Abstract
The cytoplasmic adaptor proteins TNF receptor associated factor (TRAF)3 and TRAF6 are important mediators of TLR signaling. We show here for the first time that another TRAF family member, TRAF5, is a negative regulator of TLR signaling. B lymphocytes from TRAF5−/− mice produced more IL-6, IL-12p40, IL-10, TNFα, and IgM than did WT B cells after TLR stimulation. Consistent with these data, exogenous overexpression of TRAF5 in B cells inhibited TLR-mediated cytokine and antibody production. TLR stimulation of TRAF5-deficient B cells did not affect cell survival, proliferation, or NF-κB activation, but resulted in markedly enhanced phosphorylation of the MAP kinases ERK1/2 and JNK. TRAF5 negatively regulated TLR signaling in a cell-specific manner, as TRAF5−/− macrophages and dendritic cells showed less dramatic differences in TLR-mediated cytokine production than B cells. Following TLR stimulation, TRAF5 associated in a complex with the TLR adaptor protein MyD88 and the B cell-specific positive regulator of TLR signaling TAB2. Furthermore, TRAF5 negatively regulated the association of TAB2 with its signaling partner TRAF6 after TLR ligation in B cells. These data provide the first evidence that TRAF5 acts as a negative regulator of TLR signaling.
INTRODUCTION
Toll-like receptors (TLRs) are pattern-recognition receptors, providing a first-line defense against pathogens by recognizing pathogen-associated molecular patterns (1–3). The cytoplasmic adaptor proteins tumor necrosis factor receptor associated factors (TRAFs) mediate signaling from the TNFR superfamily and the IL-1R/TLR superfamily of receptors (4). TRAF6 is recognized as an integral component of TLR signaling in multiple cell types (5). TRAF3 also mediates signaling after TLR ligation in myeloid cells, while in contrast inhibiting TLR signaling in B lymphocytes (6–8).
Of the seven known TRAF family members, TRAF5 is relatively understudied. While initially thought to be redundant with TRAF2, it is now appreciated that TRAF5 plays unique roles in CD8 T cell responses to infection, in limiting Th2 skewing, and in signaling to B cells through both CD40 and its viral mimic, latent membrane protein 1 (LMP1) (9–13). TRAF5 shares significant structural homology with TRAF3, and is composed of a C-terminal receptor binding domain (TRAF-C), a coiled-coil, leucine-zipper domain (TRAF-N), a zinc finger motif, and an N-terminal RING finger domain. TRAF5 forms heterotypic multimers with TRAF3 via TRAF-N domain interactions. This interaction is biologically important in TRAF5 recruitment to several types of membrane receptors (14–16).
TRAF5 has been implicated in the development of atherosclerosis in a mouse model (17). As TLR dysregulation is known to contribute to atherogenesis (3), we hypothesized that like TRAFs 3 and 6, TRAF5 also plays an important regulatory role in TLR signaling. To address this hypothesis, we utilized two complementary model systems. The first was a strain of genetically TRAF5-deficient mice. These mice breed and develop normally (12). Our lab previously backcrossed this strain onto the C57BL/6 genetic background, and used the mice to examine roles of TRAF5 in T cell responses to infection (11) and in LMP1-mediated B cell activation (13). The second model system inducibly overexpresses epitope-tagged TRAF5 in a well-studied B cell line to circumvent the poor quality and specificity of commercially-available TRAF5-specific antibodies, and allowed examination of the contrasting effects of TRAF5 depletion vs. excess.
Results from experiments in both models indicated that TRAF5 serves as an important negative regulator of TLR-mediated signaling, specifically in B lymphocytes. After TLR ligation, TRAF5-deficient B cells showed enhanced MAPK phosphorylation and produced more cytokines and antibody than control B cells. TRAF5 negatively regulated TLR signaling in a cell-specific manner as TRAF5-deficient dendritic cells and macrophages did not show dramatic differences in cytokine production in response to TLR agonists. Similarly, a recent study demonstrated that the TLR adaptor protein TAB2 acts in a cell-specific manner, positively regulating TLR signaling specifically in B lymphocytes. After TLR ligation, B lymphocytes from TAB2−/− mice show reduced phosphorylation of MAP kinases and produce less IL-6 and antibody (18).
We thus hypothesized that TRAF5 negatively regulates TLR signaling in B lymphocytes by acting on the positive regulator TAB2. Our results showed association of TRAF5 with TAB2 after TLR ligation in B cells. Additionally, TRAF5 negatively regulated the association of TAB2 with its known interacting partner TRAF6 after TLR ligation in B cells. These results demonstrate for the first time an important regulatory role for TRAF5 in TLR signaling.
MATERIALS AND METHODS
Mice
TRAF5−/− mice on a B6 genetic background were previously described (13). Mice were maintained under pathogen-free conditions at the University of Iowa. Use of mice in this study was according to a protocol approved by The University of Iowa Animal Care and Use Committee.
Cell lines
The mouse B cell line CH12.LX has been described previously (19). CH12.LX cells were stably transfected to inducibly express FLAG-tagged TRAF5 as previously described (20). Subclones expressing FLAG-tagged TRAF5 were maintained in B cell media (BCM10) containing RPMI 1640 (Invitrogen, Grand Island, NY) with 10 μM 2-mercaptoethanol (Invitrogen), 10% heat-inactivated FCS (Atlanta Biologicals, Atlanta, GA), antibiotics (Invitrogen), with added 400ug/mL G418 disulfate, and 200ug/ml hygromycin (Invitrogen).
Antibodies and Reagents
IPTG was purchased from Sigma Chemical Co. (St. Louis, MO). The TLR7 agonist R848 was purchased from Enzo Life Sciences (Ann Arbor, MI). The synthetic oligonucleotide CpG B 2084, a TLR9 agonist, was purchased from Integrated DNA Technologies (Coralville, IA). LPS (Escherichia coli strain 0111:B4), alkaline phosphatase substrate tablets and mouse anti-actin antibody (Ab) were purchased from Sigma-Aldrich (St. Louis, MO). ELISA Abs, PE-labeled mouse anti-IL-6 Ab, anti-CD21 and anti-IgM Abs were purchased from eBioscience (San Diego, CA). Rabbit anti-phospho-JNK Ab, rabbit anti-total JNK Ab, rabbit anti-phospho-p38 Ab, rabbit anti-phospho-ERK1/2 Ab, rabbit anti-total ERK1/2 Ab, rabbit anti-pIκBα Ab, rabbit anti-total IκBα Ab, rabbit anti-TAB2 Ab, and rabbit anti-MyD88 Ab were purchased from Cell Signaling Technology (Danvers, MA). Rabbit anti-TRAF6 Ab for Western blotting was purchased from Medical and Biological Laboratories (Japan). Anti-TRAF6 Ab for immunoprecipitation was purchased from Invitrogen. Goat anti-mouse IgG and goat anti-rabbit IgG secondary Abs were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Purified mouse IgM polyclonal Ab for ELISA standards, goat anti-mouse IgM secondary polyclonal Ab, and AP-conjugated goat anti-mouse IgM Ab were purchased from Southern Biotechnology Associates (Birmingham, AL).
Detection of Cytokine and Antibody Production In Vitro
Primary splenic B cells were isolated using an anti-CD43 negative selection kit (Miltenyi Biotec, Auburn, CA) and stimulated with 100 nM CpG-B 2084, 1ug/mL R848, or 20ug/mL LPS for 48 h. Bone marrow-derived dendritic cells were differentiated using rmGM-CSF and rmIL-4 (R&D Systems, Minneapolis, MN). Bone marrow-derived macrophages were differentiated using 30% L929 media. Differentiated cells were stimulated with TLR agonists for 24 h. After stimulation, supernatants were collected and used in cytokine-specific ELISAs. For TNFα detection, primary B cells were isolated as above and in-plate ELISAs were performed as described, as B cells rapidly bind and internalize TNF (21). For detection of Ab production, primary cells were isolated as described above and cultured at 1 × 106 cells per ml for five days in BCM10 with added TLR agonists. Ab production was determined by Ig-specific ELISA as previously described (22).
Flow Cytometry
Proliferation was measured by CFSE dilution using CellTrace (Invitrogen). Survival was measured with the FITC Annexin V/Dead Cell Apoptosis Kit (Invitrogen). For intracellular IL-6 detection, primary B cells were isolated as above and stimulated with TLR agonists for 6 h in the presence of Brefeldin-A and monensin (BD Biosciences, San Jose, CA). The Cytofix/Cytoperm Plus kit (BD Biosciences) was used to detect intracellular IL-6 by flow cytometry. For B cell subset analysis, splenocytes were stained with anti-IgM and anti-CD21. Samples were analyzed on an LSR II flow cytometer (BD Biosciences) and results were analyzed using FlowJo software (Tree Star, Ashland, OR).
Proximal signaling assays
Primary splenic B cells were isolated as for the in vitro cytokine production experiments above. 4 × 106 primary cells were resuspended in 1ml BCM10 in 1.5 mL Eppendorf tubes and stimulated with 1ug/mL R848 for 0, 2, 5, 10, 15, 30, and 60 min. After stimulation, cells were lysed in 100ul 2x SDS-PAGE loading buffer (1% SDS, 2% β-mercaptoethanol, 62.5 mM Tris, pH 6.8). Lysates were sonicated using a Branson Sonifier 250 (VWR International, Radnor, PA) with 10 pulses at 90% duty cycle, output 1.5. Samples were boiled for 10 min at 95° C.
Western Blots
10ul samples were resolved on 12% SDS-PAGE. Proteins were transferred to Immobilon-P PVDF membranes (Millipore, Billerica, MA). Membranes were blocked in 4% milk or BSA for 1 h, washed in TBST (NaCl, Tris, Tween 20, and H20), and incubated overnight at 4°C in primary Ab. Blots were washed in TBST and incubated with secondary Ab overnight at 4°C and developed using Supersignal West Pico (Thermo Fisher Scientific, Rockford, IL). Western blot chemiluminescence was read with an LAS-4000 low-light camera and analyzed with Multi Gauge software (Fujifilm Life Science, Edison, NJ).
Immunoprecipitation
FLAG-TRAF5 expression in transfected subclones of CH12.LX cells was induced by 20 hours of culture in BCM-10 with 100uM IPTG. 2 × 107 CH12.LX cells or 5 × 107 primary splenic B cells were resuspended in 1ml BCM10 in 1.5 mL Eppendorf tubes and stimulated with 1ug/mL R848 for the indicated time points. Cells were pelleted and lysed as previously described (23). 2ug/mL mouse anti-FLAG Ab (Sigma), rabbit anti-MyD88 Ab (Cell Signaling), rabbit anti-TRAF6 Ab (Invitrogen) or control mouse IgG1 Ab (MOPC21, Biolegend, San Diego, CA) were added, and samples were rotated at 4°C for 5 hours. Dynal protein G magnetic beads (Invitrogen) were added for 1 hour. Bead-bound proteins were resuspended in an equal volume of 2× SDS-PAGE loading buffer and boiled for 10 min at 95 °C.
Statistical Analysis
P values were generated by using Student’s t test (unpaired, two-tailed, at 95% confidence interval).
RESULTS
Effect of TRAF5 on TLR-mediated cytokine production
TLR ligation stimulates robust production of cytokines in both lymphoid and myeloid cells (1, 8). When stimulated through TLR4 (LPS), TLR7 (R848) or TLR9 (CpG DNA), TRAF5−/− (KO) B cells produced significantly more IL-6, IL-10, TNFα, and IL-12p40 than wild-type (WT) TRAF5+/+ B cells (Fig. 1A). Ligation of TLR2, TLR3, or TLR5 did not result in substantial B cell cytokine production. The enhanced cytokine production seen in TLR-stimulated TRAF5 KO B cells was not attributable to either enhanced cell survival or proliferation (Fig. S1). Rather, TRAF5 KO B cells produced more cytokines on a per-cell basis (Fig. S2). The enhanced cytokine production was seen in all splenic B cell subsets (Fig. S2). These data indicate that TRAF5 inhibited TLR signals to B cells.
Figure 1. Effect of TRAF5 status on TLR-mediated cytokine production by B lymphocytes.
Splenic B cell isolation and detection of cytokine production were performed as described in Materials and Methods. (A) Splenic B cells were isolated from TRAF5 KO and TRAF5 WT mice and cultured with the indicated TLR ligands for 48 hours. Cytokine production from TRAF5 KO B cells (filled bars) was compared to TRAF5 WT B cells (open bars). (B) CH12.LX B cells were cultured for 20 hours with IPTG to induce overexpression of TRAF5. Cytokine production after 48 hours of TLR stimulation from these cells (filled bars) was compared to CH12.LX cells that were not induced with IPTG (open bars). Data represent the mean values ± SD of three experiments. Statistical analysis was conducted using Student’s t test. Asterisks denote a p value of less than 0.05.
Consistent with results in TRAF5 KO B cells, CH12.LX B cells induced to overexpress transfected TRAF5 (CH12-TRAF5) produced significantly less IL-6, TNFα, and IL-12p40 than parent CH12.LX clones expressing normal levels of endogenous TRAF5 (Fig. 1B). Parent CH12.LX cells constitutively produce IL-10, so differences in IL-10 production could not be quantified reliably in the cell lines.
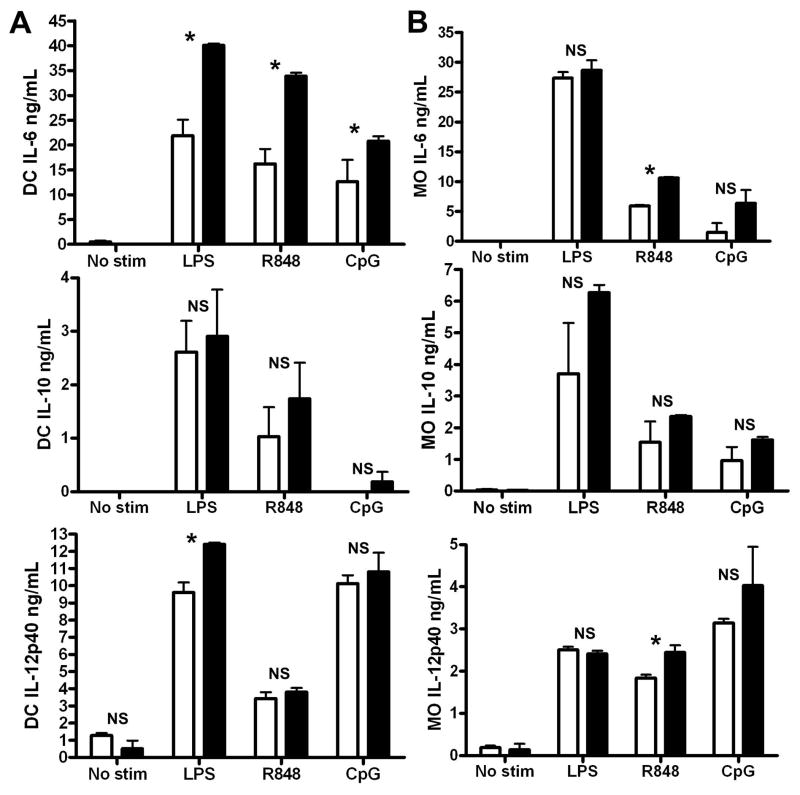
Most studies of TLRs focus on their important functions in myeloid cells. Although TRAF5-deficient B cells showed dramatic differences in cytokine production after TLR ligation, TRAF5−/− bone marrow-derived dendritic cells (BMDC) and bone marrow-derived macrophages (BMM) do not exhibit as striking a phenotype (Fig 2). Similar to B cells, TRAF5−/− BMDC produced more IL-6 after TLR stimulation, However, TRAF5-deficient BMM showed enhanced IL-6 production only after stimulation through TLR7. With the exception of IL-12p40 production after TLR4 stimulation in BMDC and after TLR7 production in BMM, no significant differences in the production of IL-10 or IL-12p40 were observed (Fig. 2).
Figure 2. Effect of TRAF5 deficiency on TLR-mediated cytokine production by bone marrow-derived dendritic cells.
BMDC (A) and BMM (B) differentiation was performed as described in Materials and Methods. BMDC and BMM derived from TRAF5 WT and TRAF5 KO mice were cultured with the indicated TLR ligands for 24 hours. Cytokine production from TRAF5 KO BMDC or BMM (filled bars) was compared to TRAF5 WT BMDC or BMM (open bars). Data represent the mean values ± SD of three experiments. Statistical analysis was conducted using Student’s t test. Asterisks denote a p value of less than 0.05.
The most striking effects of TRAF5 deficiency on TLR responses was seen in B cells, and the availability of a complementary model of TRAF5 overexpression in B cells suggested that this immune cell type was the best choice for further investigation of how TRAF5 regulates TLR responses.
Effect of TRAF5 on TLR-induced antibody production
Production of antibody is the major unique immune function of the B lymphocyte, and TLR stimulation induces robust immunoglobulin production (8). The CH12.LX B cell line produces IgM specific for phosphatidyl choline, a major constituent of erythrocyte membranes (24). IgM-secreting CH12.LX cells were enumerated as lytic plaque-forming cells on a lawn of sheep red blood cells, as previously described (25). CH12-TRAF5 cells produced significantly less antibody following TLR4, TLR7, and TLR9 stimulation than the same cells that were not induced to overexpress TRAF5, demonstrating that TRAF5 negatively regulated antibody production after TLR ligation (Fig. 3A).
Figure 3. Effect of TRAF5 status on TLR-induced antibody production.
Antibody production was measured as described in Materials and Methods. (A) CH12.LX B cells were cultured for 20 hours with IPTG to induce overexpression of TRAF5 (CH12-TRAF5). IgM production after TLR ligation from these cells (filled bars) was compared to CH12.LX cells that were not induced with IPTG (open bars). (B) Splenic B cells were isolated from TRAF5 KO and TRAF5 WT mice and cultured with the indicated TLR ligands for five days. IgM production from TRAF5 KO B cells (filled bars) was compared to TRAF5 WT B cells (open bars). Data represent the mean values ± SD of three experiments. Statistical analysis was conducted using Student’s t test. Asterisks denote a p value of less than 0.05.
Consistent with these data, TRAF5 KO B cells produced more IgM when stimulated through TLR4 or TLR7 (Fig. 3B). Together with the cytokine data from Figure 1, these results indicate that TRAF5 is a negative regulator of TLR effector functions in B cells.
TLR-induced early signaling events in TRAF5−/− B cells
As B cell survival and proliferation were unaffected by TRAF5 deficiency (Fig. S1), we hypothesized that the increases in cytokine and antibody production seen in TLR-stimulated TRAF5 KO B cells were directly attributable to enhanced signaling pathways after TLR ligation.
Fig. 1 results showed that ligation of TLR7 induced the most potent cytokine responses in both primary B cells and CH12-TRAF5 cells. The TLR7 agonist R848 was thus chosen as the optimal stimulus for characterizing the impact of TRAF5 on B cell TLR-mediated early signaling pathways. Following TLR7 ligation, TRAF5 KO B cells showed greater phosphorylation of the MAPK pathway proteins ERK1/2 and JNK compared to TRAF5 WT B cells, while phosphorylation of the MAPK p38 was unaffected (Fig. 4). TRAF5 status did not detectably impact the canonical NF-κB pathway as measured by the phosphorylation and degradation of IκBα (Fig. 5). Thus, TRAF5 regulates a distinct and specific subset of early TLR-mediated signaling pathways in B cells. Notably, this subset is distinct from that regulated by TRAF3 in B cell TLR responses (8).
Figure 4. TLR-induced MAPK activation in TRAF5-deficient B lymphocytes.
Splenic B cells were isolated from TRAF5 KO and WT mice as described in Materials and Methods and stimulated with TLR7 agonist R848 for the indicated times. Preparation of whole cell lysates and Western blotting were as described in Materials and Methods. Western blots shown are representative of three experiments, while the quantitation provided combines three separate experiments.
Figure 5. TLR-induced NF-κB activation in TRAF5-deficient B lymphocytes.
Splenic B cells were isolated from TRAF5 KO and WT mice as described in Materials and Methods and stimulated with TLR7 agonist R848 for the indicated times. Preparation of whole cell lysates and Western blotting were as described in Materials and Methods. Western blots shown are representative of three experiments, while the quantitation provided combines three separate experiments.
TLR-induced association of TRAF5 with TLR signaling proteins
Fig. 1–5 show that TRAF5 negatively regulated MAPK signaling after TLR ligation, and also restrained cytokine and antibody production in B cells, while having little effect in TLR-stimulated myeloid cells. Additionally, its manner of action is independent of cell survival, proliferation, and NF-κB activation. A recent publication identified TAB2 as a positive regulator of TLR signaling, specifically in B lymphocytes. After TLR ligation, B lymphocytes from TAB2−/− mice show reduced phosphorylation of MAP kinases and produce less IL-6 and antibody. However, TAB2-deficiency has no effect on cell survival, proliferation, or NF-κB activation and does not affect TLR signaling in macrophages. Additionally, TAB2−/− mice have decreased marginal zone B cells in the spleen, in direct opposition to the increased marginal zone B cell population observed in TRAF5−/− mice (Fig. S3) (18).
As the phenotypes of these mice are directly opposite, we examined the potential interaction of TRAF5 with TAB2 as well as with the TLR adaptor protein MyD88. A carefully orchestrated series of protein-protein interactions mediates TLR signaling, and prior reports have established that TRAFs 6 and 3 participate in these interactions. Results presented above indicate that TRAF5 is also likely to interact with signaling proteins in TLR pathways. To address this prediction, expression of FLAG-tagged TRAF5 was induced in CH12.LX cells prior to stimulation through TLR7. Cell lysates were then subjected to immunoprecipitation using α-FLAG antibody.
An association between TRAF5 and MyD88 was markedly induced early after TLR ligation (Fig. 6A), demonstrating that TRAF5 was recruited to the TLR signaling complex. The reciprocal immunoprecipitation experiment using α-MyD88 antibody confirmed the interaction between TRAF5 and MyD88 (Fig. 6B). TRAF5 also associates with TAB2 after TLR ligation (Fig. 6A). To further elucidate this interaction, TRAF6 was immunoprecipitated from both TRAF5-sufficient and TRAF5-deficient primary mouse B cells as well as parent CH12.LX and CH12-TRAF5 cells before and after TLR7 stimulation. TRAF6 is a known binding partner of TAB2. After normalization to amount of TRAF6 in the IPs, more TAB2 associated with TRAF6 in TRAF5−/− B cells thirty minutes after TLR7 ligation while samples from CH12-TRAF5 cells showed reduced association between TAB2 and TRAF6 after TLR ligation, suggesting that TRAF5 acts on TAB2 in TLR signaling in B cells (Fig. 6C). These data demonstrate that TRAF5 was recruited to TLR signaling complexes after TLR ligation where it associated with the B cell-specific positive regulator of TLR signaling, TAB2, decreasing its association with TRAF6.
Figure 6. TLR-induced association of TRAF5 with TLR signaling proteins.
CH12.LX B cells (A, B, D) were cultured for 20 hours with IPTG to induce expression of FLAG-tagged TRAF5. Splenic B cell isolation was performed as described in Materials and Methods (C). Cells were stimulated with TLR7 agonist R848 for the indicated times. Immunoprecipitation of FLAG (A), MyD88 (B), TRAF6 (C, D) and Western blotting was performed as described in Materials and Methods. Quantitation in D is shown below the blots as percent of maximum association.
DISCUSSION
Although TLRs are expressed by a variety of cell types, the majority of published reports on this important receptor family have examined their functions in myeloid cells, including the roles played by TRAF molecules in these functions (6, 7, 26–28). However, TLR functions also have major effects on B lymphocytes (29), and it has been recently demonstrated that TRAF3 plays very distinct roles in TLR signaling to B vs. myeloid cells (8). In the present study, the adaptor protein TRAF5 was revealed to inhibit TLR function in B lymphocytes, and to a lesser extent in myeloid cells. The regulatory impact was most marked in B cells, further supporting the concept that important TLR functions and regulatory mechanisms can be distinct in B lymphocytes vs. myeloid cells.
In this regard, it was shown previously that TRAF5 cooperates with TRAF3 to activate NF-κB and the transcription factor IRF3 after stimulation of the RNA helicases RIG-I and MDA-5 (30). In contrast, TRAF5 and TRAF3 affect TLR signaling pathways by distinct and non-redundant, complementary mechanisms in B cells. B lymphocytes deficient in either TRAF3 or TRAF5 show enhanced cytokine and antibody secretion after TLR ligation. However, the signaling pathways affected by these specific TRAFs show distinct differences. TRAF5−/− B cells displayed elevated ERK1/2 and JNK activation, while the canonical NF-κB pathway was unaffected (Figs. 4 and 5). In contrast, TRAF3−/− B cells show enhanced canonical NF-κB activation after TLR ligation, but do not exhibit changes in MAPK activation (8).
TRAF5 is less well examined and understood compared to other TRAF proteins, and most reports to date have focused upon its function in lymphocytes. It was shown previously that unlike TRAF5-deficient B cells, TRAF5−/− BMDC do not show defects in surface molecule upregulation after CD40 ligation (11, 12). Together with data shown here, this suggests that TRAF5 may play a lesser role in myeloid vs. lymphoid cells. This emphasizes how TRAF proteins function in both a cell-type and receptor-specific fashion (29).
Fig. 6 demonstrates association of TRAF5 with the adaptor protein MyD88, indicating that like TRAFs 3 and 6, TRAF5 is present within the TLR signaling complex. Additionally, TRAF5 associates with TAB2 after TLR ligation. A recent publication identified TAB2 as a positive regulator of TLR signaling, specifically in B lymphocytes. After TLR ligation, B lymphocytes from TAB2-deficient mice show reduced phosphorylation of MAP kinases and produce less IL-6 and antibody. However, TAB2 deficiency has no effect on cell survival, proliferation, or NF-κB activation and does not affect TLR signaling in macrophages. Additionally TAB2−/− mice have decreased marginal zone B cells in the spleen, which contrasts with the increased marginal zone B cell population that we observed in TRAF5−/− mice (Fig. S3) (18). Fig. 1–5 show that TRAF5 negatively regulated MAPK signaling after TLR ligation, resulting in restrained cytokine and antibody production in B cells, while having little effect in TLR-stimulated myeloid cells. Additionally, its manner of action was independent of cell survival, proliferation, and NF-κB activation.
As TAB2−/− and TRAF5−/− mice exhibit opposite phenotypes and TRAF5 associates with TAB2 after TLR stimulation in B cells, it is reasonable to hypothesize that TRAF5 negatively regulates TLR signaling in B lymphocytes by acting on the positive regulator TAB2. After TLR ligation, TAB2 links TRAF6 to TAK1, facilitating downstream activation of MAP kinases (31). In the absence of TRAF5, we observed greater association of TAB2 with TRAF6 after TLR7 ligation in B cells (Fig. 6C). The reciprocal experiment showed a reduced association of TAB2 with TRAF6 when TRAF5 was overexpressed in B cells (Fig. 6D). This could result from competition between TAB2 and TRAF5 for association with TRAF6. As TAB2 positively regulates MAPK pathways in B cells, its increased association with TRAF6 in the absence of TRAF5 could account for the increased MAPK phosphorylation and resulting enhancement of cytokine production seen in TLR-stimulated TRAF5-deficient B cells (Figs. 1 and 4). This agrees with a recent report in which TRAF5 negatively regulates the ERK1/2 pathway in a model of cardiac hypertrophy (32).
TLR signaling to B cells is biologically important in both normal and pathogenic immunity. Aberrant TLR signaling in B cells has been linked to autoimmune diseases, making identification of novel TLR signaling targets clinically relevant (3, 33). The present study shows for the first time that TRAF5 negatively regulates TLR signaling and downstream functions in B lymphocytes, associating with TLR signaling proteins and altering the association of TAB2 with TRAF6. Future investigations will seek to characterize in detail the mechanism(s) by which TRAF5 exerts its regulatory activities.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Timothy Rosean for his assistance with flow cytometry. This material is based upon work supported in part by the use of facilities and resources of the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development.
Footnotes
These studies were supported by grants from the NIH (R01AI28847) and the Dept. of Veterans Affairs (Merit Review 1I01BX001702) to GAB, and by the Roy J. and Lucille A. Carver Charitable Trust.
References
- 1.Aderem A, Ulevitch RJ. TLRs in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 2.Akira S. TLR signaling. J Biol Chem. 2003;278:38105–38108. doi: 10.1074/jbc.R300028200. [DOI] [PubMed] [Google Scholar]
- 3.Huang Q, Pope RM. TLR signaling: a potential link among rheumatoid arthritis, systemic lupus, and atherosclerosis. J Leukoc Biol. 2010;88:253–262. doi: 10.1189/jlb.0310126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ha H, Han D, Choi Y. TRAF-mediated TNFR-family signaling. In: Coligan John E, et al., editors. Current protocols in immunology. Unit11 19D. Chapter 11. 2009. [DOI] [PubMed] [Google Scholar]
- 5.Kirschning CJ, Wesche H, Merrill Ayres T, Rothe M. Human TLR2 confers responsiveness to bacterial LPS. J Exp Med. 1998;188:2091–2097. doi: 10.1084/jem.188.11.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hacker H, Redecke V, Blagoev B, Kratchmarova I, Hsu LC, Wang GG, Kamps MP, Raz E, Wagner H, Hacker G, Mann M, Karin M. Specificity in TLR signalling through distinct effector functions of TRAF3 and TRAF6. Nature. 2006;439:204–207. doi: 10.1038/nature04369. [DOI] [PubMed] [Google Scholar]
- 7.Oganesyan G, Saha SK, Guo B, He JQ, Shahangian A, Zarnegar B, Perry A, Cheng G. Critical role of TRAF3 in the TLR-dependent and -independent antiviral response. Nature. 2006;439:208–211. doi: 10.1038/nature04374. [DOI] [PubMed] [Google Scholar]
- 8.Xie P, Poovassery J, Stunz LL, Smith SM, Schultz ML, Carlin LE, Bishop GA. Enhanced TLR responses of TRAF3-deficient B lymphocytes. J Leukoc Biol. 2011;90:1149–1157. doi: 10.1189/jlb.0111044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akiba H, Nakano H, Nishinaka S, Shindo M, Kobata T, Atsuta M, Morimoto C, Ware CF, Malinin NL, Wallach D, Yagita H, Okumura K. CD27, a member of the TNFR superfamily, activates NF-κB and stress-activated protein kinase/JNK via TRAF2, TRAF5, and NF-κB-inducing kinase. J Biol Chem. 1998;273:13353–13358. doi: 10.1074/jbc.273.21.13353. [DOI] [PubMed] [Google Scholar]
- 10.So T, Salek-Ardakani S, Nakano H, Ware CF, Croft M. TRAF5 limits the induction of Th2 immune responses. J Immunol. 2004;172:4292–4297. doi: 10.4049/jimmunol.172.7.4292. [DOI] [PubMed] [Google Scholar]
- 11.Kraus ZJ, Haring JS, Bishop GA. TRAF5 is required for optimal T cell expansion and survival in response to infection. J Immunol. 2008;181:7800–7809. doi: 10.4049/jimmunol.181.11.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakano H, Sakon S, Koseki H, Takemori T, Tada K, Matsumoto M, Munechika E, Sakai T, Shirasawa T, Akiba H, Kobata T, Santee SM, Ware CF, Rennert PD, Taniguchi M, Yagita H, Okumura K. Targeted disruption of Traf5 gene causes defects in CD40- and CD27-mediated lymphocyte activation. Proc Natl Acad Sci U S A. 1999;96:9803–9808. doi: 10.1073/pnas.96.17.9803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kraus ZJ, Nakano H, Bishop GA. TRAF5 is a critical mediator of in vitro signals and in vivo functions of LMP1, the viral oncogenic mimic of CD40. Proc Natl Acad Sci U S A. 2009;106:17140–17145. doi: 10.1073/pnas.0903786106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pullen SS, Miller HG, Everdeen DS, Dang TT, Crute JJ, Kehry MR. CD40-TRAF interactions: regulation of CD40 signaling through multiple TRAF binding sites and TRAF hetero-oligomerization. Biochemistry. 1998;37:11836–11845. doi: 10.1021/bi981067q. [DOI] [PubMed] [Google Scholar]
- 15.Arcipowski KM, Stunz LL, Graham JP, Kraus ZJ, Vanden Bush TJ, Bishop GA. Molecular mechanisms of TRAF6 utilization by the oncogenic viral mimic of CD40, LMP1. J Biol Chem. 2011;286:9948–9955. doi: 10.1074/jbc.M110.185983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He L, Grammer AC, Wu X, Lipsky PE. TRAF3 forms heterotrimers with TRAF2 and modulates its ability to mediate NF-κB activation. J Biol Chem. 2004;279:55855–55865. doi: 10.1074/jbc.M407284200. [DOI] [PubMed] [Google Scholar]
- 17.Missiou A, Rudolf P, Stachon P, Wolf D, Varo N, Aichele P, Colberg C, Hoppe N, Ernst S, Munkel C, Walter C, Sommer B, Hilgendorf I, Nakano H, Bode C, Zirlik A. TRAF5 deficiency accelerates atherogenesis in mice by increasing inflammatory cell recruitment and foam cell formation. Circ Res. 2010;107:757–766. doi: 10.1161/CIRCRESAHA.110.219295. [DOI] [PubMed] [Google Scholar]
- 18.Ori D, Kato H, Sanjo H, Tartey S, Mino T, Akira S, Takeuchi O. Essential roles of K63-linked polyubiquitin-binding proteins TAB2 and TAB3 in B cell activation via MAPKs. J Immunol. 2013;190:4037–4045. doi: 10.4049/jimmunol.1300173. [DOI] [PubMed] [Google Scholar]
- 19.Bishop GA, Haughton G. Induced differentiation of a transformed clone of Ly-1+ B cells by clonal T cells and antigen. Proc Natl Acad Sci U S A. 1986;83:7410–7414. doi: 10.1073/pnas.83.19.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Busch LK, Bishop GA. The EBV transforming protein, LMP1, mimics and cooperates with CD40 signaling in B lymphocytes. J Immunol. 1999;162:2555–2561. [PubMed] [Google Scholar]
- 21.Hostager BS, Bishop GA. Role of TRAF2 in the activation of IgM secretion by CD40 and CD120b. J Immunol. 2002;168:3318–3322. doi: 10.4049/jimmunol.168.7.3318. [DOI] [PubMed] [Google Scholar]
- 22.Arcipowski KM, Bishop GA. TRAF binding is required for a distinct subset of in vivo B cell functions of the oncoprotein LMP1. J Immunol. 2012;189:5165–5170. doi: 10.4049/jimmunol.1201821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rowland SL, Tremblay MM, Ellison JM, Stunz LL, Bishop GA, Hostager BS. A novel mechanism for TRAF6-dependent CD40 signaling. J Immunol. 2007;179:4645–4653. doi: 10.4049/jimmunol.179.7.4645. [DOI] [PubMed] [Google Scholar]
- 24.Mercolino TJ, Arnold LW, Haughton G. Phosphatidyl choline is recognized by a series of Ly-1+ murine B cell lymphomas specific for erythrocyte membranes. J Exp Med. 1986;163:155–165. doi: 10.1084/jem.163.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bishop GA. Requirements of class II-mediated B cell differentiation for class II cross-linking and cyclic AMP. J Immunol. 1991;147:1107–1114. [PubMed] [Google Scholar]
- 26.Butt AQ, Ahmed S, Maratha A, Miggin SM. 14-3-3ε and 14-3-3σ inhibit TLR-mediated proinflammatory cytokine induction. J Biol Chem. 2012;287:38665–38679. doi: 10.1074/jbc.M112.367490. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Schneider M, Zimmermann AG, Roberts RA, Zhang L, Swanson KV, Wen H, Davis BK, Allen IC, Holl EK, Ye Z, Rahman AH, Conti BJ, Eitas TK, Koller BH, Ting JP. The innate immune sensor NLRC3 attenuates TLR signaling via modification of the signaling adaptor TRAF6 and transcription factor NF-κB. Nat Immunol. 2012;13:823–831. doi: 10.1038/ni.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi T, Walsh PT, Walsh MC, Speirs KM, Chiffoleau E, King CG, Hancock WW, Caamano JH, Hunter CA, Scott P, Turka LA, Choi Y. TRAF6 is a critical factor for dendritic cell maturation and development. Immunity. 2003;19:353–363. doi: 10.1016/s1074-7613(03)00230-9. [DOI] [PubMed] [Google Scholar]
- 29.Bishop GA. The multifaceted roles of TRAFs in the regulation of B-cell function. Nat Rev Immunol. 2004;4:775–786. doi: 10.1038/nri1462. [DOI] [PubMed] [Google Scholar]
- 30.Tang ED, Wang CY. TRAF5 is a downstream target of MAVS in antiviral innate immune signaling. PLoS One. 2010;5:e9172. doi: 10.1371/journal.pone.0009172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takaesu G, Kishida S, Hiyama A, Yamaguchi K, Shibuya H, Irie K, Ninomiya-Tsuji J, Matsumoto K. TAB2, a novel adaptor protein, mediates activation of TAK1 MAPKKK by linking TAK1 to TRAF6 in the IL-1 signal transduction pathway. Mol cell. 2000;5:649–658. doi: 10.1016/s1097-2765(00)80244-0. [DOI] [PubMed] [Google Scholar]
- 32.Bian Z, Dai J, Hiroyasu N, Guan H, Yuan Y, Gan L, Zhou H, Zong J, Zhang Y, Li F, Yan L, Shen D, Li H, Tang Q. Disruption of TRAF5 exacerbates pressure overload cardiac hypertrophy and fibrosis. J Cell Biochem. 2013 doi: 10.1002/jcb.24669. [DOI] [PubMed] [Google Scholar]
- 33.Sabroe I, Read RC, Whyte MK, Dockrell DH, Vogel SN, Dower SK. TLRs in health and disease: complex questions remain. J Immunol. 2003;171:1630–1635. doi: 10.4049/jimmunol.171.4.1630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.