Abstract
In recent years, huge advances have taken place in understanding of inner ear pathophysiology causing sensorineural hearing loss, tinnitus, and vertigo. Advances in understanding comprise biochemical and physiological research of stimulus perception and conduction, inner ear homeostasis, and hereditary diseases with underlying genetics. This review describes and tabulates the various causes of inner ear disease and defines inner ear and non-inner ear causes of hearing loss, tinnitus, and vertigo. The aim of this review was to comprehensively breakdown this field of otorhinolaryngology for specialists and non-specialists and to discuss current therapeutic options in distinct diseases and promising research for future therapies, especially pharmaceutic, genetic, or stem cell therapy.
Keywords: inner ear disease, sensorineural hearing loss, tinnitus, vertigo, genetic therapy, stem cell therapy, Meniere’s disease
Background
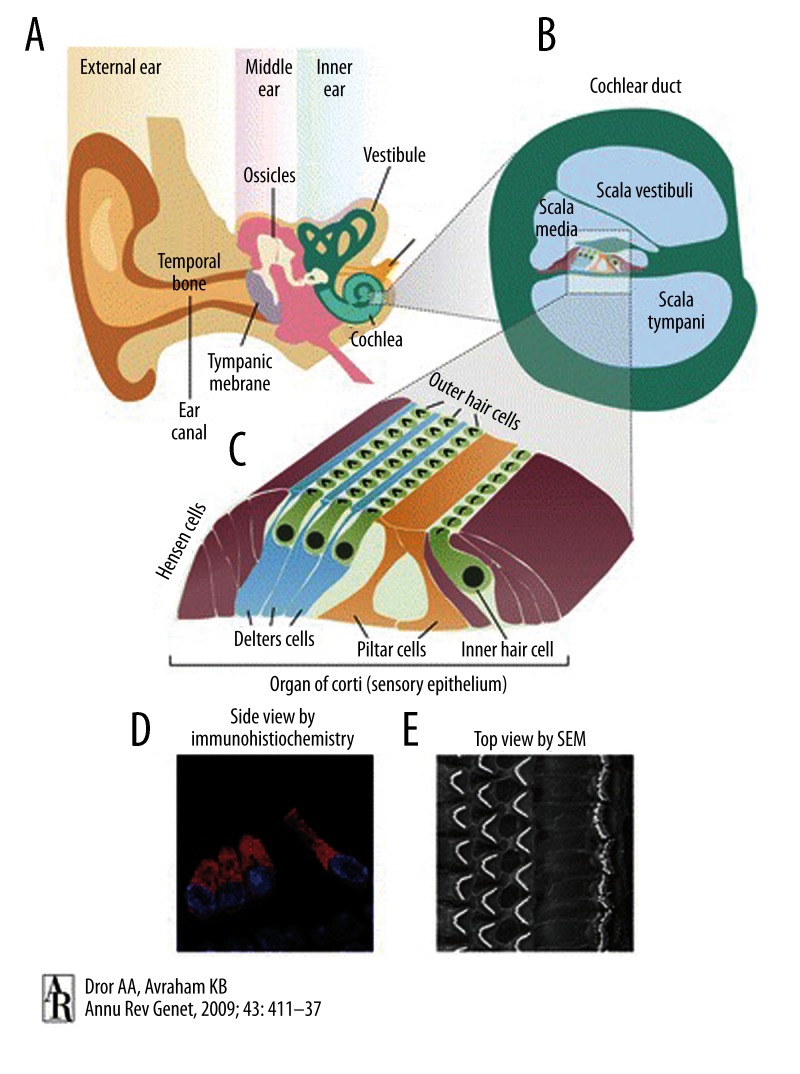
Classical inner ear disease involves the entire membranous labyrinth and is characterized by the triad of sensorineural hearing loss, tinnitus, and vertigo. Underlying pathology may involve inner ear hair cells, supporting cells, or an aberrant inner ear homeostasis resulting in altered composition of the endo- and perilymph, with direct effects on the integrity and functionality of the hair cells. Altered afferent and efferent auditory pathways may accompany a diseased inner ear or be the primary cause for inner ear symptoms. Figure 1 illustrates the structure of the human ear and inner ear.
Figure 1.
Schematic illustration of the human ear. (A) The ear consists of the outer, middle, and inner ear. (B) A section through the cochlear duct illustrates the fluid-filled compartments of the inner ear. (C) The organ of Corti resides in the scala media, with sensory hair cells surrounded by supporting cells that include Deiters’, Hensen, and pillar cells. (D) Immunohistochemistry with the inner ear hair cell marker myosin VI, marking the cytoplasm of inner and outer hair cells, and4,6-diamidino-2-phenylindole (DAPI), marking the nuclei. (E) Scanning electron microscopy image of the top view of the sensory epithelium reveals the precise arrangement of 1 row of inner hair cells and 3 rows of outer hair cells, separated by the pillar cells. (with permission from Dror AA, Avraham KB. Hearing loss: Mechanisms revealed by genetics and cell biology. Annu Rev Genet, 2009; 43: 411–37. Copyright © 2009, Annual Reviews. All rights reserved).
Prosper Ménière ascribed vertigo to the inner ear for the first time, after dissecting a hemorrhagic inner ear. Later, the syndrome of fluctuating sensorineural hearing loss, episodic vertigo, and tinnitus was named after him [1]. Aural fullness as a fourth symptom in addition to the classical triad often precedes or accompanies acute inner ear disease. However, all inner ear symptoms can appear isolated, as in atypical Ménière’s disease. It is important to distinguish between inner and non-inner ear causes of vertigo and tinnitus, which are summarized in Table 1. Tinnitus can be divided in subjective types with underlying causes in the inner ear or auditory pathway, and objective types with underlying vascular and muscular disorders, osseous diseases, or neoplasia. Subjective tinnitus is often paralleled by sensorineural hearing loss. The tinnitus prevalence increases with age and the level of hearing loss; 10% of the population over the age of 60 years state they have severe tinnitus [2–4].
Table 1.
Objective causes for tinnitus.
| Vascular disorders |
|
| Muscular disorders |
|
| Osseous diseases |
|
| Neoplasia |
|
| Miscellaneous |
|
When acute hearing loss is accompanied by vertigo, it is typically rotational vertigo. Other sensations of peripheral vestibular origin are lateropulsion, tilting, swaying, lift sensation, and falling. A strict distinction against central vestibular sensations and causes is not possible. However, nonspecific sensations like stumbling, instability, drunkenness, and dizziness often accompany central or non-vestibular causes for dizziness. Differential diagnosis of vertigo includes cerebral, musculoskeletal, and cardiovascular disorders (Table 2, inner ear vs. non-inner ear causes for dizziness). Dizziness is a non-specific term to indicate a sense of disorientation. Vertigo is a subtype of dizziness and refers to an erroneous perception of self-or object-motion or an unpleasant distortion of static gravitational orientation, which is a result of a mismatch between vestibular, visual, and somatosensory systems. The other 3 subtypes of dizziness are disequilibrium without vertigo, presyncope, and psychophysiologic dizziness.
Table 2.
Inner ear vs. non-inner ear causes of dizziness.
| Peripheral vestibular disorders |
|
| Central vestibular disorders |
|
| Cerebral disorders |
|
| Musculoskeletal disorders |
|
| Cardiovascular disorders |
|
| Miscellaneous |
|
In contrast to middle ear disease, in inner ear disease, natural hearing cannot be restored or improved by surgical reconstruction techniques. Hearing aids and implants are helpful tools for deaf patients but cannot preserve natural hearing perception when the inner ear labyrinth is highly impaired.
Hearing Disorders in Children
Hearing loss is the most common birth defect and the most prevalent sensorineural disorder in developed countries. The overall estimates of the prevalence of newborns with congenital hearing loss in Western countries are 1–6 per 1000 newborns [5–7].
Most children with congenital hearing loss have hearing impairment at birth. However, some types of congenital hearing loss may not become evident until later childhood.
The etiology of profound congenital hearing impairment is divided into 2 main causes: environmental (50%) and genetic (50%). Environmental causes include viral infections such as toxoplasma, rubella, cytomegalovirus, herpes simplex virus (TORCH). Genetic causes are divided into syndromic (30%) and non-syndromic (70%). To date, more than 300 syndromic forms of hearing loss have been described [8].
Osseous or membranous malformations of the inner ear (1:80 000) are rare compared to middle ear malformations (1:10 000) [9]. They can be the result of toxicity in the 3rd to 8th. gestational week due to causes such as pharmaceuticals, alcohol, viruses, radiation, or hypoxia. In a few cases, congenital inner ear malformations can affect the vestibular apparatus only [10]. Table 3 summarizes the most commonly used classifications of cochleovestibular malformations [11,12]. Patients with complete labyrinthine aplasia (Michel deformity) are generally not candidates for a cochlear implant. Bony cochlear aplasia and hypoplasia, common cavity of cochlea and vestibule, incomplete partition of the cochlea type 1, aplasia of the semicircular canals, and internal auditory canal malformations are correlated with vestibulocochlear nerve insufficiency [13]. However, for patients with cochlear remnants or a vestibulocochlear nerve, a cochlear implant may be considered. Another possibility for these patients is auditory brainstem implants, but most auditory brainstem recipients have only an awareness of sound and are not able to hear musical melodies, only the beat.
Table 3.
Classification of cochleovestibular malformations.
| Cochlear Malformations |
|
| Vestibular malformations | Michel deformity, common cavity, absent vestibule, hypoplastic vestibule, dilated vestibule |
| Semicircular canal malformations | Absent, hypoplastic or enlarged |
| Internal auditory canal malformations | Absent, narrow or enlarged |
| Vestibular and cochlear aqueduct findings | Enlarged |
Genetic Diseases
Profound, early-onset deafness is present in 4–11 per 10 000 children in the USA and is attributable to genetic causes in at least 50% of cases [14]; the other 50% are attributed to acquired or unknown causes. About 10–15% of hereditary hearing loss does not manifest in childhood and 10–20% are progressive. Owing to recent advances in molecular genetics, more than 130 loci and more than 50 causative genes have been identified in various populations world-wide. In general, they are involved in hair bundle morphogenesis, form constituents of the extracellular matrix, play a role in cochlear ion homeostasis (e.g. potassium channels), or serve as transcription factors. The Hereditary Hearing Loss Homepage (http://www.hereditaryhearingloss.org) gives an up-to-date overview of the genetics of hereditary hearing impairment [15].
Nonsyndromic hereditary hearing loss can be subdivided to autosomal dominant (80%) autosomal recessive (20%), X-linked (<1%), and maternally-inherited hearing loss associated with mitochondrial DNA mutations (<1%). The loci for autosomal dominant, autosomal recessive, and x-linked nonsyndromic hearing loss are designated as DFNA, DFNB, and DFNX followed by consecutive numbers, respectively. Autosomal recessive nonsyndromic hearing loss is usually prelingual and autosomal dominant nonsyndromic sensorineural hearing loss is postlingual and progressive.
Epigenetic mutations can result in progressive hearing loss in humans and mice and can be linked to inherited syndromes that can induce hearing loss, in particular, mutations in noncoding microRNA, DNA methylation, and histone modification [16]. MicroRNAs (miRNAs) are small noncoding RNAs, 21–23 nucleotides long, which regulate gene expression through the RNA interference mechanism, known to affect proliferation, differentiation, and developmental processes. Mutations in micro-RNAs were found to be responsible for non-syndromic hearing loss [17]. Inhibitors of histone deacetylation prevented hair cell death and hearing loss in aminoglycoside and cisplatin ototoxicity in animal studies [18,19].
Inner ear tissues possess a distinct pattern of barrier and transport proteins to maintain endolymph composition, generate endolymphatic potential, and facilitate sensory transduction. Connexins are gap junction proteins which constitute a major system of intercellular communication important in the exchange of electrolytes, second messengers, and metabolites. Connexin 26 accounts for up to 50% of non-syndromic autosomal recessive hearing loss in European and American populations [20–22]. Potassium is the major charge carrier for sensory transduction. It is ideal for this role, since it is by far the most abundant ion in the cytosol. Defects of the various potassium channels and the abundance of other ion carriers in the inner-ear are the causes for syndromic and non-syndromic deafness. Table 4 summarizes defective proteins in the inner-ear and related diseases.
Table 4.
Defective proteins in stria vascularis and vestibular dark cells, and related diseases.
| Gene | Description/synonyms | Related diseases |
|---|---|---|
| COL4A3, COL4A4, COL4A5 | Collagen type IV, alpha subunits III-V | Alport syndrome |
| GJA7 | Junction protein α7 /Connexin 43 | Non-syndromic deafness |
| GJB2 | Gap junction protein β2 /Connexin 26 | DFNA3/DFNB1 |
| GJB3 | Gap junction protein β3 /Connexin 31 | DFNBA2 |
| GJB6 | Gap junction protein β6 /Connexin 30 | DFNA3 |
| GJE1 | Gap junction protein ɛ1/Connexin 29 | Non-syndromic deafness |
| Cldn11 | Transmembrane protein claudin 11 | Deafness |
| Cldn14 | Transmembrane protein claudin 14 | DFNB19 |
| TMPRSS3 | Transmembrane protease, serine 3 | Deafness/DFNB8/10 |
| KCNQ1/KCNE1 | KvLQT1=voltage-activated K+ channel of long QT syndrome1 /IsK=slowly activating K+ current, minK=minimal K+ channel | Deafness/Jervell & Lange-Nielsen syndrome |
| KCNJ10 | Kir4.1=inward rectifier-type potassium channel | SeSAME or EAST syndrome |
| Slc12a2 | Na+-K+-2Cl−- cotransporter,solute carrier, family 12, member 2/NKCC1, BSC2 | Deafness |
| CLCNKA and CLCNKB | Type K chloride channel/ClC-Ka and ClC-Kb | Deafness/Bartter syndrome IV |
| ATP6V1B1, ATP6VOA4 | H+-ATPase (B1, A4) | Deafness/Distal renal tubular acidosis |
| SLC26A4 | Pendrin protein | Deafness/Pendred syndrome/DFNB4 |
| AQP4 | Aquaporin water channel protein 4 | Deafness |
Although most hereditary hearing loss causes high-frequency sensorineural hearing loss (SNHL), some deafness genes are associated with low-frequency (DFNA1, DFNA6/14/38, and probably DFNA 15/54) [23] or mid-frequency hearing loss (TECTA gene encodes for α-tectorin, a component of the tectorial membrane that overlies the sensory epithelia [24], and COL11A2 gene mutations affect the triple-helix domain of the collagen type XI, alpha 2 protein [25]).
Mutations in the WFS1 gene, encoding an 890 amino-acid transmembranous glycoprotein, wolframin, which is predominantly localized in the endoplasmic reticulum, can be responsible for nonsyndromic autosomal dominant low-frequency hearing loss (DFNA6/14/38) or cause Wolfram syndrome, which is characterized by diabetes insipidus, juvenile-onset diabetes mellitus, progressive optic atrophy, and sensorineural hearing loss. In Europe and the United States, 75% of families affected with non-syndromic autosomal dominant low-frequency SNHL carry the WFS1 mutations [26]. Mutations in the DFNB59 gene encoding the pejvakin protein was the first reported gene that leads to deafness via neuronal dysfunction along the auditory cascade. Mutations are associated with autosomal recessive auditory neuropathy with bilateral prelingual hearing loss [27].
Stereociliary Diseases
The stereocilia of the inner ear hair cells are microvilli-derived and unique cell structures that correlate anatomically with distinct cochlear functions, including mechanoelectrical transduction, cochlear amplification, adaptation, frequency selectivity, and tuning. The stereocilia have a typical staircase arrangement connected with lateral and tip links stabilizing the mature hair bundle structure. When sound is induced, fluids move through the cochlear duct and vibrate the basilar membrane with the sensory hair cells against the tectorial membrane and lead to deflection of the stereocilia and activation of the mechanoelectrical transduction channels gated by the tip links. Potassium influx is enabled, which depolarizes the hair cells.
Stereociliar function is impaired by inner ear stressors, by various types of hereditary deafness, and syndromic hearing loss [28]. Several specific molecular compounds are responsible for maintaining the complex, fragile mechanisms of dynamic stereocilia regulation. Dysfunction of any of these compounds leads to various types of inner ear impairment. Table 5 summarizes the various possible defective stereocilial molecules involved in Usher syndrome and other types of hereditary deafness.
Table 5.
Stereociliary molecules involved in Usher syndrome and other hereditary deafness types.
| Molecule | Main function | Disease Involvement | |
|---|---|---|---|
| Usher-type | Other hereditary non-syndromal deafness type | ||
| Actin | Cytoskeleton | DFNA20/26 | |
| Cadherin 23 | Cell adhesion (stereociliary links) | ID, atypical | DFNB12 |
| Clarin 1 | Transmembrane, actin organization | IIIA | |
| Espin | Actin cross-linking | DFNB36 | |
| GPR981/formerly termed VLGR12 | Ion exchange, signalling | IIC | DFNB6 |
| Harmonin | Scaffolding (homeostasis, adaptation) | IC | DFNB18 |
| HDIA3 | Actin organization | DFN1A | |
| Myosin IIIa | Motor activity, espin transport | DFNB30 | |
| Myosin VIIa | Motor activity, endocytosis (adaptation) | IB, IIA, III, atypical | DFNB2, DFNA11 |
| Myosin XV | Motor activity | DFNB3 | |
| Otoancorin | Stereocila-tectorial & otoconial membrane attachment | DFNB22 | |
| Protocadherin 15 | Cell adhesion, signalling (stereociliary links) | IF | DFNB23 |
| Radixin | Actin-plasma membrane linking | ||
| Sans protein | Membrane-associated scaffold (homeostasis) | IG | |
| Stereocilin | Stereocilia-tectorial & otoconial membrane attachment | DFNB16 | |
| TRIO and F-actin binding protein | Actin remodeling and stabilization | DFNB28 | |
| Whirlin | Scaffolding | IID | DFNB31 |
| Kaptin4 | Actin remodeling, stereocilia formation | DFNA4 | |
Usher syndrome types IE and IIIB are unknown.
GPR98=G protein coupled receptor 98.
VLGR1=very large G protein-coupled receptor-1.
HDIA=human homolog of diaphonous.
Also termed 2E4. DFNA= nonsyndromic deafness, autosomal dominant; DFNB=nonsyndromic deafness, autosomal recessive.
Usher syndrome (USH) is estimated to account for 3–6% of congenital profound deafness cases in children, and for 50% of deafness and blindness [29–31]. Three types of Usher syndrome have been distinguished, with several subclasses based on the loci of mutation. The first type is characterized by hearing impairment, vestibular dysfunction, and retinal degeneration beginning in childhood. In contrast, the second form involves normal vestibular function, less severe hearing loss, and a later onset of retinal degeneration. The third type is defined by progressive hearing loss, occasional vestibular dysfunction, and variable onset of retinal degeneration. Some patients affected with Usher syndrome show an atypical clinical designation and cannot be easily categorized into 1 of these 3 subtypes [32].
Communication Routes Between Intracranial Spaces and the Inner Ear
There are 3 communication routes between the intracranial spaces and the inner ear: the vestibular aqueduct, the cochlear aqueduct, and the internal auditory canal. The vestibular aqueduct contains the endolymphatic duct which ends in a blind pouch, the endolymphatic sac, which is embedded in 2 dural layers and is located in the epidural space. The cochlear aqueduct contains the perilymphatic duct, which communicates with the subarachnoidal space. They possess a key role in inner ear pressure regulation and fluid homeostasis and are related to inner ear diseases [33]. Enlarged vestibular aqueduct is the most common malformation of the inner ear associated with hearing loss. In the United States, 12% of deaf children at 4 years of age have an enlarged vestibular aqueduct [8]. The malformation is bilateral in 90% of cases, and patients may present with profound congenital sensorineural hearing loss, or with progressive or fluctuating hearing loss. Some patients may experience acute hearing decline related to episodes of minor head injury, overexertion, or barometric pressure changes (e.g. related to air travel, deep sea diving, or Valsalva maneuver) [34,35]. It is observed in DFNB4, Pendred syndrome, branchiootorenal syndrome, distal renal tubular acidosis, and Waardenburg syndrome.
The Pendred syndrome is the most common syndromic hearing loss (5%). It is characterized by goiter, congenital deafness, and an enlarged vestibular aqueduct, which is, together with the endolymphatic sac, primarily responsible for inner ear fluid homeostasis. It is caused by mutations of SLC26A4 gene, which codes for pendrin, an anion exchanger that seems to secrete HCO3- into the endolymph. The goitrous phenotype in Pendred syndrome is not recognized in early childhood and develops with age [36]. An enlarged vestibular aqueduct can be associated in rare cases with mutations of FOXI1 gene, which encodes the SLC26A4 transcriptional factor, and mutations of the potassium channel gene KCNJ10 [37]. The GJB2 gene encoding connexin 26 is associated with temporal bone abnormalities, including an enlarged vestibular aqueduct [38].
Table 6 summarizes the diseases associated with aberrant communication routes. In addition, abnormal communication routes may exist, such as semicircular canal dehiscence, may develop in trauma, or be caused by diseases such as cholesteatoma or inner ear malformations. Semicircular dehiscence, trauma, and cholesteatoma involving the osseous labyrinth are a few of the indications were surgical intervention is indicated and improve inner ear symptoms.
Table 6.
Diseases associated with aberrant communication routes between intracranial spaces and the inner ear, notably enlarged or obstructed aqueducts and pathologic internal auditory canal.
| Cochlear aqueduct | Vestibular aqueduct | Internal auditory canal |
|---|---|---|
| Oozer phenomenon | Gusher phenomenon | |
| Perilymphatic fistula, cochlear window rupture | ||
| Meniere’s disease | ||
| Enlarged VA as own entity Pendred syndrome Renal tubular acidosis Branchiootorenal syndrome |
||
| Pure membranous malformations (e.g. Scheibe and Alexander displasias) | ||
| Tumors or lesions of various types (e.g. endolymphatic sac tumors, nerve or vascular lesions) | ||
| Inner ear malformations (e.g. Mondini, Michel, enlarged vestibule, enlarged semicircular canal, hypoplastic cochlea) | ||
| Obstruction inside or outside the labyrinth (e.g. high jugular bulb, an aberrant vein or tumor growth) | ||
| Spread of infection between the inner ear and the brain is increased in both directions | ||
| Dysfunction results in an increased vulnerability to inner ear stressors (e.g. aminoglycosides) | ||
| Increased vulnerability to mechanical stressors (e.g. head trauma, barotrauma) | ||
| Procedures or diseases associated with intracranial hyper- or hypotension (e.g. lumbar puncture, pseudotumor cerebri) | ||
Oozer – excessive CA patency with increased communication between perilymph and liquor and a slightly increased amount of fluid flow; Gusher – IAC abnormalities resulting in excessive communication of perilymph and liquor, conductive hearing loss and free-flowing fluid during operations.
Inner Ear Homeostasis
The regulation of inner ear fluid homeostasis (its parameters are volume, concentration, osmolarity, and pressure) is the basis for adequate response to stimulation. Ion and water transport in the inner ear help maintain the proper potassium concentration required for hair cell function. Many structures are involved in the complex process of inner ear homeostasis. The stria vascularis, located at the lateral wall of the cochlear duct, and the vestibular dark cells are the 2 main structures responsible for endolymph secretion, and possess many similarities. The characteristics of these structures are the basis for regulation of inner-ear homeostasis, while impaired function is related to various diseases [39].
The stria vascularis represents one of the few epithelial types that contain capillaries. A thickening of the strial capillary basement membrane was suggested as the primary site in cochlear pathogenesis in Alport syndrome, which results from mutations in genes encoding the collagen chains alpha3 (IV), alpha4 (IV), and alpha5 (IV), preventing proper production or assembly of the type IV collagen network. The syndrome is characterized by progressive glomerular disease associated with a high-frequency sensorineural hearing loss [40]. Stria vascularis has a higher oxygen consumption than brain tissue, and the strial capillaries are larger in diameter, with a higher hematocrit and a slower flow than the capillaries of any other tissue types [41]. Schuhknecht defined the strial type of sensorineural hearing loss that is characterized by a flat stria vascularis [42,43] and reduced stria vascularis function; it has been implicated in the pathogenesis of presbyacusis [44,45]. It was shown in animal models that age-related atrophy of the stria vascularis is associated with a thickening of the basement membrane in strial capillaries. Consequently, degeneration has been attributed to decreased permeability imposed by the thickened basement membrane [46].
Vestibular dark cells and strial marginal cells are regulated by purinergic-, adrenergic-, and muscarinic receptors, steroids, vasopressin and atrial natriuretic peptide (ANP). There is evidence that the stress hormones noradrenaline and adrenaline, corticosteroids, and mineralocorticosteroids possess a key role in inner ear homeostasis and sensory transduction (Table 7). There also exists a strongly expressed and largely non-overlapping distribution pattern for different aquaporin (AQP) water channel subtypes in the inner ear, suggesting the existence of regional, subtype-specific water transport pathways [47–49]. The global regulation of water transport in the inner ear may require concerted actions of multiple types of AQPs [50].
Table 7.
Regulation of endolymph composition.
| Vasopressin1 (ADH, AVP, DDAVP) |
|
| Atrial natriuretic peptide (ANP) |
|
| Glucocorticosteroids | Na+-channels (absorption)↑ glucocorticoid inducible kinases1–3®Isc(K)↑ AQP1,3↑ Vasopressin↓ Na+/K+-ATPase activity↑ |
| Mineralocorticosteroids | Secretion↓, Isc(K)↓ Na+/K+-ATPase activity↑ |
| Adrenergic receptors | β1®K+ secretion↑®Isk(K)↑ β2®Cl- secretion↑ via cAMP (Na+ absorption, K+ secretion) β1®metabolism↑ β1®Na+/K+-ATPase activity↑ |
| Muscarinic receptors | M3, M4®K+ secretion↑ |
| ATP, UTP, purinergic receptors | K+ secretion↓, Isk(K)↓ via protein kinase C |
As Agent Vasopressin=INN, Antidiuretic hormone=ADH, AVP=arginine vasopressin, DDAVP=(one trade name of desmopressin). AQP=aquaporin; V2=antidiuretic hormone receptor 2; c-AMP=cyclic adenosine monophosphate; ES=endolymphatic sac, Isc=short circuit current; ATPase=adenosine triphosphate; Isk=short circuit current channel, ATP=adenosine triphosphate; UTP=uridine triphosphate.
The Efferent or Olivocochlear System
The efferent system of the ear possesses several distinct functions, in particular noise protection, mediation of selective attention, and improvement of signal-to-noise ratio. It also supports adaptation and frequency selectivity by modification of the micromechanical properties of outer hair cells. The myelinated medial fibers, which innervate outer hair cells, and the unmyelinated efferent fibers, which terminate under inner hair cells, together form the basis for localization of a sound stimulus and enable to function in a 3-dimensional auditory world. The efferent system is affected by inner ear stressors (e.g. noise, ototoxic drugs) and might play a key role in tinnitus generation and maintenance [51]. It is one of the main noise-protective mechanisms of the cochlea.
The excitatory glutamatergic afferent transmission of the auditory system is under inhibitory control of GABA and dopamine. Afferent dendrites can be excited via muscarinic receptors as well [52]. Neurotransmission of the efferent system takes place by inhibitory and excitatory transmitters reflecting fine regulation and noise protection. The neurotransmitters of the medial efferent fibers include ACh (acetylcholine), GABA (gamma aminobutyric acid), CGRP (calcitonin gene-related peptide), ATP (adenosine triphosphate), enkephalins, and NO [53,54]. The transmitter of the lateral efferent system include Ach, GABA, CGRP, dopamine, serotonin, and opioids like dynorphin or enkephalin. It was shown in animal experiments that dopamine agonists reduce cochlear damage by noise or ischemia [55–57] and that this transmitter may protect hair cells in inner ear stress (e.g. ischemia) [58]. The alpha 9/10 Ach-receptor suppresses the excitability of outer hair cells by mediating calcium entry into the cell, thus inducing a hyperpolarizing Ca2+-sensitive K+ current, mediated by small conductance channels (Isk). Overexpression of alpha9-Ach receptors in the outer hair cells in transgenic mice significantly reduces acoustic injury that causes either temporary or permanent damage, without changing pre-exposure cochlear sensitivity to low or moderate level sound [59]. It is interesting that regenerated nerve fibers in noise-damaged chinchilla are only afferent and have no AchE staining [60].
Meniere’s Disease
Meniere’s disease certainly represents the most impressive acute inner ear disease. However its appearance and courses are variable. In 40% to 50%, cochlear precede vestibular symptoms, in 20% to 50% the disease manifests vice versa, and in 7% to 30% cochlear and vestibular symptoms occur together initially [61,62]. If the disease progresses, permanent hearing loss develops instead of fluctuating hearing loss and hair cells go under. An endolymphatic hydrops is seen as the pathophysiological correlate of Meniere’s disease today, leading to altered hydrostatic and osmotic pressure in the endo- and perilymphatic space [63,64]. It seems that all patients with classical symptoms of Meniere’s disease have an endolymphatic hydrops, but not vice versa, as not all patients with hydrops have Meniere’s disease symptoms [65].
The degree of endolymphatic hydrops in MRI is correlated with cochlear and vestibular dysfunction [66]. Various causes for an endolymphatic hydrops have been discussed, particularly immunologic compromise, allergies, ototoxicity, quantitative or qualitative endolymph hypersecretion, and obstruction of the endolymphatic system. A genetic predisposition in combination with a multi-factor etiology was proposed [67]. The endolymphatic sac (ES) is the only structure of the inner ear with immunologic capacity. The ES is the blind ending of the endolymphatic duct, which passes along the vestibular aqueduct. It is the only structure of the inner ear that possesses a basal level of lymphocytes, leucocytes, macrophages, and Langerhans cells, and is the first structure of the inner ear that reacts to infection [68,69]. Antigen challenge to the ES in animal experiments results in endolymphatic hydrops and fluctuating hearing loss like in Meniere’s disease [70].
The ES is the regulator of the endolymph, and its parameters are pressure, volume, content, and osmolarity. Its dysfunction has significant effects on endolymph homeostasis and inner ear function. Consequently, its impaired function can lead to endolymphatic hydrops [71,72]. In Meniere’s disease, the vascularization of the pars rugosa and perisaccular tissue is impaired [73], and a saccular fibrosis was found in autopsies [74–77]. The intraosseous volume of the endolymphatic sac is reduced in later stages of Meniere’s disease [78,79].
A wide expression of the water channels aquaporin (AQP) subunits exists in the ES. So far, the AQPs 1, 2, 3, 4, 5, 6 have been found [47–49,80]. The endolymphatic volume is regulated by vasopressin, which blocks the fluid absorption mediated via V2-receptors, cAMP, and AQP2, which are expressed in the endolymphatic sac epithelium [81]. Vasopressin application leads to decreased endocytosis in the ES and endolymphatic hydrops [82,83]. The expression of AQP1 and AQP3 is elevated by corticosteroids [81,84,85]. The administration of mineralocorticoids can elevate the rate of hydrops caused by obliterated endolymphatic sacs in animal experiments [86]. Atrial natriuretic peptide (ANP) reduces the endolymph volume of the inner ear [83].
Sudden Deafness
Sudden deafness, also called idiopathic sudden sensorineural hearing loss (ISSHL), includes all causes and diseases for sudden hearing loss with unknown etiology. Discussed etiologies include vascular compromise, viral infection, endolymphatic hydrops, autoimmune diseases, and disruption of endolymphatic homeostasis triggered by stress hormones or other hormones. ISSHL is most often defined as sensorineural hearing loss of 30 dB or greater over at least 3 contiguous audiometric frequencies occurring over 72 hours [87]. Due to recent advances in gene analysis technology, various single nucleotide polymorphisms (SNPs) have been found to be closely associated with ISSHL incidence [88–91]. The incidence is 5–20 per 100 000 in Western countries [92–94]. Spontaneous remission occurs in about 45% to 65% of cases [92,95]. The prognosis is worse with higher degree of hearing loss. Low frequency hearing loss shows a better prognosis than high frequency hearing loss and better results are achieved when therapy begins in the first days after symptom onset. The current mainstay of treatment is cortisone infusions together with a rheologic agent like hydroxyethyl starch (HAES) for 3 to10 days, which lead to full remission in about 75% of cases. Addition of cortisone therapy shows a significant better outcome than rheological therapy alone [96].
It is currently believed that most cases of ISSHL are caused by circulatory disturbances, probably at the stria vascularis, which is the only epithelium that contents capillaries and has higher oxygen consumption than brain tissue. Enhanced capillary permeability can be found in acute hyper-or hypotension [97]. Suckfüll could improve hearing impairment by low-density lipoprotein apheresis [98]. Pentoxifylline, a commonly used drug for ISSHL, increases cochlear blood flow and significantly improves acute hearing loss, tinnitus, and vertigo compared to placebo [99]. Ischemic damage could be prevented in animal models for cochlear ischemia by various compounds like insulin-like growth factor (IGF-1), AM-111 (an apoptosis inhibitor), prednisolone, edarabone (a free radical scavenger), ginsenoside RB1 (Kappo), glia-cell derived neurotrophic factor (GDNF), hematopoietic stem cells, and liposome-encapsulated hemoglobin (artificial red blood cells) [100].
Noise
Noise-induced hearing loss may occur suddenly as acoustic trauma with mechanical overload or be gradual due to repeated exposure and regarded as constant metabolic stress. Generally speaking, the ear can be exposed to short periods in excess of 120 dB without permanent harm, although with discomfort. Long-term exposure to sound levels over 80 dB can cause permanent hearing loss. Since decibels are based on a logarithmic scale, every increase of 3 decibels results in a doubling of intensity. In contrast to a temporary threshold shift, also called auditory fatigue, which usually recovers in 24–48 hours, permanent threshold shift is characterized by degeneration of hair cells and ganglion cells with a higher vulnerability of outer hair cells than inner hair cells. Sound pressure levels (SPL) over 150 dB and 1.5 ms duration at minimum result in mechanical damage to the middle and inner ear (e.g. hemorrhage, rupture of the basilar or Reissner’ membrane, and damage to the organ of Corti). Damage to the middle ear leads to combined hearing loss patterns.
The pathophysiological mechanisms of hearing loss caused by noise, ototoxic agents, in presbyacusis, or ISSHL are alike. The 2 key mechanisms are formation of reactive oxygen species (free radicals, ROS) and reactive nitrogen species (RNS), followed by activation of apoptotic signaling pathways of cell death [101,102]. ROS emerge immediately after noise exposure [103] and persist for 7–10 days thereafter [104], which might correspond to the time-window for post-exposure intervention and containment of the extent of hearing loss. Another consequence of noise exposure is an increase of free Ca2+ in outer hair cells immediately after acoustic trauma [105], which might trigger ROS production and induces apoptotic pathways [106]. In addition, noise decreases cochlear blood flow [107], which is suggested to be caused by vasoactive lipid peroxidation products such as isoprostanes [108]. The magnesium supplementation protective effects in noise trauma might arise from reduction of calcium influx into the cell and consequent decrease of apoptosis pathways in hair cells. It can also limit ischemia by inducing vasodilatation of cochlear arterioles [109]. The clinical value of magnesium supplementation for noise-induced hearing loss protection is well explored [110].
Ototoxicity
Medications with ototoxic adverse effects include aminoglycosides, loop diuretics, cytostatics (cisplatin, cyclophosphamide), tuberculostatics (streptomycin, rifampicin, capreomycin), quinine, chloroquine, salicylic acid, and phenothiazines. Ototoxic agents may impair the function of various inner ear structures and alter the fine tuning of mechanoelectrical transduction, resulting in inner ear symptoms. As described above, molecular irregularities within the stereocilia lead to increased inner ear vulnerability. For example, reduction or omission of otocadherin (also known as CDH23, and encoded by the Ahl gene) weakens the cell and may make stereocilia more vulnerable to physical damage from noise and ageing [111]. Stereocilia are the first structures to be damaged by inner ear stressors such as noise or aminoglycosides. Noise affects the stereociliary carbohydrate metabolism, resulting in degeneration of ciliary interconnections together with disarrangement and detachment of cilia [112]. Aminoglycosides alter the carbohydrate metabolism, which leads to deterioration of the glycocalyx and weakening of the ciliary interconnections and tip links, resulting in stereociliary fusion [113,114]. An alteration in stereociliary stiffness leads to an increase in the hair cell discharge rate, which has been linked to tinnitus generation [115].
Numerous gene polymorphisms are relevant for susceptibility to noise-induced hearing loss and ototoxicity. Among them, polymorphisms of glutathione-S-transferase and mitochondrial MTRNR1 mutation 1555G>A are most explored. The latter causes the structure of the mitochondrial 12S ribosomal RNA to be more similar to that of bacterial rRNA, thus making the mitochondrial ribosomal decoding site more accessible to aminoglycoside antibiotics, which exert their antibacterial effect by specifically binding to the bacterial ribosome. It accounts for 20% of patients with aminoglycoside ototoxicity and carriers of this mutation may sustain profound deafness after a single injection. Patients with this mutation may develop spontaneous hearing loss as well [116,117].
Basically, interventions to prevent or attenuate ototoxic and noise-induced adverse effects take 1 of 2 approaches: the augmentation of protective pathways or the inhibition of cell death pathways. Human trials have shown prevention of aminoglycoside-induced hearing loss by ROS scavenger glutathione [118] and RNS scavenger salicylate [119]. One small study with 11 patients treated by transtympanic injection of apoptosis inhibitor D-JNKI-1 (AM-111) within 24 hours after acoustic trauma showed therapeutic effectiveness in noise-induced hearing loss [120].
Therapy
Surgical therapeutic options are only chosen for distinct diseases with progression and excessive symptoms like neoplasia, dehiscence of the semicircular canals (resurfacing of the dehiscence or plugging of the semicircular canal), high jugular bulb (jugular bulb compression, jugular vein ligation or embolization), vascular loops at the cerebellopontine angle (vascular decompression of the vestibulocochlear nerve). Middle ear implants are used for mid-grade sensorineural hearing loss of about 40–80 dB in middle frequencies when conventional hearing aids cannot be used due to pathologies or diseases of the external ear canal like chronic otitis externa, canal stenosis, eczema or psoriasis, absence of the pinna, mandibular fractures, excessive cerumen production, or perspiration. Cochlear implants are used for severe to profound sensorineural hearing loss. It can be considered in all cases of congenital inner ear malformations when cochlear remnants or a vestibulocochlear nerve exist.
Numerous pharmaceutical agents have been explored for idiopathic sudden sensorineural hearing loss, for protection, and therapy of noise trauma or ototoxicity. Most of them have not entered clinical usage as efficacy has been shown in animal experiments in most cases. A comprehensive list of tested compounds can be found in reviews by Lynch and Kil [121], Rybak and Whitworth [122], Ohlemiller [123], Iishi and Schacht [124], and Tabuchi et al. [125]. They comprise radical scavengers like N-acetylcysteine, D-methionine, α-lipoic acid, ebselen, resveratrol, gingko biloba, vitamin C, vitamin E, water-soluble coenzyme Q10, ferulic acid; minerals like magnesium, selenium, zinc; receptor agonists and antagonists like A1 adenosine receptor agonists, glutamate antagonists, calcium channel blockers; growth and neurotrophic factors like glial cell line-derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNTF), fibroblast growth factor (FGF), neurotrophin 3 (NT3); hormones like corticosteroids, estradiol, dehydroepiandrosterone (DHEA) and anti-apoptotic substances blocking apoptotic cascades. However, it appears that a combination of substances might be more effective than a single compound (e.g. complementary therapies to modulate oxidative stress, exotoxicity, blood flow, calcium and stimulation overload, apoptotic pathways, neurotrophic or hormonal control mechanisms). In this context, pharmacogenetics will increase therapeutic efficacy as certain supplementations will be effective in only a subset of the population and protection can be tailored to genetic variants (e.g. the necessity of administration of N-acetylcysteine for protection of noise trauma is dependent on genetic polymorphisms of the glutathione S-transferase [GST]) [126].
Pharmaceutical Therapy
Standard therapy for acute inner ear symptoms are cortisone infusions together with a rheologic agent. Addition of radical scavengers orally or intravenously to the therapy regimen like vitamin C, vitamin E, or L-N-acetylcysteine has shown improved hearing outcome [127–129]. Treatment considerations include stress reduction, cardiovascular improvement, and cervical physiotherapy.
Betahistine (H1 receptor agonist, H3 receptor antagonist) and Arlevert® (fixed combination of cinnarizine, a calcium channel blocker, and dimenhydrinate, a H1-receptor antagonist) are drugs often used as mainstay therapy for vertigo control in Meniere’s disease and can reduce number and intensity of vertigo episodes [130,131]. If numerous recurrent vertigo attacks occur, transtympanic gentamicin application is used to destroy vestibular hair cells, as gentamicin is 4 times more vestibulotoxic than cochleotoxic. Complete vertigo control can be achieved in about 75% and substantial vertigo control in about 93% of cases [132]. Radical scavengers like vitamin C, glutathione, and thioctic acid showed improvement of hearing loss, tinnitus, and vertigo in Meniere’s disease [133,134].
Arlevert® and Gingko biloba have shown efficacy for compensation of chronic vertigo (e.g. residual vestibular deficit) [135,136]. Current standard therapy for decompensated chronic tinnitus is tinnitus retraining therapy (TRT) or tinnitus desensitization therapy (TDT). Tinnitus masking can be added and increases success rates [137].
Stem Cell Therapy
Birds, fish, and other non-mammalian vertebrates can regenerate inner hair cells throughout life via 2 mechanisms; the non-mitotic transdifferentiation of supporting cells, and the mitotic proliferation and differentiation of a subset of supporting cells. In human utricles, supporting cells appear to hold the potential as progeny because some are able to respond to trauma by dividing [138]. A possible third mechanism is that non-mammalian vertebrates may regenerate hair cells through the differentiation of resident stem cells, but it is still controversial whether stem cells in adult sensory epithelia are from a subtype of supporting cells. Introducing exogenous stem cells into the degenerated ear is the other major approach for inner ear therapy [139]. Challenges in stem cell therapy are uncontrolled cell growth, variable human response to resorption, recellularization, regeneration and potential disastrous consequences (e.g. malignant transformation) [140].
There have been significant strides made in regenerating cochlear hair cells by mesenchymal-derived stem cells from induced pluripotent (iPSC) precursors from both mouse embryonic stem cells [141,142] and human fibroblasts or from more abundant sources such as human adipocytes [143–164]. However, considerable efforts have been directed towards stimulating mammalian hair cell regeneration and related studies have shown promising results. The Math1 gene encodes a basic helix-loop-helix transcription factor (bHLH), which is necessary for terminal differentiation of otic epithelial progenitor cells into hair cells [147,148]. Overexpression of Math1 was first shown to induce conversion of nonsensory cells into hair cells via plasmid vector in vitro[149]. Mouse atonal homolog, Math1, and human atonal homolog HATH1 were demonstrated via adenoviral vector delivery, effecting phenotypic conversion of supporting cells to hair cells in vivo[150,151]. The first reported demonstration of gene therapy-mediated recovery of hearing loss was reported in 2005. Normal-hearing adult guinea pigs were ototoxically deafened and then inoculated with adenovector-delivered Math-1 [152]. The next challenge in stem cell research is to induce full functional and organized hair cells. Numerous growth factors are utilized in the developmental processes of stem cells and it is conceivable that delivery of these growth factors in vivo with a form of inner drug delivery will be necessary.
Genetic Therapy
Gene therapy offers the potential for more direct manipulation of gene expression in the target cells by directly inhibiting expression of a deleterious allele or by inserting and forcing expression of a missing or down-regulated gene. These tasks can be accomplished by gene transfer technology. The challenges of gene therapy include aspects of delivery, specificity to targets, adverse effects, and regulation of quantity and duration of gene expression.
One potentially groundbreaking method for changing the outcome of inner ear disease is genetic manipulation by RNA interference (RNAi) by microRNAs (miRNAS) and gene-specific small interfering RNAs (siRNA), which inactivate messenger RNA (mRNA). Animal studies used siRNA against TRPV1 (transient receptor potential vanilloid receptor 1) or NOX3, which are induced by cisplatin in a ROS-dependent manner, via round window application or transtympanic application, respectively, to successfully reduce cisplatin-related hearing loss [153,154]. In addition, numerous animal studies showed elevation of hearing thresholds, hair cell protection, and prevention of neuron loss from ototoxic drug application for viral vector-mediated delivery of genes for apoptosis inhibitors and growth factors [155–159].
The above-mentioned studies dealt with the task of protecting and repairing inner ear sensory epithelia. In addition, some animal studies have shown success with gene replacement or suppression in animal models for hereditary hearing loss. Hearing in deaf connexin 30 null mice could be restored by genetically overexpressing the connexin 26 gene [160]. Synaptic transmission and hearing could be restored after viral-mediated gene delivery of vesicular glutamate receptor-3 VGLUT3 in mouse mutants [161]. SiRNA-technology has been used in a mouse model for connexin 26 mutation-related hearing loss to block expression of a dominant connexin 26 gene [162].
Drug therapy of the inner ear involves specific difficulties due to its bony isolation and the blood-inner ear barrier. One of the most important issues before full clinical application is the development of smart delivery systems that can carry a variety drugs, proteins, and nucleic acids such as DNA and siRNA, and its controlled release. The use of viral vectors is associated with higher transfection efficiency, but there are toxicity and safety problems, such as immunogenicity and insertional mutagenesis [163,164].
Nanoparticles promise improved biocompatibility, in vivo stability, target specify, cell/tissue uptake, and internalization of the encapsulated therapeutic agents and fewer adverse effects than viral vectors. They vary in size from 10 to 1000 nm and, depending on the end use, may or may not contain a drug molecule. Various nanoparticle systems with specific characteristics exist: poly (D;L-lactic/glycolic acid) nanoparticles, magnetic nanoparticles, lipid nanoparticles, liposomes, polymersomes, hydroxyapatite nanoparticles, and silica nanoparticles [165]. The feasibility of liposome-mediated gene-transfer was shown more than a decade ago. Transgenic gene expression in the neurosensory epithelia and surrounding tissue of the guinea pig cochlea without toxicity and inflammation in the target organ was achieved for up to 14 days after direct microinjection into the cochlea [166]. The most promising route of drug delivery to the inner ear seems to be via the round window membrane, and numerous studies have been successful in detecting various nanoparticles in the organ of Corti and spiral ganglion neurons after round window application [167–169]. The round window membrane serves as a barrier providing protection for the inner ear by limiting transfer of molecules as a function of factors such as size, electrical charge, and concentration [170]. A new type of nanocarrier is nanogel, which consist of a jelly form of nanoparticle reservoirs that adheres to the round window membrane [171]. However, the most immediate challenge for nanoparticle-based gene therapy to overcome is to achieve a high transfection rate, especially in cells that do not divide, such as neurons and the hair cells of the inner ear. Newly discovered transposons, genetic elements that can relocate between genomic sites using a ‘cut and paste’ mechanism, may be useful [172].
Conclusions
The genetic and pathophysiological causes of inner ear symptoms, notably sensorineural hearing loss, tinnitus, and vertigo, are numerous. Currently, there are no therapeutic options to restore hearing perception. Hearing aids and implants are useful tools for deaf patients but cannot restore natural hearing perception. However, the fundamentals of pathophysiological understanding have been laid for advances in pharmaceutical, genetic, and stem cell therapy.
Footnotes
Source of support: Self financing
References
- 1.Ménière P. Pathologie auriculaire: memoire sur des lesions de l’oreille interne donant lieu a des symptomes de congestion cerebrale apoplectiforme. Gaz Med. 1861;16:597–601. [in French] [Google Scholar]
- 2.Chung DY, Gannon RP, Mason K. Factors affecting the prevalence of tinnitus. Audiology. 1984;23(5):441–52. doi: 10.3109/00206098409070084. [DOI] [PubMed] [Google Scholar]
- 3.National Center for Health Statistics. Vital and Health Statistics, Series 11 No. 32. 1968. pp. 1–28. [PubMed] [Google Scholar]
- 4.Nadol JB., Jr Hearing loss. N Engl J Med. 1993;329:1092–102. doi: 10.1056/NEJM199310073291507. [DOI] [PubMed] [Google Scholar]
- 5.Fortnum H, Summerfield A, Marshall D, et al. Prevalence of permanent childhood hearing impairment in the United Kingdom and implications for universal neonatal hearing screening: questionnaire based ascertainment study. BMJ. 2001;323:536–40. doi: 10.1136/bmj.323.7312.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kemper AR, Down SM. A cost-effectiveness analysis of newborn hearing screening strategies. Arch Pediatr Adolesc Med. 2000;154:484–88. doi: 10.1001/archpedi.154.5.484. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham M, Cox EO. Hearing assessment in infants and children: Recommendations beyond neonatal screening. Pediatrics. 2003;111:436–40. doi: 10.1542/peds.111.2.436. [DOI] [PubMed] [Google Scholar]
- 8.Morton CC, Nance WE. Newborn hearing screening: a silent revolution. N Engl J Med. 2006;354:2151–64. doi: 10.1056/NEJMra050700. [DOI] [PubMed] [Google Scholar]
- 9.Gross M, Lange K, Spormann-Lagodzinski M. Congenital hearing loss in children. 2: Genetic hearing loss. HNO. 2001;49:602–17. doi: 10.1007/s001060170056. [DOI] [PubMed] [Google Scholar]
- 10.Oezki M, Kato Z, Sasai H, et al. Congenital inner ear malformations without sensorineural hearing loss in children. Int J Ped Otorhinolaryngol. 2009;73:1484–87. doi: 10.1016/j.ijporl.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Jackler RK, Luxford WM, House WF. Congenital malformations of the inner ear: a classification based on embryogenesis. Laryngoscope. 1987;97:2–14. doi: 10.1002/lary.5540971301. [DOI] [PubMed] [Google Scholar]
- 12.Sennaroglu L, Saatci I. A new classification for cochleovestibular malformations. Laryngoscope. 2002;112:2230–41. doi: 10.1097/00005537-200212000-00019. [DOI] [PubMed] [Google Scholar]
- 13.Giesemann AM, Kontorinis G, Zajaczek J, et al. The vestibulocochlear nerve: aplasia and hypoplasia in combination with inner ear malformations. Eur Radiol. 2012;22:519–24. doi: 10.1007/s00330-011-2287-z. [DOI] [PubMed] [Google Scholar]
- 14.Marazita ML, Ploughman LM, Rawlings B, et al. Genetic epidemiological studies of early-onset deafness in the U.S. School-age population. Am J Med Genet. 1993;46:486–91. doi: 10.1002/ajmg.1320460504. [DOI] [PubMed] [Google Scholar]
- 15.Van Camp G, Smith RJH. Homepage on the Internet. Hereditary Hearing Loss Homepage. Available from: http//web01.ua.ac.be/hhh/
- 16.Friedman LM, Avraham KB. MicroRNAs and epigenetic regulation in the mammalian inner ear: implications for deafness. Mamm Genome. 2009;20:581–603. doi: 10.1007/s00335-009-9230-5. [DOI] [PubMed] [Google Scholar]
- 17.Mencia A, Modamio-Hoybjor S, Redshaw N, et al. Mutations in the seed region of human miR-96 are responsible for nonsyndromic progressive hearing loss. Nat Genet. 2009;41:609–13. doi: 10.1038/ng.355. [DOI] [PubMed] [Google Scholar]
- 18.Drottar M, Liberman MC, Ratan RR, Roberson DW. The histone deacetylase inhibitor sodium butyrate protects against cisplatin-induced hearing loss in guinea pigs. Laryngoscope. 2006;116:292–96. doi: 10.1097/01.mlg.0000197630.85208.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen FQ, Schacht J, Sha SH. Aminoglycoside-induced histone deacetylation and hair cell death in the mouse cochlea. J Neurochem. 2009;108:1226–36. doi: 10.1111/j.1471-4159.2009.05871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zelente L, Gasparini P, Estivill X, et al. Connexin 26 mutations associated with the most common form of non-syndromic neurosensory autosomal recessive deafness (DFNB1) in Mediterraneans. Hum Mol Genet. 1997;6:1605–9. doi: 10.1093/hmg/6.9.1605. [DOI] [PubMed] [Google Scholar]
- 21.Estivill X, Fortina P, Surrey S, et al. Connexin-26 mutations in sporadic and inherited sensorineural deafness. Lancet. 1998;351:394–98. doi: 10.1016/S0140-6736(97)11124-2. [DOI] [PubMed] [Google Scholar]
- 22.Kelley P, Harris D, Corner B, et al. Novel mutations in the connexin 26 gene (GJB2) that cause autosomal recessive (DFNB1) hearing loss. Am J Hum Genet. 1998;62:792–99. doi: 10.1086/301807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gurtler N, Kim Y, Mhatre A, et al. DFNA 54, a third locus for low-frequency hearing loss. J Mol Med. 2004;82(11):775–80. doi: 10.1007/s00109-004-0597-1. [DOI] [PubMed] [Google Scholar]
- 24.Verhoeven K, Van Laer L, Kirschhofer K, et al. Mutations in the human alpha-tectorin gene cause autosomal dominant nonsyndromic hearing impairment. Nat Genet. 1998;19(1):60–62. doi: 10.1038/ng0598-60. [DOI] [PubMed] [Google Scholar]
- 25.McGuirt WT, Prasad SD, Griffith AJ, et al. Mutations in COL11A2 cause nonsyndromic hearing loss (DFNA13) Nat Genet. 1999;23:413–19. doi: 10.1038/70516. [DOI] [PubMed] [Google Scholar]
- 26.Cryns K, Pfister M, Pennings R, et al. Mutations in the WFS1 gene that cause low-frequency sensorineural hearing loss are small non-inactivating mutations. Hum Genet. 2002;110:389–94. doi: 10.1007/s00439-002-0719-1. [DOI] [PubMed] [Google Scholar]
- 27.Delmaghani S, del Castillo FJ, Michel V, et al. Mutations in the gene encoding pejvakin, a newly identified protein of the afferent auditory pathway, cause DFNB59 auditory neuropathy. Nat Genet. 2006;38:770–78. doi: 10.1038/ng1829. [DOI] [PubMed] [Google Scholar]
- 28.Ciuman RR. Auditory and vestibular hair cell stereocilia: relationship between functionality and inner ear disease. J Laryngol Otol. 2011;125:991–1003. doi: 10.1017/S0022215111001459. [DOI] [PubMed] [Google Scholar]
- 29.Kimberling WJ, Moller C. Clinical and molecular genetics of Usher syndrome. J Am Acad Audiol. 1995;6:63–72. [Google Scholar]
- 30.Bougham JA, Vernon M, Shaver KA. Usher syndrome: definition and estimate of prevalence from two high risk populations. J Chron Dis. 1983;36:595–603. doi: 10.1016/0021-9681(83)90147-9. [DOI] [PubMed] [Google Scholar]
- 31.Vernon M. Usher syndrome-deafness and progressive blindness. Clinical cases, prevention, theory and literature survey. J Chron Dis. 1969;22:133–51. doi: 10.1016/0021-9681(69)90055-1. [DOI] [PubMed] [Google Scholar]
- 32.Otterstede CR, Spandau U, Blankenagel A, et al. A new clinical classification for Usher’s syndrome based on a new subtype of Usher’s syndrome type 1. Laryngoscope. 2001;111:84–86. doi: 10.1097/00005537-200101000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Ciuman RR. Communication routes between intracranial spaces and inner ear; function, pathophysiologic importance and relations with inner ear diseases. Am J Otolaryngol. 2009;30:193–202. doi: 10.1016/j.amjoto.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Swartz JD. An overview of congenital/developmental sensorineural hearing loss with emphasis on the vestibular aqueduct syndrome. Semin Ultrasound CT MR. 2004;25:353–68. doi: 10.1053/j.sult.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Reardon W, Omahoney CF, Trembath R, et al. Enlarged vestibular aqueduct: a radiological marker of Pendred syndrome, and mutation of the PDS gene. QJM. 2000;93:99–104. doi: 10.1093/qjmed/93.2.99. [DOI] [PubMed] [Google Scholar]
- 36.Everett LA, Glaser B, Beck JC, et al. Pendred syndrome is caused by mutations in a punative sulphate transporter gene (PDS) Nat Genet. 1997;17:411–22. doi: 10.1038/ng1297-411. [DOI] [PubMed] [Google Scholar]
- 37.Chen K, Wang X, Sun L, Jiang H. Screening of SLC26A4, FOXI1, KCNJ10, and GJB2 in bilateral deafness patients with inner ear malformation. Otolaryngol Head Neck Surg. 2012;146:972–78. doi: 10.1177/0194599812439670. [DOI] [PubMed] [Google Scholar]
- 38.Propst EJ, Blaser S, Stockley TL, et al. Temporal bone imaging in GJB2 deafness. Laryngoscope. 2006;116:2178–86. doi: 10.1097/01.mlg.0000244389.68568.a7. [DOI] [PubMed] [Google Scholar]
- 39.Ciuman RR. Stria vascularis and vestibular dark cells: characterisation of main structures responsible for inner-ear homeostasis, and their pathophysiological relations. J Laryngol Otol. 2009;123:151–62. doi: 10.1017/S0022215108002624. [DOI] [PubMed] [Google Scholar]
- 40.Gratton MA, Rao VH, Meehan DT, et al. Matrix metalloproteinase dysregulation in the stria vascularis of mice with Alport syndrome: implications for capillary basement membrane pathology. Am J Pathol. 2005;166:1465–74. doi: 10.1016/S0002-9440(10)62363-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hawkins JE., Jr Microcirculation in the labyrinth. Arch Otorhinolaryngol. 1976;212:241–51. doi: 10.1007/BF00453672. [DOI] [PubMed] [Google Scholar]
- 42.Castaldo A, Linthicum FH., Jr Stria vascularis hearing loss. Otol Neurotol. 2006;27:285–86. doi: 10.1097/00129492-200602000-00025. [DOI] [PubMed] [Google Scholar]
- 43.Schuknecht HF. Pathology of the ear. 2nd ed. Malvern (PA): Lea & Febiger; 1993. [Google Scholar]
- 44.Johnsson LG, Hawkins JE., Jr Sensory and neural degeneration with aging, as seen in microdissection in the human inner ear. Ann Otol Rhinol Laryngol. 1972;81:179–93. doi: 10.1177/000348947208100203. [DOI] [PubMed] [Google Scholar]
- 45.Nadol JB., Jr Electron microscopic findings in presbyacusic degeneration of the basal turn of the cochlea. Otolaryngol Head Neck Surg. 1979;87:818–36. doi: 10.1177/019459987908700617. [DOI] [PubMed] [Google Scholar]
- 46.Thomopoulos GN, Spicer SS, Gratton MA, Schulte BA. Age-related thickening of basement membrane in stria vascularis capillaries. Hear Res. 1997;111:31–41. doi: 10.1016/s0378-5955(97)00080-4. [DOI] [PubMed] [Google Scholar]
- 47.Löwenheim H, Hirt B. Aquaporine. Discovery, function, and significance for otorhinolaryngology. HNO. 2004;52(8):673–78. doi: 10.1007/s00106-004-1128-7. [DOI] [PubMed] [Google Scholar]
- 48.Beitz E, Kumagami H, Krippeit-Drews P, et al. Expression pattern of aquaporin water channels in the inner ear of the rat. The molecular basis for a water regulation system in the endolymphatic sac. Hear Res. 1999;132:76–84. doi: 10.1016/s0378-5955(99)00036-2. [DOI] [PubMed] [Google Scholar]
- 49.Beitz E, Zenner HP, Schultz JE. Aquaporin-mediated fluid regulation in the inner ear. Cell Mol Neurobiol. 2003;23:315–29. doi: 10.1023/A:1023636620721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang D, Chen P, Chen S, et al. Expression patterns of aquaporins in the inner ear: evidence for concerted actions of multiple types of aquaporins to facilitate water transport in the cochlea. Hear Res. 2002;165:85–95. doi: 10.1016/s0378-5955(02)00288-5. [DOI] [PubMed] [Google Scholar]
- 51.Ciuman RR. The efferent system or olivocochlear function bundle-fine regulator and protector of hearing perception. Int J Biomed Sci. 2010;4:276–88. [PMC free article] [PubMed] [Google Scholar]
- 52.Oestreicher E, Wolfgang A, Felix D. Neurotransmission of the cochlear inner hair cell synapse-implications for inner ear therapy. Adv Otorhinolaryngol. 2002;59:131–39. doi: 10.1159/000059245. [DOI] [PubMed] [Google Scholar]
- 53.Schrott-Fischer A, Kammen-Jolly K, Scholtz A, et al. Efferent neurotransmission in the human cochlea and vestibule. Acta Otolaryngol. 2007;127:13–19. doi: 10.1080/00016480600652123. [DOI] [PubMed] [Google Scholar]
- 54.Puel JL. Chemical synaptic transmission in the cochlea. Prog Neurobiol. 1995;47:449–76. doi: 10.1016/0301-0082(95)00028-3. [DOI] [PubMed] [Google Scholar]
- 55.Pujol R, Puel JL, D’Aldin C, Eybalin M. Pathophysiology of the glutamatergic synapses in the cochlea. Acta Otolaryngol. 1993;113:330–34. doi: 10.3109/00016489309135819. [DOI] [PubMed] [Google Scholar]
- 56.D’Aldin C, Puel JL, Leducq R, et al. Effects of a dopaminergic agonist in the guinea pig cochlea. Hear Res. 1995;90:202–11. doi: 10.1016/0378-5955(95)00167-5. [DOI] [PubMed] [Google Scholar]
- 57.D’Aldin C, Eybalin M, Puel JL, et al. Synaptic connections and putative functions of the dopaminergic innervation of the guinea pig cochlea. Eur Arch Otorhinolaryngol. 1995;252:270–74. doi: 10.1007/BF00185388. [DOI] [PubMed] [Google Scholar]
- 58.Halmos G, Doleviczenyi Z, Repassy G, et al. D2 autoreceptor inhibition reveals oxygen-glucose deprivation-induced release of dopamine in guinea-pig cochlea. Neuroscience. 2005;132(3):801–9. doi: 10.1016/j.neuroscience.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 59.Maison SF, Luebke AF, Liberman MC, Zuo J. Efferent protection from acoustic injury is mediated via alpha9 nicotinic acetylcholine receptors on outer hair cells. J Neurosci. 2002;22(24):10838–46. doi: 10.1523/JNEUROSCI.22-24-10838.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Strominger RN, Bohne BA, Harding GW. Regenerated nerve fibers in the noise-damaged chinchilla cochlea are not efferent. Hear Res. 1995;92(1–2):52–62. doi: 10.1016/0378-5955(95)00196-4. [DOI] [PubMed] [Google Scholar]
- 61.Schmidt PH, Brunsting RC, Antvelink JB. Meniere’s disease: etiology and natural history. Acta Otolaryngol. 1979;87:410–12. doi: 10.3109/00016487909126442. [DOI] [PubMed] [Google Scholar]
- 62.Haye R, Quist-Hanssen SV. The natural course of Meniere’s disease. Acta Otolaryngol. 1976;82:289–93. doi: 10.3109/00016487609120908. [DOI] [PubMed] [Google Scholar]
- 63.Hallpike CS, Cairns H. Observations on the pathology of Meniere’s syndrome. J Laryngol Otol. 1938;53:625–55. [PMC free article] [PubMed] [Google Scholar]
- 64.Yamakawa K. Über die pathologische Veränderung bei einem Meniere-Kranken. Z Otol. 1938;11:192–93. [Google Scholar]
- 65.Merchant SN, Adams JC, Nadol JB., Jr Pathophysiology of Meniere’s syndrome: are symptoms caused by endolymphatic hydrops? Otol Neurotol. 2005;26:74–81. doi: 10.1097/00129492-200501000-00013. [DOI] [PubMed] [Google Scholar]
- 66.Gürkov R, Flatz W, Louza J, et al. In vivo visualized endolymphatic hydrops and inner ear functions in patients with electrocochleographically confirmed Meniere’s disease. Otol Neurotol. 2012;33:1040–45. doi: 10.1097/MAO.0b013e31825d9a95. [DOI] [PubMed] [Google Scholar]
- 67.Arweiler DJ, Jahnke K, Grosse-Wilde H. Meniere disease as an autosomal dominant hereditary disease. Laryngorhinootologie. 1995;74:512–15. doi: 10.1055/s-2007-997791. [DOI] [PubMed] [Google Scholar]
- 68.Altermatt HJ, Gebbers JO, Müller C, et al. Human endolymphatic sac: Evidence for a role in inner ear immune defence. ORL J Otorhinolaryngol Relat Spec. 1990;52:143–48. doi: 10.1159/000276124. [DOI] [PubMed] [Google Scholar]
- 69.Tomiyama S, Harris JP. The endolymmphatic sac: its importance in inner ear immune responses. Laryngoscope. 1986;96:685–91. doi: 10.1288/00005537-198606000-00018. [DOI] [PubMed] [Google Scholar]
- 70.Tomiyama S, Kinoshita T, Jinnouchi K. Fluctuating hearing loss following immune reaction in the endolymphatic sac of guinea pigs. ORL J Otorhinolaryngol Relat Spec. 1995;57:122–28. doi: 10.1159/000276724. [DOI] [PubMed] [Google Scholar]
- 71.Manni JJ, Kuijpers W. Longitudinal flow of macromolecules in the endolymphatic space of the rat: An autoradiographical study. Hear Res. 1987;26:229–37. doi: 10.1016/0378-5955(87)90059-1. [DOI] [PubMed] [Google Scholar]
- 72.Thalmann R, Thalmann I. Source and role of endolymph macromolecules. Acta Otolaryngol. 1999;119:293–96. doi: 10.1080/00016489950181260. [DOI] [PubMed] [Google Scholar]
- 73.Ikeda M, Sando I. Endolymphatic duct and sac in patients with Meniere’s disease. Ann Otol Rhinol Laryngol. 1984;93:540–46. doi: 10.1177/000348948409300603. [DOI] [PubMed] [Google Scholar]
- 74.Lim DJ, Glasscock ME. Fine morphology of the endolymphatic sac in Meniere’s disease. In: Vosteen KH, Schuknecht H, Pfaltz CR, et al., editors. Meniere’s disease Pathogenesis, Diagnosis and treatment. New York: Georg Thieme Verlag; 1981. p. 115. [Google Scholar]
- 75.Arenberg IK, Marovitz WF, Shambaugh GE. The role of the endolymphatic sac in the pathogenesis of endolymphatic hydrops in man. Acta Otolaryngol Suppl. 1970;275:1–49. [PubMed] [Google Scholar]
- 76.Saito H, Kitahara M, Yazawa Y, Matsumoto M. Histopathologic findings in surgical specimens of endolymphatic sac in Meniere’s disease. Acta Otolaryngol. 1977;83:465–69. doi: 10.3109/00016487709128872. [DOI] [PubMed] [Google Scholar]
- 77.Yasawa Y, Kitahara M. Electron microscopic studies of the endolymphatic sac in Meniere’s disease. ORL J Otorhinolaryngol Relat Spec. 1981;43:121–30. doi: 10.1159/000275533. [DOI] [PubMed] [Google Scholar]
- 78.Shambaugh GE., Jr Surgery of the endolymphatic sac. Arch Otolaryngol. 1966;83:305–15. doi: 10.1001/archotol.1966.00760020307003. [DOI] [PubMed] [Google Scholar]
- 79.Xenellis JE, Linthicum FH, Jr, Webster P, Lopez R. Basilar membrane displacement related to endolymphatic sac volume. Laryngoscope. 2004;114(11):1953–59. doi: 10.1097/01.mlg.0000147927.98766.e1. [DOI] [PubMed] [Google Scholar]
- 80.Zhong SX, Liu ZH. Expression of aquaporins in the cochlea and endolymmphatic sac of guinea pig. ORL J Otorhinlaryngol Relat Spec. 2003;65:284–89. doi: 10.1159/000075227. [DOI] [PubMed] [Google Scholar]
- 81.Fukushima M, Kitahara T, Fuse Y. Changes in aquaporin expression in the inner ear of the rat after i.p. injection of steroids. Acta Otolaryngol Suppl. 2004;553:13–18. doi: 10.1080/03655230410017599. [DOI] [PubMed] [Google Scholar]
- 82.Takeada T, Takeda S, Kitano H, et al. Endolymphatic hydrops induced by chronic administration of vasopressin. Hear Res. 2000;140:1–6. doi: 10.1016/s0378-5955(99)00180-x. [DOI] [PubMed] [Google Scholar]
- 83.Kumagami H, Löwenheim H, Beitz E, et al. The effect of antidiuretic hormone on the endolymphatic sac and the inner ear. Pflugers Arch. 1998;436:970–75. doi: 10.1007/s004240050731. [DOI] [PubMed] [Google Scholar]
- 84.Kitahara T, Fukushima M, Uno Y, et al. Up-regulation of cochlear aquaporin-3 mRNA after intra-endolymphatic sac application of dexamethasone. Neurol Res. 2003;25:865–70. doi: 10.1179/016164103771953989. [DOI] [PubMed] [Google Scholar]
- 85.Fukushima M, Kitahara T, Uno Y, et al. Effects of intratympanic injection of steroids on changes in rat inner ear aquaporin expression. Acta Otolaryngol. 2002;122:600–6. doi: 10.1080/000164802320396268. [DOI] [PubMed] [Google Scholar]
- 86.Dunnebier EA, Segenhout JM, Wit HP, Albers FW. Two-phase endolymphatic hydrops: a new dynamic guinea pig model. Acta Otolaryngol. 1997;117:13–19. doi: 10.3109/00016489709117984. [DOI] [PubMed] [Google Scholar]
- 87.Wilson WR, Byl FM, Laird N. The efficacy of steroids in the treatment of idiopathic sudden hearing loss. A double-blind clinical study. Acta Otolaryngol. 1980;106:772–76. doi: 10.1001/archotol.1980.00790360050013. [DOI] [PubMed] [Google Scholar]
- 88.Görür K, Tuncer U, Eskandari G, et al. The role of factor V Leiden and prothrombin G20210A mutations in sudden sensorineural hearing loss. Otol Neurotol. 2005:599–601. doi: 10.1097/01.mao.0000178120.46290.6c. [DOI] [PubMed] [Google Scholar]
- 89.Capaccio P, Ottaviani F, Cuccarini V, et al. Sudden hearing loss and MTHFR 677C>t/1298A>C gene polymorphisms. Genet Med. 2005;7:206–8. doi: 10.1097/01.gim.0000157817.92509.45. [DOI] [PubMed] [Google Scholar]
- 90.Rudack C, Langer C, Stoll W, et al. Vascular risk factors in sudden hearing loss. Thromb Haemost. 2006;95:454–61. doi: 10.1160/TH05-08-0554. [DOI] [PubMed] [Google Scholar]
- 91.Capaccio P, Cuccarini V, Ottaviani F, et al. Prothrombotic gene mutations in patients with sudden sensorineural hearing loss and cardiovascular thrombotic disease. Ann Otol Rhinol Laryngol. 2009;118:205–10. doi: 10.1177/000348940911800308. [DOI] [PubMed] [Google Scholar]
- 92.Byl FM., Jr Sudden hearing loss: Eight years experience and suggested prognostic table. Laryngoscope. 1984;94:647–61. [PubMed] [Google Scholar]
- 93.Fetterman BL, Saunders JE, Luxford WM. Prognosis and treatment of sudden sensorineural hearing loss. Am J Otol. 1996;17:529–36. [PubMed] [Google Scholar]
- 94.Hughes GB, Freedman MA, Haberkamp TJ, Guay ME. Sudden sensorineural hearing loss. Otolaryngol Clin North Am. 1996;29:393–405. [PubMed] [Google Scholar]
- 95.Mattox DE, Simmons FB. Natural history of sudden sensorineural hearing loss. Ann Otol Rhinol Laryngol. 1977;86:463–80. doi: 10.1177/000348947708600406. [DOI] [PubMed] [Google Scholar]
- 96.Ziegler EA, Hohlweg-Majert B, Maurer J, Mann WJ. Epidemiological data of patients with sudden hearing loss – a retrospective study over a period of three years. Laryngorhinootologie. 2003;82:4–8. doi: 10.1055/s-2003-36904. [DOI] [PubMed] [Google Scholar]
- 97.Sakagami M, Sano M, Tamaki H, Matsunaga T. Ultrastructural study of effect of acute hyper- and hypotension on the stria vascularis and spiral ligament. Acta Otolaryngol Suppl. 1984;406:256–62. doi: 10.3109/00016488309123046. [DOI] [PubMed] [Google Scholar]
- 98.Suckfüll M. Fibrinogen and LSL apheresis in treatment of sudden hearing loss: a randomised multicentre trial. Lancet. 2002;360:1811–17. doi: 10.1016/S0140-6736(02)11768-5. [DOI] [PubMed] [Google Scholar]
- 99.Cesarone MR, Incandela L, Belcaro G, et al. Treatment of vascular inner ear disease in vascular patients with pentoxifylline: a controlled, randomized trial. Angiology. 2002;53(Suppl 1):S23–26. [PubMed] [Google Scholar]
- 100.Gyo K. Experimental study of transient cochlear ischemia as a cause of sudden deafness. World J Otorhinlaryngol. 2013;3:1–15. [Google Scholar]
- 101.Tadros SF, D’Souza M, Zhu X, Frisina RD. Apoptosis-related genes change their expression with age and hearing loss in the mouse cochlea. Apoptosis. 2008;13:1303–21. doi: 10.1007/s10495-008-0266-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sha SH, Chen FQ, Schacht J. PTEN attenuates PIP3/Akt signaling in the cochlea of the aging CBA/J mouse. Hear Res. 2010;264:86–92. doi: 10.1016/j.heares.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yamane H, Nakai Y, Takayama M, et al. Appearance of free radicals in the guinea pig inner ear after noise-induced acoustic trauma. Eur Arch Otorhinolaryngol. 1995;252:504–8. doi: 10.1007/BF02114761. [DOI] [PubMed] [Google Scholar]
- 104.Yamashita D, Jiang HY, Schacht J, Miller JM. Delayed production of free radicals following noise exposure. Brain Res. 2004;1019:201–9. doi: 10.1016/j.brainres.2004.05.104. [DOI] [PubMed] [Google Scholar]
- 105.Fridberger A, Flock A, Ulfendahl M, Flock B. Acoustic overstimulation increases outer hair cell Ca2+ concentrations and causes dynamic concentrations of the hearing organ. Proc Natl Acad Sci USA. 1998;95:7127–32. doi: 10.1073/pnas.95.12.7127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis links. Nat Rev Mol Cell Biol. 2003;4:552–65. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 107.Thorne PR, Nuttall AL. Laser Doppler measurements of cochlear blood flow during loud sound exposure in the guinea pig. Hear Res. 1987;27:1–10. doi: 10.1016/0378-5955(87)90021-9. [DOI] [PubMed] [Google Scholar]
- 108.Ohinata Y, Miller JM, Altschuler RA, Schacht J. Intense noise induces formation of vasoactive lipid peroxidation products in the cochlea. Brain Res. 2000;878:163–73. doi: 10.1016/s0006-8993(00)02733-5. [DOI] [PubMed] [Google Scholar]
- 109.Abaamrane L, Raffin F, Gal M, et al. Long-term administration of magnesium after acoustic trauma caused by gunshot noise in guinea pigs. Hear Res. 2009;247:137–45. doi: 10.1016/j.heares.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 110.Attias J, Weisz G, Almong S, et al. Oral magnesium intake reduces permanent hearing loss induced by noise exposure. Am J Otolaryngol. 1994;15:26–32. doi: 10.1016/0196-0709(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 111.Davis RR, Kozel P, Erway LC. Genetic influences in individual susceptibility to noise: a review. Noise Health. 2003;5:19–28. [PubMed] [Google Scholar]
- 112.Takumida M, Fredelius L, Bagger-Sjoback D, et al. Effect of acoustic overstimulation on the glycocalyx and the ciliary interconnections in the organ of Corti: high resolution scanning electron microscopic investigation. J Laryngol Otol. 1989;103:1125–29. doi: 10.1017/s002221510011117x. [DOI] [PubMed] [Google Scholar]
- 113.Takumida M, Urquiza R, Bagger-Sjoback D, Wersall J. Effect of gentamicin on the carbohydrates of the vestibular end organs: an investigation by the use of FITC-lectins. J Laryngol Otol. 1989;103:357–62. doi: 10.1017/s0022215100108953. [DOI] [PubMed] [Google Scholar]
- 114.Takumida M, Bagger-Sjoback D, Wersall J, Harada Y. The effect of gentamicin on the glycocalyx and the ciliary interconnections in vestibular sensory cells: a high resolution scanning electron microscopic investigation. Hear Res. 1989;37:163–70. doi: 10.1016/0378-5955(89)90037-3. [DOI] [PubMed] [Google Scholar]
- 115.Schwaber MK. Medical evaluation of tinnitus. Otolaryngol Clin North Am. 2003;36:287–92. doi: 10.1016/s0030-6665(02)00161-5. [DOI] [PubMed] [Google Scholar]
- 116.Prezant TR, Agapian JV, Bohlman MC, et al. Mitochondrial ribosomal RNA mutation associated with both antibiotic-induced and non-syndromic deafness. Nat Genet. 1993;4:289–94. doi: 10.1038/ng0793-289. [DOI] [PubMed] [Google Scholar]
- 117.Fischel-Ghodsian N. Genetic factors in aminoglycoside toxicity. Pharmagenomics. 2005;6:27–36. doi: 10.1517/14622416.6.1.27. [DOI] [PubMed] [Google Scholar]
- 118.Feldman L, Efrati S, Eviatar E, et al. Gentamicin-induced ototoxicity in hemodialysis patients is ameliorated by N-acetylcysteine. Kidney Int. 2007;72:359–63. doi: 10.1038/sj.ki.5002295. [DOI] [PubMed] [Google Scholar]
- 119.Sha SH, Qiu JH, Schacht J. Aspirin to prevent gentamicin-induced hearing loss. N Engl J Med. 2006;354:1856–57. doi: 10.1056/NEJMc053428. [DOI] [PubMed] [Google Scholar]
- 120.Suckfuell M, Canis M, Strieth S, et al. Intratympanic treatment of acoustic trauma with a cell-permeable JNK ligand: a prospective randomized phase I/II study. Acta Otolaryngol. 2007;127:938–42. doi: 10.1080/00016480601110212. [DOI] [PubMed] [Google Scholar]
- 121.Lynch ED, Kil J. Compounds for the prevention and treatment of noise-induced hearing loss. Drug Discov Today. 2005;10:1291–98. doi: 10.1016/S1359-6446(05)03561-0. [DOI] [PubMed] [Google Scholar]
- 122.Rybak LP, Whitworth CA. Ototoxicity: therapeutic opportunities. Drug Discov Today. 2005;10:1313–21. doi: 10.1016/S1359-6446(05)03552-X. [DOI] [PubMed] [Google Scholar]
- 123.Ohlemiller KK. Recent findings and emerging questions in cochlear noise injury. Hear Res. 2008;245:5–17. doi: 10.1016/j.heares.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Oishi N, Schacht J. Emerging treatments for noise-induced hearing loss. Expert Opin Emerg Drugs. 2011;16:235–45. doi: 10.1517/14728214.2011.552427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tabuchi K, Nishimura B, Nakamagoe M, et al. Ototoxicity: mechanisms of cochlear impairment and its prevention. Curr Med Chem. 2011;18:4866–71. doi: 10.2174/092986711797535254. [DOI] [PubMed] [Google Scholar]
- 126.Lin CY, Wu JL, Shih TS, et al. N-Acetyl-cysteine against noise-induced temporary threshold shift in male workers. Hear Res. 2010;269:42–47. doi: 10.1016/j.heares.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 127.Kang HS, Park JJ, Ahn SK, et al. Effect of high dose intravenous vitamin C on idiopathic sudden sensorineural hearing loss: a prospective single-blind randomized controlled trial. Eur Arch Otorhinolaryngol. 2013;270:2631–36. doi: 10.1007/s00405-012-2294-y. [DOI] [PubMed] [Google Scholar]
- 128.Hatano M, Uramoto N, Okabe Y, et al. Vitamin E and vitamin C in the treatment of idiopathic sudden sensorineural hearing loss. Acta Otolaryngol. 2008;128:116–21. doi: 10.1080/00016480701387132. [DOI] [PubMed] [Google Scholar]
- 129.Angell SI, Abi-Hachem RN, Vivero RJ, et al. L-N-Acetylcysteine treatment is associated with improved hearing outcome in sudden idiopathic sensorineural hearing loss. Acta Otolaryngol. 2012;132:369–76. doi: 10.3109/00016489.2011.647359. [DOI] [PubMed] [Google Scholar]
- 130.Novotny M, Kostrica R. Fixed combination of cinnarizine and dimenhydrinate versus betahistine dimesylate in the treatment of Meniere’s disease: a randomized, doubleblind, parallel group clinical study. Int Tinnitus J. 2002;8:115–23. [PubMed] [Google Scholar]
- 131.Claes J, Van de Heyning PH. Medical treatment of Meniere’s disease: a review of literature. Acta Otolaryngol Suppl. 1997;526:37–42. doi: 10.3109/00016489709124019. [DOI] [PubMed] [Google Scholar]
- 132.Cohen-Kerem R, Kisilevsky V, Einarson TR, et al. Intratympanic gntamicin for Meniere’s disease. A meta-analysis. Laryngoscope. 2004;114:2085–91. doi: 10.1097/01.mlg.0000149439.43478.24. [DOI] [PubMed] [Google Scholar]
- 133.Raponi G, Alpini D, Volonte S, et al. The role of free radicals and plasmatic antioxidant in Meniere’s syndrome. Int Tinnitus J. 2003;9:104–8. [PubMed] [Google Scholar]
- 134.Takumida M, Anniko M, Ohtani M. Radical scavengers for Meniere’s disease after failure of conventional therapy. A pilot study. Acta Otolaryngol. 2003;123:697–703. doi: 10.1080/00016480310000728a. [DOI] [PubMed] [Google Scholar]
- 135.Walther LE. Procedures for restoring vestibular disorders. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2005;4 Doc05. [PMC free article] [PubMed] [Google Scholar]
- 136.Hamann K. Special Gingko extract in cases of vertigo: a systematic review of randomised, double-blind, placebo-controlled clinical examinations. HNO. 2007:258–63. doi: 10.1007/s00106-006-1440-5. [DOI] [PubMed] [Google Scholar]
- 137.Von Wedel H, Von Wedel UC. An assessment of tinnitus retraining therapy. HNO. 2000;48:887–901. doi: 10.1007/s001060050685. [DOI] [PubMed] [Google Scholar]
- 138.Marano RJ, Redmond SL. In vitro cultured primary cells from a human utricle explant possesses hair cell like characteristics. J Mol Histol. 2011;42:365–70. doi: 10.1007/s10735-011-9333-7. [DOI] [PubMed] [Google Scholar]
- 139.Hu Z. A stem cell based replacement for the inner ear. J Otology Rhinology [serial online] 2012;1(1) [Google Scholar]
- 140.Zuliani GF. Stem cell based regenerative medicine: What is science? What is fiction? And what is science fiction? J Otology Rhinology [serial online] 2012;1(1) [Google Scholar]
- 141.Oshima K, Shin K, Diensthuber M, et al. Mechanosensitive hair cell-like embryonic and induced pluripotent stem cells. Cell. 2010;141:704–16. doi: 10.1016/j.cell.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Koehler KR, Mikosz AM, Molosh AI, et al. Generation of inner ear sensory epithelia from pluripotent stem cells in 3D culture. Nature. 2013;500:217–21. doi: 10.1038/nature12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Duran Alonso MB, Fejoo-Redondo A, Conde de Felipe M, et al. Generation of inner ear sensory cells from bone marrow-derived human mesenchymal stem cells. Regen Med. 2012;7:769–83. doi: 10.2217/rme.12.65. [DOI] [PubMed] [Google Scholar]
- 144.Boddy SL, Chen W, Romero-Guevara R, et al. Inner ear progenitor cells can be generated in vitro from human bone marrow mesenchymal stem cells. Regen Med. 2012;7:757–67. doi: 10.2217/rme.12.58. [DOI] [PubMed] [Google Scholar]
- 145.Yuan XD, Yan X, Yang H, et al. In vitro study of directional inducible differentiation of inner hair cells from human adipose-derived mesenchymal stem cells. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2009;44:323–28. [PubMed] [Google Scholar]
- 146.Mosna F, Sensebe L, Krampera M. Human bone marrow and adipose tissue mesenchymal stem cells: a user’s guide. Stem Cells Dev. 2010;19:1449–70. doi: 10.1089/scd.2010.0140. [DOI] [PubMed] [Google Scholar]
- 147.Bermingham NA, Hassan BA, Price SD, et al. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–41. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- 148.Chen P, Johnson JE, Zoghbi HY, Segil N. The role of Math1 in inner ear development: uncoupling the establishment of the sensory primordium from hair cell fate determination. Development. 2002;129:2495–505. doi: 10.1242/dev.129.10.2495. [DOI] [PubMed] [Google Scholar]
- 149.Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3:580–86. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- 150.Kawamoto K, Ishimoto S, Minoda R, et al. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci. 2003;23:4395–400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shou J, Zheng JL, Gao WQ. Robust generation of new hair cells in the mature mammalian inner ear by adenoviral expression of Hath1. Mol Cell Neurosci. 2003;23:169–79. doi: 10.1016/s1044-7431(03)00066-6. [DOI] [PubMed] [Google Scholar]
- 152.Izumikawa M, Minoda R, Kawamoto K, et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11:271–76. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- 153.Mukherjea D, Jajoo S, Whitworth, et al. Short interfering RNA against transient receptor potential vanilloid 1 attenuates cisplatin-induced hearing loss in the rat. J Neurosci. 2008;28:13056–65. doi: 10.1523/JNEUROSCI.1307-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mukherjea D, Jajoo S, Kaur T, et al. Transtympanic administration of short interfering siRNA for the NOX3 isoform of NADPH oxidase protects against cisplatin-induced hearing loss in the rat. Antioxid Redox Signal. 2010;13:589–98. doi: 10.1089/ars.2010.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pfannenstiel SC, Praetorius M, Plinkert PK, et al. Bcl-2 gene therapy prevents aminoglycoside-induced degeneration of auditory and vestibular hair cells. Audiol Neurootol. 2009;14:254–66. doi: 10.1159/000192953. [DOI] [PubMed] [Google Scholar]
- 156.Yagi M, Magal E, Sheng Z, et al. Hair cell protection from aminoglycoside ototoxicity by adenovirus-mediated overexpression of glial cell line-derived neurotrophic factor. Hum Gene Ther. 1999;10:813–23. doi: 10.1089/10430349950018562. [DOI] [PubMed] [Google Scholar]
- 157.Kawamato K, Yagi M, Stover T, et al. Hearing and hair cells are protected by adenoviral gene therapy with TGF-beta1 and GDNF. Mol Ther. 2003;7:484–92. doi: 10.1016/s1525-0016(03)00058-3. [DOI] [PubMed] [Google Scholar]
- 158.Cooper LB, Chan DK, Roediger FC, et al. AAV-mediated delivery of the caspase inhibitor XIAP protects against cisplatin ototoxicity. Otol Neurotol. 2006;27:484–90. doi: 10.1097/01.mao.0000202647.19355.6a. [DOI] [PubMed] [Google Scholar]
- 159.Liu Y, Okada T, Simzaki K, et al. Protection against aminoglycoside-induced ototoxicity by regulated AAV vector-mediated GDNF gene transfer into the cochlea. Mol Ther. 2008;16:474–80. doi: 10.1038/sj.mt.6300379. [DOI] [PubMed] [Google Scholar]
- 160.Ahmad S, Tang W, Chang Q, et al. Restoration of connexin 26 protein level in the cochlea completely rescues hearing in a mouse model of human connexin30-linked deafness. Proc Natl Acad Sci USA. 2007;104:1337–41. doi: 10.1073/pnas.0606855104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Akil O, Seal RP, Burke K, et al. Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene therapy. Neuron. 2012;75:283–93. doi: 10.1016/j.neuron.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Maeda Y, Fukushima K, Nishizaki K, Smith RJ. In vitro and in vivo suppression of GJB2 expression by RNA interference. Hum Mol Genet. 2005;14:1641–50. doi: 10.1093/hmg/ddi172. [DOI] [PubMed] [Google Scholar]
- 163.Raper SE, Chirmule N, Lee FS, et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80:148–58. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 164.Thomas CE, Erhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4:148–58. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 165.Chen G, Zhang X, Yang F, Mu L. Disposition of nanoparticle-based delivery system via inner ear administration. Curr Drug Metab. 2010;11:886–97. doi: 10.2174/138920010794479673. [DOI] [PubMed] [Google Scholar]
- 166.Wareing M, Mhatre AN, Pettis R, et al. Cationic liposome mediated transgene expression in the guinea pig cochlea. Hear Res. 1999;128:61–69. doi: 10.1016/s0378-5955(98)00196-8. [DOI] [PubMed] [Google Scholar]
- 167.Buckiova D, Ranjan S, Newman TA, et al. Minimally invasive drug delivery to the cochlea through application of nanoparticles to the round window membrane. Nanomedicine (Lond) 2012;7:1339–54. doi: 10.2217/nnm.12.5. [DOI] [PubMed] [Google Scholar]
- 168.Ge X, Jackson RL, Liu J, et al. Distribution of PLGA nanoparticles in chinchilla cochleae. Otolaryngol Head Neck Surg. 2007;137:619–23. doi: 10.1016/j.otohns.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 169.Tamura T, Kita T, Nakagawa T, et al. Drug delivery to the cochlea using PLGA nanoparticles. Laryngoscope. 2005;115:2000–5. doi: 10.1097/01.mlg.0000180174.81036.5a. [DOI] [PubMed] [Google Scholar]
- 170.Goycoolea MV. Clinical aspects of round window membrane permeability under normal and pathological conditions. Acta Otolaryngol. 2001;121:437–47. doi: 10.1080/000164801300366552. [DOI] [PubMed] [Google Scholar]
- 171.Kabanov AV, Vinogradov SV. Nanogels as pharmaceutical carriers: finite networks of infinite capabilities. Angew Chem Int Ed Engl. 2009;48:5418–29. doi: 10.1002/anie.200900441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kim A, Pykko I. Size matters: versatile use of PiggyBac transposons as a genetic manipulation tool. Mol Cell Biochem. 2011;354:301–9. doi: 10.1007/s11010-011-0832-3. [DOI] [PubMed] [Google Scholar]