exhibits injury at the cerebral cortex (motor, cingulum) and corpus callosum.
Panel B: In addition to injury at the cortex (retrosplenial granular) and corpus callosum (red 1), there is also injury at the anterior dorsal hippocampus (red 2).
Panel C: At more caudal levels, the posterior and ventral hippocampus (red 3) appears without macroscopic injury.
The asterisk (red *) indicates a rostrocaudal cut done intentionally prior to cutting the brain, to mark the left hemisphere.
Panel A: sc-16216 antibody recognizes cortical neurons in both vehicle –injected PN5 pups treated according to the multiple-hit protocol (VEH), PN5 pups after sham surgery with only right intracerebral vehicle injections (SHAM). The negative control (no anti-GalR1 primary antibody used) does not show any immunoreactivity. The bar scale indicates a 100μm distance. Similar expression patterns were obtained from a total of five PN4–5 male rats/group (VEH versus SHAM).
Panel B: The sc-16216 antibody recognizes epitopes in the cerebral cortex (layer 5), the dentate gyrus, and CA1 (milder staining), but not in CA3 region of a 13 week old female GalR1 wild type mouse. Reduced but visible (see CA3 and CA1 regions) staining is seen in a GalR1 knockout same age female mouse (B6.129S-Galr1tm1Tpi/J, Jackson Laboratories, Bar Harbor Maine; provided by Dr Brian Klein), suggesting the presence of non-GalR1 related epitopes. This knockout mouse has been reported to have no GalR1 expression in the homozygous mutant mouse (Jacoby et al., 2002). The scale bar indicates 100μm distance.




