Abstract
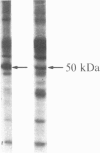
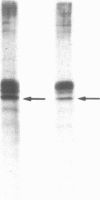
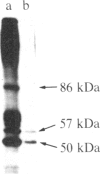
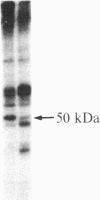
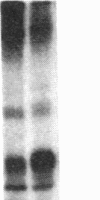
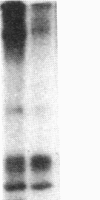
After prolonged visual adaptation of Drosophila, dramatic long-term changes of in vitro phosphorylation of a 50-kDa brain protein that is identical to the Ca2+/calmodulin-dependent protein kinase (EC 2.7.1.37) can be measured in isolated heads. By selective receptor cell desensitization in blue light, subcellular distribution of the 50-kDa kinase in fly brain is modified, and Ca2+-stimulated in vitro phosphorylation is increased. Concomitantly the 50-kDa kinase is translocated by in vitro phosphorylation from the membrane-cytoskeleton complex into the cytoplasm. After adaptation, association of the enzyme to the membrane shows long-term modification. In yellow light, which reverts receptor cell adaptation within seconds, the changes in kinase activity and distribution remain for about 2 hr, corresponding to the duration of behavioral modification induced by blue light. Reducing protein synthesis with cycloheximide inhibits the induction of behavioral modification as well as the prolonged modulation of the 50-kDa kinase by blue light. From our simple assay to measure biochemical changes induced in the intact organism by sensory stimulation, we propose that Ca2+/calmodulin-dependent kinase II is involved in long-term modulation of synaptic transmission.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aceves-Piña E. O., Booker R., Duerr J. S., Livingstone M. S., Quinn W. G., Smith R. F., Sziber P. P., Tempel B. L., Tully T. P. Learning and memory in Drosophila, studied with mutants. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):831–840. doi: 10.1101/sqb.1983.048.01.086. [DOI] [PubMed] [Google Scholar]
- Alkon D. L. Calcium-mediated reduction of ionic currents: a biophysical memory trace. Science. 1984 Nov 30;226(4678):1037–1045. doi: 10.1126/science.6093258. [DOI] [PubMed] [Google Scholar]
- Bennett M. K., Erondu N. E., Kennedy M. B. Purification and characterization of a calmodulin-dependent protein kinase that is highly concentrated in brain. J Biol Chem. 1983 Oct 25;258(20):12735–12744. [PubMed] [Google Scholar]
- Dingley F., Smith J. M. Temperature acclimatization in the absence of protein synthesis in Drosophila subobscura. J Insect Physiol. 1968 Aug;14(8):1185–1194. doi: 10.1016/0022-1910(68)90058-9. [DOI] [PubMed] [Google Scholar]
- Dudaí Y., Uzzan A., Zvi S. Abnormal activity of adenylate cyclase in the Drosophila memory mutant rutabaga. Neurosci Lett. 1983 Dec 2;42(2):207–212. doi: 10.1016/0304-3940(83)90408-1. [DOI] [PubMed] [Google Scholar]
- Farley J., Alkon D. L. Cellular mechanisms of learning, memory, and information storage. Annu Rev Psychol. 1985;36:419–494. doi: 10.1146/annurev.ps.36.020185.002223. [DOI] [PubMed] [Google Scholar]
- Huttner W. B., Schiebler W., Greengard P., De Camilli P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J Cell Biol. 1983 May;96(5):1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E. R., Abrams T., Bernier L., Carew T. J., Hawkins R. D., Schwartz J. H. Classical conditioning and sensitization share aspects of the same molecular cascade in Aplysia. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):821–830. doi: 10.1101/sqb.1983.048.01.085. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Schwartz J. H. Molecular biology of learning: modulation of transmitter release. Science. 1982 Oct 29;218(4571):433–443. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lisman J. E. A mechanism for memory storage insensitive to molecular turnover: a bistable autophosphorylating kinase. Proc Natl Acad Sci U S A. 1985 May;82(9):3055–3057. doi: 10.1073/pnas.82.9.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., McGuinness T. L., Leonard C. S., Sugimori M., Greengard P. Intraterminal injection of synapsin I or calcium/calmodulin-dependent protein kinase II alters neurotransmitter release at the squid giant synapse. Proc Natl Acad Sci U S A. 1985 May;82(9):3035–3039. doi: 10.1073/pnas.82.9.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H., Pak W. L. Light-induced phosphorylation of retina-specific polypeptides of Drosophila in vivo. Science. 1984 Jan 13;223(4632):184–186. doi: 10.1126/science.6419348. [DOI] [PubMed] [Google Scholar]
- Miller S. G., Kennedy M. B. Regulation of brain type II Ca2+/calmodulin-dependent protein kinase by autophosphorylation: a Ca2+-triggered molecular switch. Cell. 1986 Mar 28;44(6):861–870. doi: 10.1016/0092-8674(86)90008-5. [DOI] [PubMed] [Google Scholar]
- Quinn W. G., Sziber P. P., Booker R. The Drosophila memory mutant amnesiac. Nature. 1979 Jan 18;277(5693):212–214. doi: 10.1038/277212a0. [DOI] [PubMed] [Google Scholar]
- Saitoh T., Schwartz J. H. Phosphorylation-dependent subcellular translocation of a Ca2+/calmodulin-dependent protein kinase produces an autonomous enzyme in Aplysia neurons. J Cell Biol. 1985 Mar;100(3):835–842. doi: 10.1083/jcb.100.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh T., Schwartz J. H. Serotonin alters the subcellular distribution of a Ca2+/calmodulin-binding protein in neurons of Aplysia. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6708–6712. doi: 10.1073/pnas.80.21.6708. [DOI] [PMC free article] [PubMed] [Google Scholar]