Abstract
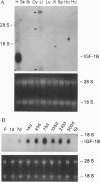
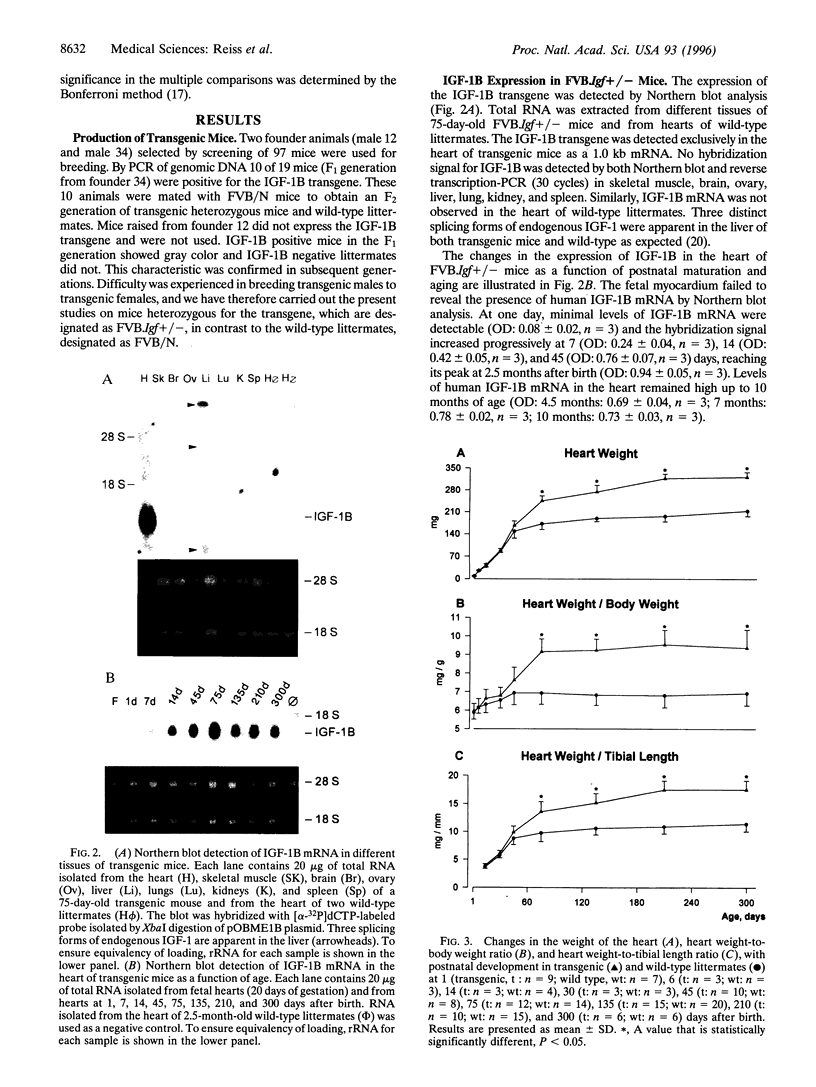
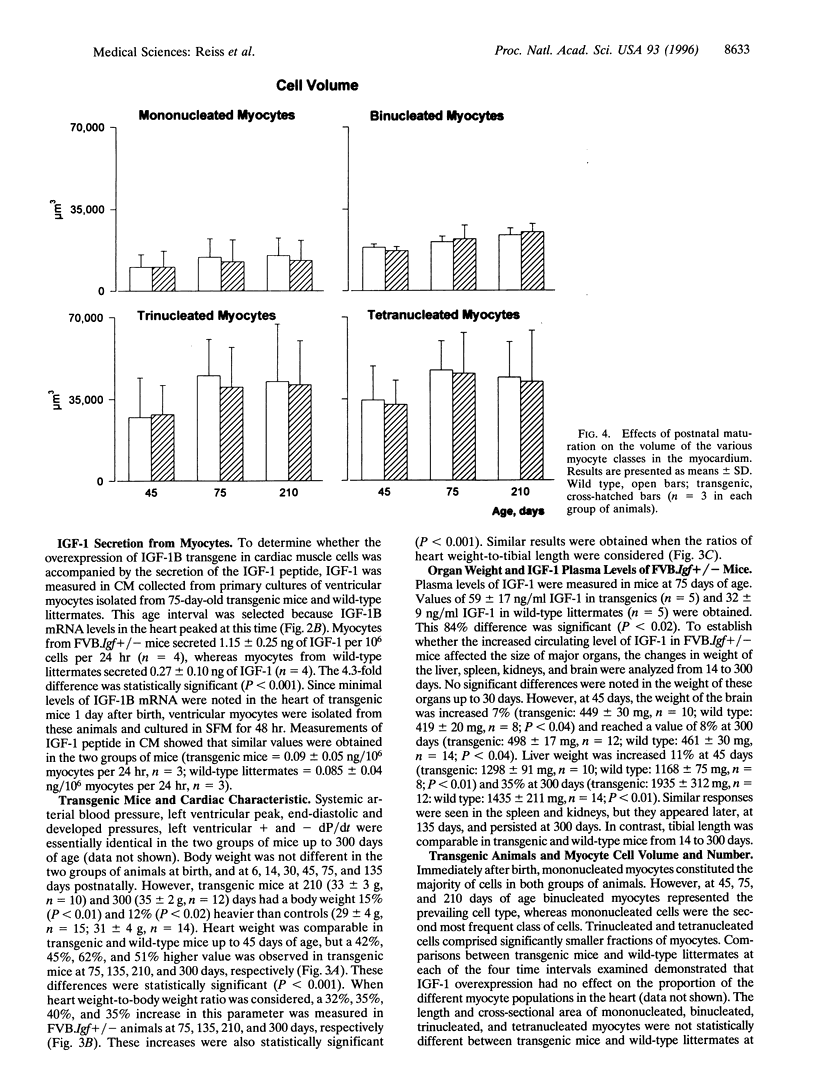
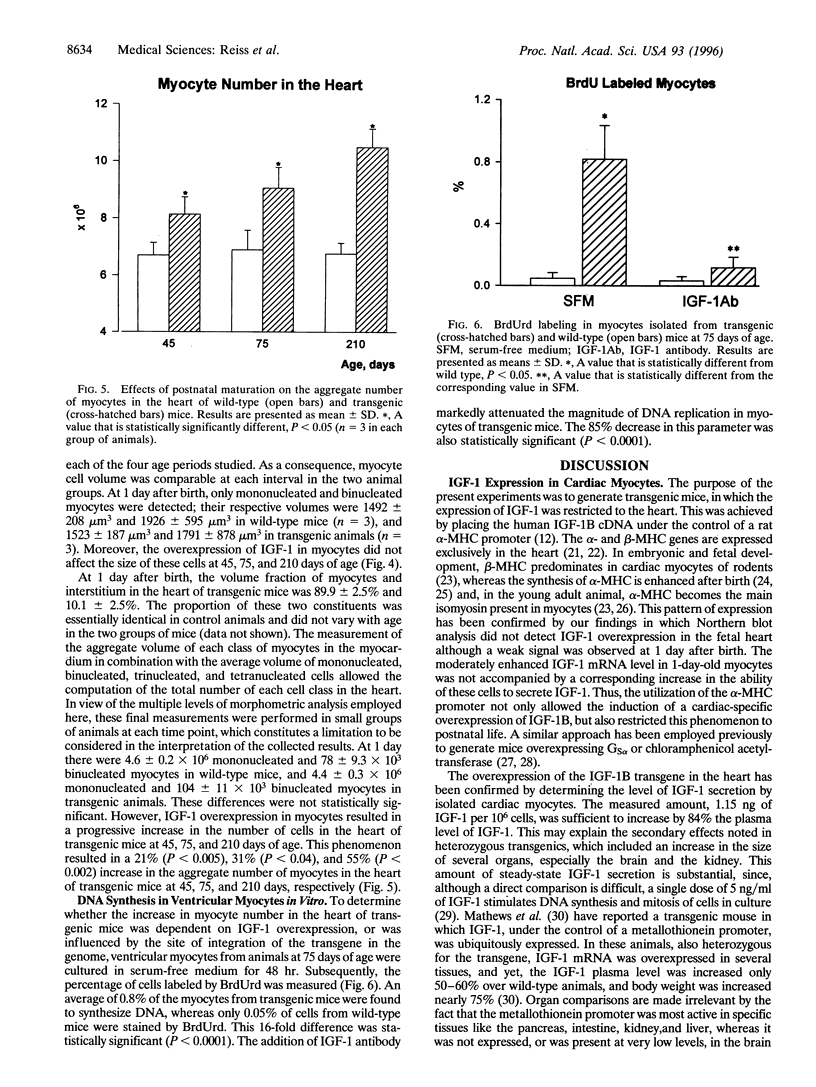
Transgenic mice were generated in which the cDNA for the human insulin-like growth factor 1B (IGF-1B) was placed under the control of a rat alpha-myosin heavy chain promoter. In mice heterozygous for the transgene, IGF-1B mRNA was not detectable in the fetal heart at the end of gestation, was present in modest levels at 1 day after birth, and increased progressively with postnatal maturation, reaching a peak at 75 days. Myocytes isolated from transgenic mice secreted 1.15 +/- 0.25 ng of IGF-1 per 10(6) cells per 24 hr versus 0.27 +/- 0.10 ng in myocytes from homozygous wild-type littermates. The plasma level of IGF-1 increased 84% in transgenic mice. Heart weight was comparable in wild-type littermates and transgenic mice up to 45 days of age, but a 42%, 45%, 62%, and 51% increase was found at 75, 135, 210, and 300 days, respectively, after birth. At 45, 75, and 210 days, the number of myocytes in the heart was 21%, 31%, and 55% higher, respectively, in transgenic animals. In contrast, myocyte cell volume was comparable in transgenic and control mice at all ages. In conclusion, overexpression of IGF-1 in myocytes leads to cardiomegaly mediated by an increased number of cells in the heart.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almendral J. M., Huebsch D., Blundell P. A., Macdonald-Bravo H., Bravo R. Cloning and sequence of the human nuclear protein cyclin: homology with DNA-binding proteins. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1575–1579. doi: 10.1073/pnas.84.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anversa P., Fitzpatrick D., Argani S., Capasso J. M. Myocyte mitotic division in the aging mammalian rat heart. Circ Res. 1991 Oct;69(4):1159–1164. doi: 10.1161/01.res.69.4.1159. [DOI] [PubMed] [Google Scholar]
- Anversa P., Olivetti G., Loud A. V. Morphometric study of early postnatal development in the left and right ventricular myocardium of the rat. I. Hypertrophy, hyperplasia, and binucleation of myocytes. Circ Res. 1980 Apr;46(4):495–502. doi: 10.1161/01.res.46.4.495. [DOI] [PubMed] [Google Scholar]
- Baserga R., Rubin R. Cell cycle and growth control. Crit Rev Eukaryot Gene Expr. 1993;3(1):47–61. [PubMed] [Google Scholar]
- Baserga R. The insulin-like growth factor I receptor: a key to tumor growth? Cancer Res. 1995 Jan 15;55(2):249–252. [PubMed] [Google Scholar]
- Cheng W., Reiss K., Kajstura J., Kowal K., Quaini F., Anversa P. Down-regulation of the IGF-1 system parallels the attenuation in the proliferative capacity of rat ventricular myocytes during postnatal development. Lab Invest. 1995 Jun;72(6):646–655. [PubMed] [Google Scholar]
- Chizzonite R. A., Zak R. Regulation of myosin isoenzyme composition in fetal and neonatal rat ventricle by endogenous thyroid hormones. J Biol Chem. 1984 Oct 25;259(20):12628–12632. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Donath M. Y., Zapf J., Eppenberger-Eberhardt M., Froesch E. R., Eppenberger H. M. Insulin-like growth factor I stimulates myofibril development and decreases smooth muscle alpha-actin of adult cardiomyocytes. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1686–1690. doi: 10.1073/pnas.91.5.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue T. J., Dworkin L. D., Lango M. N., Fliegner K., Lango R. P., Benstein J. A., Slater W. R., Catanese V. M. Induction of myocardial insulin-like growth factor-I gene expression in left ventricular hypertrophy. Circulation. 1994 Feb;89(2):799–809. doi: 10.1161/01.cir.89.2.799. [DOI] [PubMed] [Google Scholar]
- Gaudin C., Ishikawa Y., Wight D. C., Mahdavi V., Nadal-Ginard B., Wagner T. E., Vatner D. E., Homcy C. J. Overexpression of Gs alpha protein in the hearts of transgenic mice. J Clin Invest. 1995 Apr;95(4):1676–1683. doi: 10.1172/JCI117843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulick J., Subramaniam A., Neumann J., Robbins J. Isolation and characterization of the mouse cardiac myosin heavy chain genes. J Biol Chem. 1991 May 15;266(14):9180–9185. [PubMed] [Google Scholar]
- Hanson M. C., Fath K. A., Alexander R. W., Delafontaine P. Induction of cardiac insulin-like growth factor I gene expression in pressure overload hypertrophy. Am J Med Sci. 1993 Aug;306(2):69–74. doi: 10.1097/00000441-199308000-00001. [DOI] [PubMed] [Google Scholar]
- Ito H., Hiroe M., Hirata Y., Tsujino M., Adachi S., Shichiri M., Koike A., Nogami A., Marumo F. Insulin-like growth factor-I induces hypertrophy with enhanced expression of muscle specific genes in cultured rat cardiomyocytes. Circulation. 1993 May;87(5):1715–1721. doi: 10.1161/01.cir.87.5.1715. [DOI] [PubMed] [Google Scholar]
- Kajstura J., Cheng W., Reiss K., Anversa P. The IGF-1-IGF-1 receptor system modulates myocyte proliferation but not myocyte cellular hypertrophy in vitro. Exp Cell Res. 1994 Dec;215(2):273–283. doi: 10.1006/excr.1994.1343. [DOI] [PubMed] [Google Scholar]
- Kajstura J., Zhang X., Liu Y., Szoke E., Cheng W., Olivetti G., Hintze T. H., Anversa P. The cellular basis of pacing-induced dilated cardiomyopathy. Myocyte cell loss and myocyte cellular reactive hypertrophy. Circulation. 1995 Oct 15;92(8):2306–2317. doi: 10.1161/01.cir.92.8.2306. [DOI] [PubMed] [Google Scholar]
- Kajstura J., Zhang X., Reiss K., Szoke E., Li P., Lagrasta C., Cheng W., Darzynkiewicz Z., Olivetti G., Anversa P. Myocyte cellular hyperplasia and myocyte cellular hypertrophy contribute to chronic ventricular remodeling in coronary artery narrowing-induced cardiomyopathy in rats. Circ Res. 1994 Mar;74(3):383–400. doi: 10.1161/01.res.74.3.383. [DOI] [PubMed] [Google Scholar]
- Kardami E. Stimulation and inhibition of cardiac myocyte proliferation in vitro. Mol Cell Biochem. 1990 Feb 9;92(2):129–135. doi: 10.1007/BF00218130. [DOI] [PubMed] [Google Scholar]
- Lompré A. M., Nadal-Ginard B., Mahdavi V. Expression of the cardiac ventricular alpha- and beta-myosin heavy chain genes is developmentally and hormonally regulated. J Biol Chem. 1984 May 25;259(10):6437–6446. [PubMed] [Google Scholar]
- Mahdavi V., Chambers A. P., Nadal-Ginard B. Cardiac alpha- and beta-myosin heavy chain genes are organized in tandem. Proc Natl Acad Sci U S A. 1984 May;81(9):2626–2630. doi: 10.1073/pnas.81.9.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdavi V., Periasamy M., Nadal-Ginard B. Molecular characterization of two myosin heavy chain genes expressed in the adult heart. Nature. 1982 Jun 24;297(5868):659–664. doi: 10.1038/297659a0. [DOI] [PubMed] [Google Scholar]
- Mathews L. S., Hammer R. E., Behringer R. R., D'Ercole A. J., Bell G. I., Brinster R. L., Palmiter R. D. Growth enhancement of transgenic mice expressing human insulin-like growth factor I. Endocrinology. 1988 Dec;123(6):2827–2833. doi: 10.1210/endo-123-6-2827. [DOI] [PubMed] [Google Scholar]
- Ng W. A., Grupp I. L., Subramaniam A., Robbins J. Cardiac myosin heavy chain mRNA expression and myocardial function in the mouse heart. Circ Res. 1991 Jun;68(6):1742–1750. doi: 10.1161/01.res.68.6.1742. [DOI] [PubMed] [Google Scholar]
- Pietrzkowski Z., Lammers R., Carpenter G., Soderquist A. M., Limardo M., Phillips P. D., Ullrich A., Baserga R. Constitutive expression of insulin-like growth factor 1 and insulin-like growth factor 1 receptor abrogates all requirements for exogenous growth factors. Cell Growth Differ. 1992 Apr;3(4):199–205. [PubMed] [Google Scholar]
- Reiss K., Kajstura J., Capasso J. M., Marino T. A., Anversa P. Impairment of myocyte contractility following coronary artery narrowing is associated with activation of the myocyte IGF1 autocrine system, enhanced expression of late growth related genes, DNA synthesis, and myocyte nuclear mitotic division in rats. Exp Cell Res. 1993 Aug;207(2):348–360. doi: 10.1006/excr.1993.1202. [DOI] [PubMed] [Google Scholar]
- Reiss K., Kajstura J., Zhang X., Li P., Szoke E., Olivetti G., Anversa P. Acute myocardial infarction leads to upregulation of the IGF-1 autocrine system, DNA replication, and nuclear mitotic division in the remaining viable cardiac myocytes. Exp Cell Res. 1994 Aug;213(2):463–472. doi: 10.1006/excr.1994.1224. [DOI] [PubMed] [Google Scholar]
- Schwartz K., Lompre A. M., Bouveret P., Wisnewsky C., Whalen R. G. Comparisons of rat cardiac myosins at fetal stages in young animals and in hypothyroid adults. J Biol Chem. 1982 Dec 10;257(23):14412–14418. [PubMed] [Google Scholar]
- Sinha A. M., Umeda P. K., Kavinsky C. J., Rajamanickam C., Hsu H. J., Jakovcic S., Rabinowitz M. Molecular cloning of mRNA sequences for cardiac alpha- and beta-form myosin heavy chains: expression in ventricles of normal, hypothyroid, and thyrotoxic rabbits. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5847–5851. doi: 10.1073/pnas.79.19.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussenbach J. S., Steenbergh P. H., Jansen E., Holthuizen P., Meinsma D., van Dijk M. A., Gloudemans T. Structural and regulatory aspects of the human genes encoding IGF-I and -II. Adv Exp Med Biol. 1991;293:1–14. doi: 10.1007/978-1-4684-5949-4_1. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Yamamoto H., Takeuchi S., Takeuchi T. Melanization in albino mice transformed by introducing cloned mouse tyrosinase gene. Development. 1990 Feb;108(2):223–227. doi: 10.1242/dev.108.2.223. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Zetterberg A., Larsson O. Coordination between cell growth and cell cycle transit in animal cells. Cold Spring Harb Symp Quant Biol. 1991;56:137–147. doi: 10.1101/sqb.1991.056.01.018. [DOI] [PubMed] [Google Scholar]