Abstract
The Osmanthus fragrans flower, a popular herb in Eastern countries, contains several antioxidant compounds. Ben Cao Gang Mu, traditional Chinese medical literature, describes the usefulness of these flowers for phlegm and stasis reduction, arrest of dysentery with blood in the bowel, and stomachache and diarrhea treatment. However, modern evidence regarding the therapeutic efficacy of these flowers is limited. This study was aimed at assessing the antioxidative effects of the ethanol extract of O. fragrans flowers (OFE) in vivo and evaluating its antioxidant maintenance and therapeutic effect on an allergic airway inflammation in mice. After OFE's oral administration to mice, the values obtained in the oxygen radical absorbance capacity assay as well as the glutathione concentration in the lungs and spleens of mice increased while thiobarbituric acid reactive substances decreased significantly, indicating OFE's significant in vivo antioxidant activity. OFE was also therapeutically efficacious in a mouse model of ovalbumin-induced allergic airway inflammation. Orally administered OFE suppressed ovalbumin-specific IgE production and inflammatory cell infiltration in the lung. Moreover, the antioxidative state of the mice improved. Thus, our findings confirm the ability of the O. fragrans flowers to reduce phlegm and suggest that OFE may be useful as an antiallergic agent.
1. Introduction
Osmanthus fragrans, known as sweet olive, tea olive, and fragrant olive, is a species of Oleaceae native to southwestern China [1]. It is widely cultivated as an ornamental plant for its fragrant flowers in Taiwan, southern Japan, southern China, Europe, North America, and elsewhere. The flower of O. fragrans, called Kwai-fah in China, has been used as a beverage and as an additive for tea and foods such as cake, pastry, paste, vinegar, and liqueurs. It is popular because of its delicate fruity/floral aroma. Various volatile components of the flowers are also used, primarily for perfumes, flavors, and aromatherapy. It was recorded in Ben Cao Gang Mu that the O. fragrans flower was used to reduce phlegm and stasis as well as to arrest dysentery with blood in the bowel. Traditional Chinese medicine has also suggested the use of O. fragrans to treat weakened vision, halitosis, panting, asthma, cough, toothache, stomachache, diarrhea, and hepatitis. However, modern evidence for the biomedical use of the ethanol extract of O. fragrans flowers (OFE) is limited.
Overproduction of free radicals can cause oxidative damage and may eventually lead to chronic diseases. Many plants contain free radical-scavenging molecules such as phenolic acids and flavonoids, which show strong antioxidant activity. There are some reports of antioxidant activity of O. fragrans. Lee et al. demonstrated that the dried flowers of O. fragrans contain abundant phenols and flavonoids and exhibit antioxidant activity in the ferric reducing ability of plasma (FRAP) assay, 1,1-diphenyl-2-picryl-hydrazyl (DPPH) radical-scavenging activity, and hydroxyl anion scavenging ability [2]. Our group has also analyzed many antioxidant components of OFE and found that the antioxidant activityof O. fragrans is slightly weaker than that of green tea [3]. In the aforementioned study, five phenolic compounds, tyrosyl acetate (1), (+)-phillygenin (2), (8E)-ligustroside (3), rutin (4), and verbascoside (5), were isolated from the CHCl3 layer of O. fragrans. Evaluation of the antioxidative properties of the isolated compounds 2, 4, and 5 revealed strong DPPH radical-scavenging activity, with IC50 values of 19.1, 10.3, and 6.2 μM, respectively. These isolates also exhibited an H2O2-scavenging ability, with IC50 values of 10.5, 23.4, and 13.4 μM, respectively.
Although modern evidence for the in vivo therapeutic activity of O. fragrans is yet to be provided, a few studies have examined the in vitro bioactivity of OFE. For example, OFE inhibited lipid peroxidation occurring through ferrous chloride in the mitochondria in rat brain, liver, heart, and kidney [2]. OFE may also exert neuroprotective actions through the upregulation of the AKT survival pathway, which attenuates neurotoxicity [2]. Several lignans isolated from the flowers of O. fragrans var. aurantiacus inhibited nitric oxide production in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages [4].
Asthma is a chronic respiratory disease characterized by airway inflammation and airway hyperresponsiveness (AHR). Previous reviews have highlighted the importance of reactive oxygen/nitrogen species in airway inflammation [5, 6]. Reactive oxygen species (ROS) also contribute to lipid peroxidation. The pathologic effects of lipid peroxidation, including epithelial cell activation, disruption, or death, smooth muscle contraction, airway hypersensitivity, and increased mucus secretion, are all observed in asthma [7]. Losing control of intracellular oxidants to environmental cellular stress also may lead to allergic disorders [8]. For example, the polarization of the helper T cells Th1/Th2 balance is dependent on the intracellular thiol-redox status of macrophages [9]. In another study, Pazmandi et al. observed that H2O2-exposed plasmacytoid dendritic cells (pDCs) provided stronger stimuli for Th2 than for Th1 differentiation upon autologous activation (cocultivation with naïve autologous T cells) when compared to untreated pDCs [10]. Furthermore, enhanced oxidative stress may contribute to the progression or perpetuation of existing airway inflammation through enhanced AHR [11, 12] via airway smooth muscle contraction [13], mucus hypersecretion [14], epithelial shedding [15], vascular exudation [16, 17], and induction of various proinflammatory chemical mediators because of accumulation of inflammatory cells in the lower respiratory tract [18]. All of these actions are believed to be related to severe asthma [19–21].
The increase in ROS during an asthma exacerbation might overwhelm endogenous antioxidant defenses. Glutathione (GSH) is a key antioxidant in the lining fluid of the respiratory tract. Disturbed GSH status is reported in asthma for total [22] and for oxidized [23] GSH. Total GSH concentration, which includes the oxidized form GSSG, is higher in the bronchial and alveolar fluid in patients with mild asthma [22]. A recent report suggested that increasing GSH levels and the GSH/GSSG ratio with γ-glutamylcysteine ethyl ester (γ-GCE) ameliorated bronchial asthma by altering the Th1/Th2 cell imbalance through interleukin (IL-) 12 production from antigen presenting cells (APC) and by suppressing chemokine production and eosinophil migration [24]. Enhancing intracellular GSH can also decrease the release of cytokines from lung cells by decreasing NF-κB activation [25, 26]. In addition, GSH has recently been reported to attenuate IL-13 induced asthma in mice [27].
Liao et al. investigated the total antioxidant capability in serum and the activity of the antioxidant enzymes superoxide dismutase (SOD) and glutathione peroxidase in 46 asthmatic children and 52 normal controls [28]. The total antioxidant capability in the serum of asthmatic children is significantly less than that found in the serum of controls. Plasma levels of lipid peroxides are also significantly increased in patients with asthma [29, 30].
The concentration of ascorbic acid and α-tocopherol in lung lining fluid is reportedly low in patients with mild asthma [23, 31]. Low dietary intake of vitamin C and manganese was found to be associated with an increased risk of bronchial activity [30]. Dietary supplements of vitamin C and vitamin E to asthmatics decrease the severity of pollutant-induced bronchial responsiveness [32]. Chang and Crapo [33] found that intratracheal treatment of ovalbumin (OVA-) challenged mice with the catalytic antioxidant, manganese (III) meso-tetrakis-(N-methylpyridinium-2-yl) porphyrin, having high SOD activity drastically reduced the severity of airway inflammation as evidenced by the reduced numbers of eosinophils, neutrophils, and lymphocytes found in bronchoalveolar lavage fluid.
The aim of the current study was to examine the mechanism of the preventive effect of OFE on allergic airway inflammation. The study comprised two parts: the first was to evaluate the antioxidation state following OFE administration and the second was to examine the immunomodulatory effects of OFE. We evaluated the antioxidative effects of OFE in naïve mice using the following methods: assessing the total antioxidant capacity in the lung and spleen by using the values obtained in the oxygen radical absorbance capacity (ORAC) assay, assessing the free radical-scavenging effects on DPPH, monitoring lipid peroxidation by measuring thiobarbituric acid reactive substances (TBARS), and determining the concentration of GSH. We used a mouse model of allergic airway inflammation to investigate the antiallergic and antioxidant effects of OFE as well as to determine organ weights.
2. Materials and Methods
2.1. Plant Material
Dried flowers of O. fragrans were collected from Nanto, Taiwan, in 2005-2006. They were examined and authenticated by Professor C. S. Kuoh, Department of Biology, National Cheng Kung University. A voucher specimen (no. Hung-0201) was deposited in the Herbarium of Chung Hwa University of Medical Technology.
2.2. Preparation of OFE
The dried flowers of O. fragrans were ground into a fine powder using a mill (RT-08, Rong Tsong, Taiwan), collected and sealed in a polyethylene plastic bag, and then stored at 0–4°C for further use. O. fragrans flowers (200 g) were soaked (72 h) in 75% ethanol (3 L) twice and filtered through Whatman no. 1 filter paper. The combined extracts were concentrated under reduced pressure and freeze-dried to provide a dark syrup that was stored at −20°C for further use. The extract was endotoxin free (≤0.1 E.U.) according to the Limulus amebocyte lysate (LAL) assay (Cambrex, Walkersville, MD).
2.2.1. Determination of Total Phenolic Content in OFE
Following the method described by Yen and Hung [34], the sample solution in methanol (0.1 mL, 1 mg/mL) was well mixed with 2% Na2CO3 (2 mL). After 3 min, 50% Folin-Ciocalteu agent (0.1 mL) was added. The mixture was allowed to stand at room temperature (RT) for 30 min with intermittent mixing. The absorbance at 750 nm was recorded. A standard curve using gallic acid was prepared. The total phenolic content was expressed as gallic acid equivalents (mg of GAE per g extract).
2.2.2. Determination of Total Flavonoid Content in OFE
Following the methods described by Woisky and Salatino [35] and also by Chang et al. [36], the sample solution (0.5 mL) was mixed with 95% EtOH (1.5 mL), 10% AlCl3 (0.1 mL), 1 M KOAc (0.1 mL), and distilled water (2.8 mL). The mixture was allowed to stand at RT for 30 min, and the absorbance was measured at 415 nm. The amount of sample solution was substituted by the same amount of a quercetin solution (0–200 μg/mL) as a standard. The amount of 10% aluminum chloride was substituted by the same amount of distilled water to serve as a blank. The total flavonoid content was calculated from the plot of absorbance against quercetin concentration using linear regression analysis and expressed as quercetin equivalents (μg of QE per g extract).
2.3. DPPH Free Radical-Scavenging Assay
DPPH is a stable free radical with a purple color that is reduced by antioxidants to a colorless compound. We employed DPPH in an assay modified from the method of Shimada et al. [37]. MeOH (3.8 mL), sample solution in methanol (0.2 mL, 1 mg/mL), and 1 mM DPPH solution (1.0 mL) were mixed well and left to stand in the dark at RT for 30 min. The final concentration of the sample was 40 μg/mL. The absorbance at 517 nm was measured. The sample in methanol was used as a blank, while DPPH radical in methanol solution was used as a control. The DPPH radical scavenging activity was calculated according to the following equation:
| (1) |
where A is the absorbance at 517 nm.
The concentration providing 50% inhibition (IC50) of the DPPH radical-scavenging activity was calculated from the plot of the percent inhibition against sample concentration by using a linear regression analysis.
2.4. Animals
Female BALB/c mice between six and eight weeks of age were purchased from the National Laboratory Animal Center in Taiwan. The animal room was kept on a 12 h light : dark cycle and maintained at a constant temperature (25°C ± 2°C) and humidity. Animal care and handling conformed to the NIH Guide for the Care and Use of Laboratory Animals. All experiments were performed under protocols approved by the biotechnology department of National Formosa University's affidavit of approval of animal use protocol. Pentobarbital (intraperitoneal, i.p.) injections were used to anesthetize (10 mg/mL, 60 μL per mouse) or sacrifice (10 mg/mL, 200 μL per mouse) the mice.
For the results obtained in the antioxidant evaluation assays, mice were divided into two groups comprised of four BALB/c mice each. Group 1 (control) received only distilled water. Group 2 (OFE) received 1000 mg/kg body weight OFE daily for 14 days by oral gavage.
2.5. Organ Collection, Preparation, and Protein Quantization
After sacrifice, organs from the mice were collected and weighed. The spleens and lungs (1 g) were homogenized in PBS (pH 7.2) on ice by using a homogenizer (Motor Drives, Glas-Col Inc., Terre Haute, IN). The homogenate was then centrifuged at 2200 ×g for 10 min, and the filtrate was collected. Protein was determined by using the BCA assay kit (Pierce, Rockford, IL) using bovine serum albumin as a standard.
2.6. Determination of Antioxidant Activity
2.6.1. Oxygen Radical Absorbance Capacity Assay (ORAC)
The total antioxidant activity of the organ samples was measured by using the oxygen radical absorbance capacity (ORAC) assay according to Chung et al. [38]. This assay was carried out in black-walled, 96-well plates at 37°C. All solutions were prepared in 75 mM phosphate buffer (Na2HPO4 : NaH2PO4, pH 7.0) and preincubated at 37°C for 30 min before use. Fifteen μL of organ homogenate (diluted 100 times) and 100 μL of 0.1 μM β-PE (β-phycoerythrin) were transferred directly into the well to incubate for 10 min using the FLUOstar OPTIMA microplate reader system (Galaxy BMG LABTECH Inc., Cary, NC). We rapidly added 75 mM 2,2′-azobis (2-amidinopropane) dihydrochloride (AAPH, 85 μL) and immediately measured the resulting fluorescence by using fluorescence filters with an excitation wavelength of 480 nm and an emission wavelength of 520 nm. The fluorescence was recorded at 5 min intervals for 120 min until the final value was less than 5% of the initial value. ORAC values from samples were calculated by using the following equation and expressed as Trolox equivalents: ORAC value (mM) = 20 × k × (S sample − S blank)/(S Trolox − S blank), where k was the sample dilution factor. The area under the curve (S) was calculated by the following equation:
| (2) |
where f 0 was the initial fluorescence reading at 0 min and f n represented the measurement at time n.
2.6.2. TBARS Assay
Lipid peroxidation was measured by determining the formation of malondialdehyde based on the presence of TBARS in the lung and spleen [39]. A standard curve was prepared using 1,1,3,3-tetraethoxypropane (Sigma, St. Louis, Mo). The organ filtrate (1 mL) and standard were mixed with 10% trichloroacetic acid (1 mL) 0.4% thiobarbituric acid (1 mL) and 0.2% butylated hydroxy toluene (BHT) (0.1 mL) (both reagents from Sigma, St. Louis, Mo). The reaction mixture was incubated at 95°C for 1 h, cooled under light-protected conditions, and then centrifuged at 2200 ×g for 10 min. The absorbance of the supernatant was measured using a microplate fluorescence reader (F-2500, Hitachi, Japan) in 96-well format with the excitation and emission filters set at 515 nm and 550 nm, respectively.
2.6.3. Measurement of Glutathione
The glutathione concentration was determined according to the method described by Sedlak and Lindsay [40] with little modification. The organ homogenate (150 μL) was mixed with 5% TCA solution (450 μL). The resulting solution was centrifuged at 16770 ×g for 10 min to remove protein. The supernatant (30 μL) and 0.01 M 5,5′-dithio-bis-(2-nitrobenzoic acid) (DTNB, 10 μL) were added to 0.4 M Tris buffer (140 μL, pH 8.9). The solution remained at RT for 5 min. The absorbance at 412 nm was measured by n ELISA Reader (VersaMax, Molecular Devices Inc., Sunnyvale, CA). A GSH solution (0–12 μM) was used as standard. Tris buffer was used as blank. The plasma GSH content was calculated from the plot of absorbance against GSH concentration.
2.7. Establishment of the Animal Model of Allergic Airway Inflammation
The schedule for OVA sensitization and challenge as well as the oral administration schedule of OFE are summarized in Figure 1. Briefly, the mice were sensitized with i.p. injections of OVA (20 μg in 1x PBS; Sigma) mixed with alum (2 mg; Pierce, Rockford, III) as an adjuvant on day 0. On day 14, the mice were boosted with OVA (50 μg) mixed with alum (2 mg). The mice that were given i.p. injections of 1x PBS served as negative controls. Either one of two doses of OFE in normal saline (200 μL) (high OFE, 1000 mg/kg body weight or low OFE, 100 mg/kg body weight) or normal saline (for positive and negative control groups) was orally administered daily from day 1 to day 29. Each group consisted of six mice. On days 35–38, the mice were exposed to aerosolized 5% OVA for a 20 min period by placing them in a chamber. The aerosols were generated and conducted into the chamber using an ultrasonic nebulizer (DeVibiss, PA). The output of the nebulizer was 0.3 mL/min and the particles size produced was 0.5–5 μm.
Figure 1.
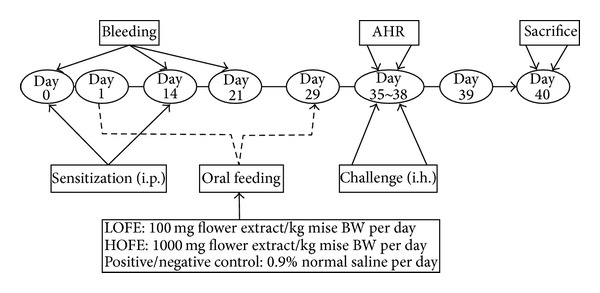
The schedule for the development of the OVA animal model of allergic airway inflammation and OFE administration. i.p.: intraperitoneal injection; i.h.: inhalation with OVA.
2.7.1. Serum Levels of Anti-OVA Antibodies
After all groups of mice were anesthetized, they were bled from the retro-orbital venous plexus. Serum was collected after centrifugation at 12,000 ×g for 10 min. Sera anti-OVA IgE and IgG2a antibody titers were determined by ELISA. Briefly, 96-well microtiter plates were coated with OVA (10 μg/mL). Serum samples were added to each well for an overnight period. Then biotin-conjugated anti-mouse IgE or IgG2a was added for 1 h at 37°C. Streptavidin-conjugated alkaline phosphatase was added for an additional 2 h at RT. Finally, the reaction was developed by 2,2′-azino-bis (3-ethylbenz-thiazoline-6-sulfonic acid), and the absorbance was determined at 420 nm in a microplate reader.
2.7.2. Bronchoalveolar Lavage and Cell Differential Counts
After all groups of mice were anesthetized, they were bled from the retroorbital venous plexus and then sacrificed. Using a cannula, their lungs were immediately lavaged through the trachea three times with 1x HBSS (1 mL) minus ionized calcium and magnesium. The lavaged fluid was centrifuged at 400 ×g for 10 min at 4°C. After washing, the cells were resuspended in 1x HBSS (1 mL) and the total cell counts were determined using a hemocytometer. Cytocentrifuged preparations were stained with Liu's stain for differential cell counts. Based on standard morphologic criteria, a minimum of 200 cells was counted and classified as monocytes, lymphocytes, neutrophils, or eosinophils.
2.7.3. Histopathological Study of Lung Organs
To evaluate the effects of OFE treatment, the lungs were immediately removed after lavage and fixed in a solution of 3% v/v formalin (in 0.01 M phosphate buffer, pH 7.2). The tissues were subsequently embedded in paraffin, cut into 5-μm-thick sections, stained with hematoxylin-eosin, and examined by light microscopy for histopathological changes.
2.8. Statistical Analysis
Data from the ORAC and the TBARS assay as well the GSH concentration and various treatments are presented as mean ± SD (standard deviation). The Student's t test was used for comparison between treatments. Differences between two treatment groups or a treatment group compared with a negative or positive control group were considered statistically significant at P values less than 0.05, 0.01, or 0.001.
3. Results and Discussion
3.1. The Total Phenolic and Flavonoid Content in OFE
The total phenolic content in OFE was 367.9 ± 13.4 mg GAE/g extract, while the total flavonoid content was 45.0 ± 2.0 μg QE/g extract. Flavonoids have been reported to induce antiallergic and anti-inflammatory effects [41]. For example, flavonoids showed a strong inhibition of IL-4 and IL-13 production, histamine release, and CD40 ligand expression in basophils [42]. The antiasthmatic effect by phenylpropanoid glycosides such as verbascoside has also been reported [43].
3.2. Antioxidant Activity and Free Radical Scavenging Capacity of OFE as Determined in the ORAC and DPPH Assays
The total antioxidant capacity of ethanol extract of OFE as determined in the ORAC assay was 0.4 ± 0.0 mM Trolox equivalents. The DPPH IC50 was 8.4 μg/mL, which was better than that of the methanol extract (12.8 μg/mL) of O. fragrans flowers [3], but less than Trolox (4.9 μg/mL). Green tea, which has been proven to have a strong scavenging effect against DPPH radicals, has been reported to have antiallergic activity [44]. We found that in addition to the other antioxidant phenolic compounds, verbascoside and rutin are two major compounds in the ethanol extract of OFE (unpublished data). The antiallergic effects of verbascoside [43] and rutin [45] have also been reported. Together, these results support our decision to evaluate the antiallergic effects of the ethanol extract of OFE.
3.3. The Effects of Orally Administered OFE on Total Antioxidant Capacity, Lipid Peroxidation, and GSH Concentration in Lung and Spleen of BALB/c Mice
The oxidation of lipids, nucleic acids, or proteins may be involved in the oxidative damage of biological molecules. Additionally, oxidation is believed to play a role in the development of inflammatory diseases. For this reason, the total antioxidant capacity (as measured in the ORAC assay), GSH concentration, and lipid peroxidation (as measured in the TBARS assay) in mouse lung and spleen were investigated. There are no previous reports on the in vivo antioxidant effects of OFE.
We first evaluated the antioxidant capacity of the OFE extract in mice after oral administration of OFE for 14 days. Lungs and spleens from the OFE group demonstrated significantly higher values in the ORAC assay and higher concentrations of GSH than those in the control group (normal saline) (Table 1). In contrast, values derived from the TBARS assay were significantly decreased in the OFE group compared with those in the control group. Taken together, these results indicate that oral administration of OFE promotes antioxidant capacity and reduces lipid peroxidation in the lung and spleen of mice.
Table 1.
Effects of orally administered OFE on oxidative status in the lungs and spleen of mice as determined in three different assays.
| Tissue | ORAC (Trolox equivalents, mM) | TBARS (nM/mg protein) | GSH (μM/mg protein) | |||
|---|---|---|---|---|---|---|
| Control | OFE | Control | OFE | Control | OFE | |
| Lung | 2.42 ± 0.61 | 3.60 ± 0.24* | 0.20 ± 0.02 | 0.12 ± 0.02* | 15.27 ± 3.14 | 24.36 ± 3.01*** |
| Spleen | 2.10 ± 0.68 | 3.97 ± 0.79*** | 0.57 ± 0.01 | 0.46 ± 0.05*** | 15.97 ± 1.13 | 25.23 ± 1.19*** |
OFE (1000 mg OFE/kg body weight in 200 μL normal saline) was orally administered daily for 14 days.
Data are expressed as mean ± standard deviation; n = 4; *P < 0.05, ***P < 0.01 as compared to (control) BALB/c mice given normal saline.
The phenolic antioxidants in OFE include tyrosyl acetate, (+)-phillygenin, (8E)-ligustroside, rutin, and verbascoside [3]. The verbascoside obtained from OFE has been reported to protect cell lines from free radical-induced oxidative stress as measured by TBARS [46] and β-amyloid-induced cell injury by attenuating ROS production [46]. However, the in vivo evaluation of the antioxidant effects of these five compounds needs further study. Oxidative stress is an important factor that contributes to the pathologic development of asthma. Given our results for the antioxidant capacity of OFE, we next evaluated OFE for its preventive effects in an animal model of allergic airway inflammation.
3.4. OVA-Specific Serum Antibody Levels in an Animal Model of Allergic Airway Inflammation
Following the OFE preventive protocol (Figure 1), OFE oral administration inhibited OVA-specific IgE production (Figure 2(a)) and enhanced the production of OVA-specific IgG2a (Figure 2(b)). IgE suppression can alleviate the development of asthma [47]. In contrast, IgG2a is the major isotype of the Th1 immune response. The effect of antioxidants to regulate the immune response has been an important issue in western society [48]. The strong Th2 inhibition by flavonoids [42] and phenolic compounds [49] contributes to the suppression of Th2 inflammation. The polarization effect of antioxidants on Th1 and Th2 has been discussed [50]. In a study of the antiallergic effects of curcumin, IFN-γ was increased and IgE was suppressed in the latex allergy model [51]. In our study, the increase in anti-OVA IgG2a and decrease in IgE expression indicate that OFE prevents development of Th2 suppression. A higher GSH induction also contributes to the development of Th1 as suggested by the Th1-promoting effect of GSH [24].
Figure 2.

The effects of daily oral administration of OFE on the OVA-specific IgE and IgG2a antibody production in the OVA animal model of allergic airway inflammation. PC: OVA immunized group; NC: negative control group; HOFE: high dose of OFE; LOFE: low dose of OFE. Significant increase *P < 0.05,**P < 0.01, and ***P < 0.001 or decrease # P < 0.05, ## P < 0.01, and ### P < 0.001, as compared to NC or PC groups.
3.5. Cellular Composition in Bronchoalveolar Fluid in an Animal Model of Allergic Airway Inflammation and the Histopathologic Effects of OFE on Mouse Lung Tissue
In order to evaluate the effects of OFE on the recruitment of inflammatory cells into airway, cells in bronchoalveolar(BAL) fluid were determined by Liu's stain. Compared with the positive control group, oral administration of a high dose (HOFE) but not a low dose (LOFE) of OFE could suppress the recruitment of eosinophils, neutrophils, and lymphocytes (Figure 3). In the histopathological study, extensive cellular infiltration of the periairway region in lung sections of positive control-group mice was found. However, lung tissue from groups administered OFE demonstrated less severe inflammation (Figure 4). Fewer inflammatory cells infiltrated the periairway region in lungs of mice in the HOFE group. This result is consistent with the cellular composition of BAL fluid in the HOFE group and with the observed antiallergic effect of OFE. Results from previous studies examining the antiallergic effects of verbascoside [43] and rutin [45] demonstrated that these compounds in OFE contribute to the inhibition of inflammatory cell infiltration in lung.
Figure 3.

The effects of OFE on cell (eosinophils, neutrophils, monocytes, or lymphocytes) infiltration in bronchoalveolar fluid. Significant increase *P < 0.05, **P < 0.01, and ***P < 0.001 or decrease # P < 0.05, ## P < 0.01, and ### P < 0.001 as compared to NC or PC groups.
Figure 4.

Lung sections were prepared from mice subjected to the OVA animal model of allergic airway inflammation after daily oral OFE or saline (control) administration. Arrows indicate immune cell infiltration by hematoxylin-eosin staining (magnification 200x).
3.6. Effects of Oral OFE Administration on Relative Organ Weight in an Animal Model of Allergic Airway Inflammation
A change in organ weights is a good indicator of chemically- or biologically-induced damage to organs [52]. Oral administration of OFE (100, 800, or 1000 mg/kg) for 28 days did not alter organ weights (liver, spleen, lung, and kidney) in mice (data not shown). However, HOFE (1000 mg/kg) administration for 28 days to mice subjected to the OVA animal model of allergic airway inflammation lowered the relative kidney weight to body weight percentage when compared with mice in the negative control group (NC group, treated with saline) but not when compared with mice in the positive control (PC group, OVA-immunized and treated with saline) (Table 2). In contrast, there were no significant differences among groups in the relative organ weight to body weight percentages for liver and lung. Recently, we evaluated a medium dose of OFE (500 mg/kg) in the OVA-induced allergic airway inflammation model and found that medium dose of OFE also protected against the infiltrated cells in BAL fluid and maintained the antioxidative state in lung (data not shown). Further investigation will be required to clarify the organ cytotoxicity effect by a medium dose of OFE orally administered in this animal model of allergic airway inflammation.
Table 2.
Effects of OFE on relative organ weights for mice in an animal model of allergic airway inflammation.
| Relative organ weight to body weight (%) | ||||
|---|---|---|---|---|
| Group | ||||
| Organ | NC | PC | LOFE | HOFE |
| Liver | 6.00 ± 0.86 | 5.04 ± 0.36 | 5.34 ± 0.68 | 5.44 ± 0.80 |
| Lung | 1.38 ± 0.31 | 1.13 ± 0.14 | 1.33 ± 0.21 | 1.34 ± 0.17 |
| Kidney | 1.75 ± 0.20 | 1.48 ± 0.05 | 1.51 ± 0.14 | 1.42 ± 0.10# |
Evaluation of OFE cytotoxicity in an animal model of allergic airway inflammation. Mice (n = 4) were injected (i.p.) with OVA for positive control (PC), a low dose of OFE (LOFE) or a high dose of HOFE. Negative control (NC) mice were injected with normal saline. During the establishment of the allergic airway inflammation model, mice were treated for 28 days with saline (NC and PC), 1000 mg OFE/kg body weight (HOFE, in 200 μL saline), or 100 mg OFE/kg body weight (LOFE, in 200 μL saline). After sacrifice on Day 40, organs were collected and weighed. Data are expressed as mean ± standard deviation. Significant decrease # P < 0.05 compared to NC group mice.
3.7. OFE Improves Antioxidative Status in Mouse Lung following OVA Administration
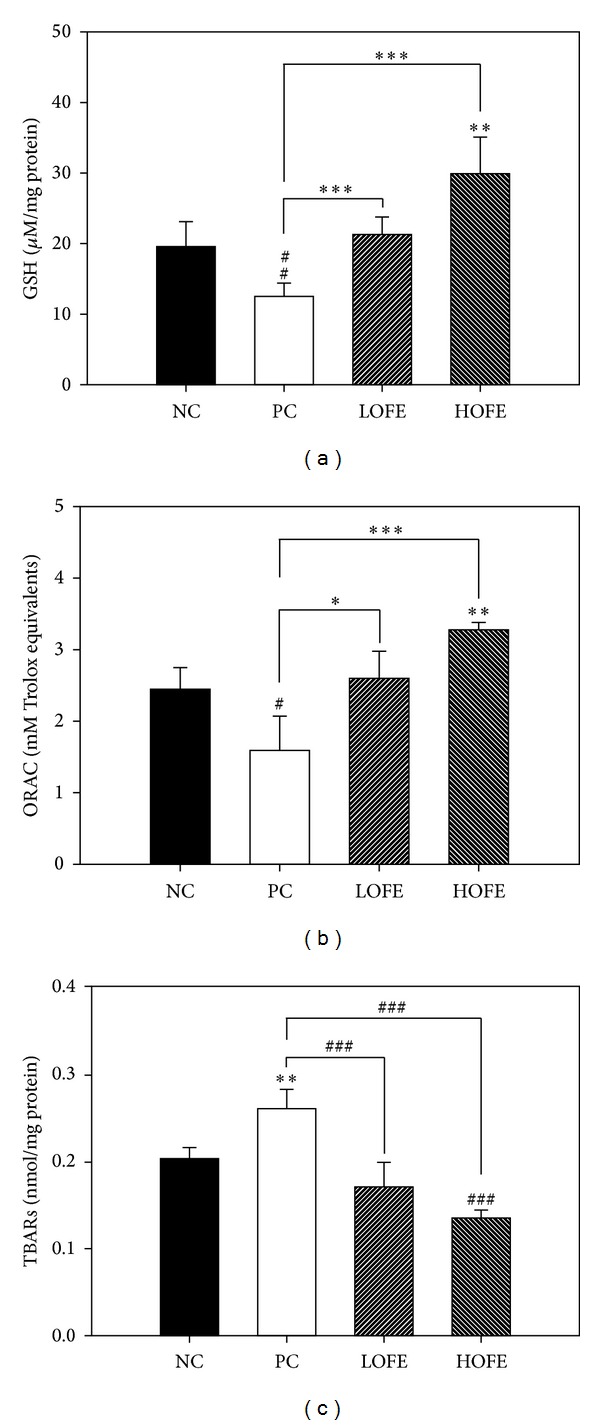
We measured the severity of the oxidative damage in lungs of mice from each group. In lungs taken from animals subjected to the mouse model of allergic airway inflammation, the GSH concentration and the total antioxidant capacity values obtained from the ORAC assay were significantly decreased compared with those of the lungs from mice in the negative control group. This suggests that airway inflammation may lead to oxidative stress in lungs. However, these phenomena were improved following oral administration of OFE (Figures 5(a) and 5(b)). In contrast, the value obtained in the TBARS assay for mice treated with OFE was significantly lower than that obtained for mice in the positive control group (P < 0.01, Figure 5(c)). Taken together, these data indicate that OFE improves the total antioxidant capacity in lung and that this improvement is correlated with a lower severity of signs in the OFE-treated mice subjected to the OVA-induced animal model of allergic airway inflammation. Recent reports have suggested that increasing the GSH levels could attenuate Th2 development [24–27], which offers a possible mechanism for the alleviation in the severity of airway inflammation observed in the present study following OFE administration.
Figure 5.
4. Conclusion
The therapeutic success of many antioxidant agents in allergic airway inflammation in the clinic is moderate at best [53, 54]. Conversely, researchers have found that antioxidants such as fruit juice [55] and plant extracts [56] interfere with the Th1 immune response in human peripheral blood mononuclear cells. Such results reflect the need for determining the appropriate antioxidants for treatment in different types of inflammatory disease. The present report was the first to evaluate the antioxidant and immunomodulatory effects of OFE, applying traditional Chinese medicinal knowledge regarding the O. fragrans flower to modern scientific study. We found that OFE, which contains many antioxidants, promotes a positive antioxidative state in an animal model of allergic airway inflammation. It also has protective effects including decreasing the OVA-specific IgE production and inflammatory cell infiltration in lung.
Conflict of Interests
The authors declare that they have no competing interests and that no financial relationship exists with any company mentioned in this paper.
Acknowledgments
This study was supported by the National Science Council of the Republic of China. (NSC 94-2313-B-273-004). The authors thank Editage for providing editorial assistance. They also appreciate the help of Dong-Jer Shiou (Department of Biotechnology, National Formosa University) for checking the references in this paper.
References
- 1.Larsen K. Flora of Taiwan. Nordic Journal of Botany. 1995;15(6):p. 574. [Google Scholar]
- 2.Lee H-H, Lin C-T, Yang L-L. Neuroprotection and free radical scavenging effects of Osmanthus fragrans . Journal of Biomedical Science. 2007;14(6):819–827. doi: 10.1007/s11373-007-9179-x. [DOI] [PubMed] [Google Scholar]
- 3.Hung CY, Tsai YC, Li KY. Phenolic antioxidants isolated from the flowers of Osmanthus fragrans . Molecules. 2012;17(9):10724–10737. doi: 10.3390/molecules170910724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee D-G, Lee S-M, Bang M-H, et al. Lignans from the flowers of Osmanthus fragrans var. aurantiacus and their inhibition effect on NO production. Archives of Pharmacal Research. 2011;34(12):2029–2035. doi: 10.1007/s12272-011-1204-y. [DOI] [PubMed] [Google Scholar]
- 5.Cho YS, Moon H-B. The role of oxidative stress in the pathogenesis of asthma. Allergy, Asthma and Immunology Research. 2010;2(3):183–187. doi: 10.4168/aair.2010.2.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Folkerts G, Kloek J, Muijsers RBR, Nijkamp FP. Reactive nitrogen and oxygen species in airway inflammation. European Journal of Pharmacology. 2001;429(1–3):251–262. doi: 10.1016/s0014-2999(01)01324-3. [DOI] [PubMed] [Google Scholar]
- 7.Wood LG, Gibson PG, Garg ML. Biomarkers of lipid peroxidation, airway inflammation and asthma. European Respiratory Journal. 2003;21(1):177–186. doi: 10.1183/09031936.03.00017003a. [DOI] [PubMed] [Google Scholar]
- 8.Matsue H, Edelbaum D, Shalhevet D, et al. Generation and function of reactive oxygen species in dendritic cells during antigen presentation. Journal of Immunology. 2003;171(6):3010–3018. doi: 10.4049/jimmunol.171.6.3010. [DOI] [PubMed] [Google Scholar]
- 9.Murata Y, Shimamura T, Hamuro J. The polarization of Th1/Th2 balance is dependent on the intracellular thiol redox status of macrophages due to the distinctive cytokine production. International Immunology. 2002;14(2):201–212. doi: 10.1093/intimm/14.2.201. [DOI] [PubMed] [Google Scholar]
- 10.Pazmandi K, Magyarics Z, Boldogh I, Csillag A, Rajnavolgyi E, Bacsi A. Modulatory effects of low-dose hydrogen peroxide on the function of human plasmacytoid dendritic cells. Free Radical Biology and Medicine. 2012;52(3):635–645. doi: 10.1016/j.freeradbiomed.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katsumata U, Miura M, Ichinose M, et al. Oxygen radicals produce airway constriction and hyperresponsiveness in anesthetized cats. The American Review of Respiratory Disease. 1990;141(5):1158–1161. doi: 10.1164/ajrccm/141.5_Pt_1.1158. [DOI] [PubMed] [Google Scholar]
- 12.Weiss EB, Bellino JR. Leukotriene-associated toxic oxygen metabolites induce airway hyperreactivity. Chest. 1986;89(5):709–716. doi: 10.1378/chest.89.5.709. [DOI] [PubMed] [Google Scholar]
- 13.Rhoden KJ, Barnes PJ. Effect of hydrogen peroxide on guinea-pig tracheal smooth muscle in vitro: role of cyclo-oxygenase and airway epithelium. British Journal of Pharmacology. 1989;98(1):325–330. doi: 10.1111/j.1476-5381.1989.tb16898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adler KB, Holden-Stauffer WJ, Repine JE. Oxygen metabolites stimulate release of high-molecular-weight glycoconjugates by cell and organ cultures of rodent respiratory epithelium via an arachidonic acid-dependent mechanism. The Journal of Clinical Investigation. 1990;85(1):75–85. doi: 10.1172/JCI114436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doelman CJA, Leurs R, Oosterom WC, Bast A. Mineral dust exposure and free radical-mediated lung damage. Experimental Lung Research. 1990;16(1):41–55. doi: 10.3109/01902149009064698. [DOI] [PubMed] [Google Scholar]
- 16.del Maestro RF, Bjork J, Arfors K-E. Increase in microvascular permeability induced by enzymatically generated free radicals. I. In vivo study. Microvascular Research. 1981;22(3):239–254. doi: 10.1016/0026-2862(81)90095-9. [DOI] [PubMed] [Google Scholar]
- 17.Tate RM, Vanbenthuysen KM, Shasby DM. Oxygen-radical-mediated permeability edema and vasoconstriction in isolated perfused rabbit lungs. The American Review of Respiratory Disease. 1982;126(5):802–806. doi: 10.1164/arrd.1982.126.5.802. [DOI] [PubMed] [Google Scholar]
- 18.Rahman I, Yang S-R, Biswas SK. Current concepts of redox signaling in the lungs. Antioxidants and Redox Signaling. 2006;8(3-4):681–689. doi: 10.1089/ars.2006.8.681. [DOI] [PubMed] [Google Scholar]
- 19.Comhair SAA, Ricci KS, Arroliga M, et al. Correlation of systemic superoxide dismutase deficiency to airflow obstruction in asthma. American Journal of Respiratory and Critical Care Medicine. 2005;172(3):306–313. doi: 10.1164/rccm.200502-180OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fitzpatrick AM, Teague WG, Holguin F, Yeh M, Brown LAS. Airway glutathione homeostasis is altered in children with severe asthma: evidence for oxidant stress. The Journal of Allergy and Clinical Immunology. 2009;123(1):146.e8–152.e8. doi: 10.1016/j.jaci.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fitzpatrick AM, Brown LAS, Holguin F, Teague WG. Levels of nitric oxide oxidation products are increased in the epithelial lining fluid of children with persistent asthma. The Journal of Allergy and Clinical Immunology. 2009;124(5):990.e9–996.e9. doi: 10.1016/j.jaci.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith LJ, Houston M, Anderson J. Increased levels of glutathione in bronchoalveolar lavage fluid from patients with asthma. The American Review of Respiratory Disease. 1993;147(6, part 1):1461–1464. doi: 10.1164/ajrccm/147.6_Pt_1.1461. [DOI] [PubMed] [Google Scholar]
- 23.Kelly FJ, Mudway I, Blomberg A, Frew A, Sandstrom T. Altered lung antioxidant status in patients with mild asthma. The Lancet. 1999;354(9177):482–483. doi: 10.1016/S0140-6736(99)01812-7. [DOI] [PubMed] [Google Scholar]
- 24.Koike Y, Hisada T, Utsugi M, et al. Glutathione redox regulates airway hyperresponsiveness and airway inflammation in mice. American Journal of Respiratory Cell and Molecular Biology. 2007;37(3):322–329. doi: 10.1165/rcmb.2006-0423OC. [DOI] [PubMed] [Google Scholar]
- 25.Antonicelli F, Parmentier M, Drost EM, et al. Nacystelyn inhibits oxidant-mediated interleukin-8 expression and NF-κB nuclear binding in alveolar epithelial cells. Free Radical Biology and Medicine. 2002;32(6):492–502. doi: 10.1016/s0891-5849(01)00820-6. [DOI] [PubMed] [Google Scholar]
- 26.Aoki T, Suzuki Y, Suzuki K, et al. Modulation of ICAM-1 expression by extracellular glutathione in hyperoxia-exposed human pulmonary artery endothelial cells. American Journal of Respiratory Cell and Molecular Biology. 1996;15(3):319–327. doi: 10.1165/ajrcmb.15.3.8810635. [DOI] [PubMed] [Google Scholar]
- 27.Lowry MH, McAllister BP, Jean J-C, et al. Lung lining fluid glutathione attenuates IL-13-induced asthma. American Journal of Respiratory Cell and Molecular Biology. 2008;38(5):509–516. doi: 10.1165/rcmb.2007-0128OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao M-F, Chen C-C, Hsu M-H. Evaluation of the serum antioxidant status in asthmatic children. Acta Paediatrica Taiwanica. 2004;45(4):213–217. [PubMed] [Google Scholar]
- 29.Tsukagoshi H, Shimizu Y, Iwamae S, et al. Evidence of oxidative stress in asthma and COPD: potential inhibitory effect of theophylline. Respiratory Medicine. 2000;94(6):584–588. doi: 10.1053/rmed.2000.0785. [DOI] [PubMed] [Google Scholar]
- 30.Soutar A, Seaton A, Brown K. Bronchial reactivity and dietary antioxidants. Thorax. 1997;52(2):166–170. doi: 10.1136/thx.52.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olusi SO, Ojutiku OO, Jessop WJE, Iboko MI. Plasma and white blood cell ascorbic acid concentrations in patients with bronchial asthma. Clinica Chimica Acta. 1979;92(2):161–166. doi: 10.1016/0009-8981(79)90110-4. [DOI] [PubMed] [Google Scholar]
- 32.Trenga CA, Koenig JQ, Williams PV. Dietary antioxidants and ozone-induced bronchial hyperresponsiveness in adults with asthma. Archives of Environmental Health. 2001;56(3):242–249. doi: 10.1080/00039890109604448. [DOI] [PubMed] [Google Scholar]
- 33.Chang L-Y, Crapo JD. Inhibition of airway inflammation and hyperreactivity by an antioxidant mimetic. Free Radical Biology and Medicine. 2002;33(3):379–386. doi: 10.1016/s0891-5849(02)00919-x. [DOI] [PubMed] [Google Scholar]
- 34.Yen G-C, Hung C-Y. Effects of alkaline and heat treatment on antioxidative activity and total phenolics of extracts from Hsian-tsao (Mesona procumbens Hemsl.) Food Research International. 2000;33(6):487–492. [Google Scholar]
- 35.Woisky RG, Salatino A. Analysis of propolis: some parameters and procedures for chemical quality control. Journal of Apicultural Research. 1998;37(2):99–105. [Google Scholar]
- 36.Chang C-C, Yang M-H, Wen H-M, Chern J-C. Estimation of total flavonoid content in propolis by two complementary colometric methods. Journal of Food and Drug Analysis. 2002;10(3):178–182. [Google Scholar]
- 37.Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cycodextrin emulsion. Journal of Agricultural and Food Chemistry. 1992;40:945–948. [Google Scholar]
- 38.Chung K-Y, Lee S-J, Chung S-M, Lee M-Y, Bae O-N, Chung J-H. Generation of free radical by interaction of iron with thiols in human plasma and its possible significance. Thrombosis Research. 2005;116(2):157–164. doi: 10.1016/j.thromres.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 39.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 40.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Analytical Biochemistry. 1968;25(1):192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 41.Bellik Y, Boukraâ L, Alzahrani HA, et al. Molecular mechanism underlying anti-inflammatory and anti-allergic activities of phytochemicals: an update. Molecules. 2012;18(1):322–353. doi: 10.3390/molecules18010322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawai M, Hirano T, Higa S, et al. Flavonoids and related compounds as anti-allergic substances. Allergology International. 2007;56(2):113–123. doi: 10.2332/allergolint.R-06-135. [DOI] [PubMed] [Google Scholar]
- 43.Lee JY, Lee JG, Sim SS, Whang W-K, Kim CJ. Anti-asthmatic effects of phenylpropanoid glycosides from Clerodendron trichotomum leaves and Rumex gmelini herbes in conscious guinea-pigs challenged with aerosolized ovalbumin. Phytomedicine. 2011;18(2-3):134–142. doi: 10.1016/j.phymed.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 44.Heo J-C, Rho JR, Kim T-H, Kim S-Y, Lee S-H. An aqueous extract of green tea Camellia sinensis increases expression of Th1 cell-specific anti-asthmatic markers. International Journal of Molecular Medicine. 2008;22(6):763–767. [PubMed] [Google Scholar]
- 45.Jung CH, Lee JY, Cho CH, Kim CJ. Anti-asthmatic action of quercetin and rutin in conscious guinea-pigs challenged with aerosolized ovalbumin. Archives of Pharmacal Research. 2007;30(12):1599–1607. doi: 10.1007/BF02977330. [DOI] [PubMed] [Google Scholar]
- 46.Wang H, Xu Y, Yan J, et al. Acteoside protects human neuroblastoma SH-SY5Y cells against β-amyloid-induced cell injury. Brain Research. 2009;1283(4):139–147. doi: 10.1016/j.brainres.2009.05.101. [DOI] [PubMed] [Google Scholar]
- 47.Lebman DA, Coffman RL. Interleukin 4 causes isotype switching to IgE in T cell-stimulated clonal B cell cultures. The Journal of Experimental Medicine. 1988;168(3):853–862. doi: 10.1084/jem.168.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johanna G, Christian C, Kathrin B, Dietmar F, Robert S. Immunoregulatory impact of food antioxidants. doi: 10.2174/13816128113199990047. Current Pharmaceutical Design. In press. [DOI] [PubMed] [Google Scholar]
- 49.Chung S-Y, Champagne ET. Reducing the allergenic capacity of peanut extracts and liquid peanut butter by phenolic compounds. Food Chemistry. 2009;115(4):1345–1349. [Google Scholar]
- 50.Murr C, Schroecksnadel K, Winkler C, Ledochowski M, Fuchs D. Antioxidants may increase the probability of developing allergic diseases and asthma. Medical Hypotheses. 2005;64(5):973–977. doi: 10.1016/j.mehy.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 51.Kurup VP, Barrios CS, Raju R, Johnson BD, Levy MB, Fink JN. Immune response modulation by curcumin in a latex allergy model. Clinical and Molecular Allergy. 2007;5, article 1 doi: 10.1186/1476-7961-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Michael B, Yano B, Sellers RS, et al. Evaluation of organ weights for rodent and non-rodent toxicity studies: a review of regulatory guidelines and a survey of current practices. Toxicologic Pathology. 2007;35(5):742–750. doi: 10.1080/01926230701595292. [DOI] [PubMed] [Google Scholar]
- 53.Omenaas E, Fluge Ø, Buist AS, Vollmer WM, Gulsvik A. Dietary vitamin C intake is inversely related to cough and wheeze in young smokers. Respiratory Medicine. 2003;97(2):134–142. doi: 10.1053/rmed.2003.1439. [DOI] [PubMed] [Google Scholar]
- 54.Grievink L, Smit HA, Ocké MC, van ’T Veer P, Kromhout D. Dietary intake of antioxidant (pro)-vitamins, respiratory symptoms and pulmonary function the MORGEN study. Thorax. 1998;53(3):166–171. doi: 10.1136/thx.53.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neurauter G, Wirleitner B, Schroecksnadel K, Schennach H, Fuchs D. Wine and grape juice modulate interferon-γ-induced neopterin production and tryptophan degradation in human PBMC. Pteridines. 2004;15(1):1–9. doi: 10.1016/j.intimp.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 56.Winkler C, Wirleitner B, Schroecksnadel K, Schennach H, Mur E, Fuchs D. In vitro effects of two extracts and two pure alkaloid preparations of Uncaria tomentosa on peripheral blood mononuclear cells. Planta Medica. 2004;70(3):205–210. doi: 10.1055/s-2004-815536. [DOI] [PubMed] [Google Scholar]


