Abstract
Background and aim: Νon-motor symptoms in Parkinson’s disease (PD) are very common and contribute to the severity of patient’s disability. We evaluated the frequency of nonmotor symptoms in patients with PD and we explored the influence of disease characteristics on the presence of these symptoms.
Patients and methods: One hundred sixty six patients and sixty six matched controls were enrolled in the study. The Non-Motor Symptoms Questionnaire (NMSQuest), a 30-item self-completed questionnaire, was used for the evaluation of nonmotor symptoms.
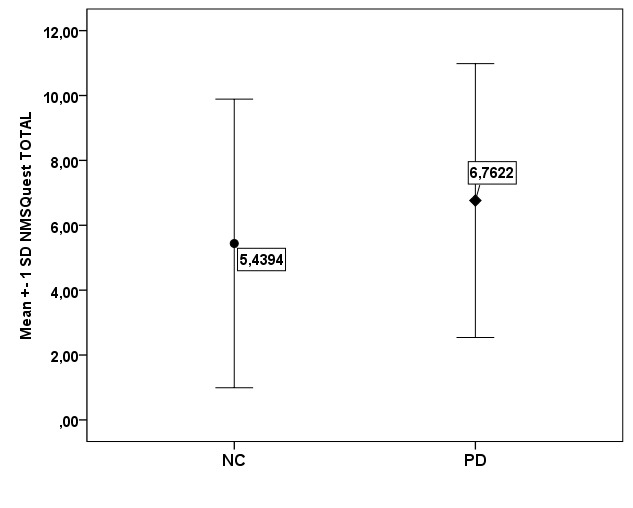
Results: Non-motor symptoms were more common in PD patients than controls. Mean ± SD NMSQuest score was 6.76 ± 4.22 in PD patients and 5.44 ± 4.45 in controls (p=0.035). The more common non-motor symptoms in PD patients were urinary urgency (54.3%), nocturia (51.8%), constipation (45.7%) and sadness (42.1%). There was a correlation between NMSQuest score and severity of the disease.
Conclusion: Non-motor symptoms in PD are too important to remain undetected. By incorporating into every day practice the use of suitable, reliable questionnaires, we will be able to facilitate detection and management of these symptoms.
Keywords: Parkinson disease, non-motor symptoms, NMS Questionnaire
Introduction
Parkinson’s disease (PD) is a common neurodegenerative disorder affecting about 1% of the population over the age of 65 years. PD is characterized by motor symptomatology including tremor at rest, rigidity, bradykinesia, postural instability and freezing episodes. However the clinical spectrum of PD is more extensive covering a wide range of nonmotor symptoms including neuropsychiatric manifestations ( cognitive impairment, depression, anxiety, psychosis, apathy, compulsive disorders ), sleep disorders, autonomic dysfunction (constipation, urinary and sexual dysfunction ), sensory symptoms and fatigue1,2.
Nonmotor symptoms are very common in PD, occurring throughout the course of the disease, from the early stages to the advanced disease1,2. Furthermore, some of them might precede the expression of the motor symptomatology1,2. Non-motor symptoms remain under-recognized since neurologists focus primarily on the motor aspects of the disease and the patients do not report them, because they do not realize that non-motor symptoms are connected with PD. Shulman et al3 have reported that the non-motor symptoms are not identified by neurologists in over 50% of consultations. Furthermore, non-motor symptoms contribute to the severity of disability, to institutionalization and to impaired quality of life. In order to enhance the recognition of the non-motor symptoms in PD two instruments have been developed: 1) the Non-Motor Symptoms Questionnaire (NMSQuest)4 that detects the presence of non-motor symptoms and 2) the Non-Motor Symptoms Scale (NMSS)5 for the evaluation of severity and frequency of various domains of non-motor symptoms.
The purpose of our study was the evaluation of occurrence of non-motor symptoms in Greek patients with Parkinson disease and the assessment of the influence of different disease characteristics on the presence of nonmotor symptoms.
Patients and Methods
One hundred sixty six consecutive PD patientsattending the outpatient clinic for PD of the 3rd University Department of Neurology participated in the study. Their diagnosis of the illness was based on the U.K. Parkinson’s Disease Society Brain Bank, clinical diagnostic criteria6. Exclusion criteria were: dementia, concomitant severe illness (stroke, injury e.t.c.), sensorial deficit (blindness), pharmacological effect (dopamine antagonists e.t.c.) and inability to reply questionnaires.
Sixty six matched healthy subjects served as the control population (NC). Informed consent was obtained from both patients and controls.
PD patients completed a demographic questionnaire including age, gender, education and pertinent information about the disease (age at onset, duration of disease, type of treatment). Clinical assessment was made using the Hoehn and Yahr stage classification system7.
The NMSQuest4 was used for the evaluation of nonmotor symptoms. This is a 30-item self-completed questionnaire for comprehensive indication of presence or absence of non-motor symptoms in PD. The items of the NMSQuest are grouped to nine domains: gastrointestinal (8 items), urinary tract (2 items), sexual function (2 items), cardiovascular (2 items), apathy/attention/memory (3 items), hallucinations/delusions (2 items), depression/anxiety/anhedonia (2 items), sleep/fatigue (5 items), pain (1 item) and miscellaneous (3 items). For each item there is a yes or no answer.
The NMSQuest was translated into Greek by two independent native Greek doctors with excellent knowledge of English. The Greek version of the NMSQuest, obtained by consensus, was back translated into English by an English native doctor without access to the original English version of the questionnaire. The back-translation was compared with the original version and modifications were made to eliminate all discrepancies between the original and the back-translated version.
PD patients and healthy controls completed the questionnaire. Furthermore, 54 stable patients completed the questionnaire for a second time, approximately 15 days following the first evaluation, for test-retest reliability evaluation.
Data analysis
Initially, a reliability analysis of the NMSQuest scale was performed using the calculation of Cronbach’s alpha and item to total coefficients. Test - retest NMSQuest total scores concordance was evaluated by means of the intraclass correlation coefficient. Comparison between PD patients and NC mean NMSQuest scores was performed with the t-test for two independent samples, since Levene’s test for homogeneity of variances, demonstrated that variances between the two groups were equal. The same principle applied to all other bivariate comparisons; otherwise the Man-Whitney U test was used. Comparisons between Yes/No response frequencies of the two study groups in every NMSQuest item was performed by means of the Pearson Chi-Square (χ2).The same test was used for comparisons of every other frequency differences (e.g. sex distribution). Exploration of differences between mean NMSQuest scores of PD patients, in relation to stage of the disease, was performed by means of the Kruskal-Wallis H test. Correlation between total NMSQuest score and disease duration was calculated by means of the Pearson’s r correlation coefficient. Partial correlation coefficients were used for correlations between NMSQuest total score and levodopa dose as well levodopa treatment duration, after controlling for duration of the disease. Statistical analysis was performed by means of the IBM SPSS statistics package, version 19.0 (SPSS Inc., Chicago, IL).
Results
A. Clinical and demographic data
In the PD patients group there were 109 men (66.5%) and 55 women (33.5%), while in the NC group there were 35 men (53%), and 31women (47%). The difference in sex proportions between groups was not significant (p=0.057). PD patients and controls were matched for age. The mean age for the PD group was 59.5 ± 9.3 years and for the controls it was 56.4 ± 8.8 years (p=0.085). The mean level of education was 9.1 years for PD patients and 9.8 years for NCs (p=0.09).
PD patients had a mean duration of the disease of 7.09 ± 5.31 years. Duration of the disease was 7.27 ± 5.58 years in men and 6.73 ± 4.78 years in women (p=0.553). Distribution according to stage of the disease (Hoehn and Yahr classification)7 was as follows: In stage 1, there were 8.3%; in stage 2, 58.3%; in stage 3, 23.7% and in stage 4, 9.6% of PD patients. Distribution of stage frequencies in relation to gender (percentage of men versus women) was as follows: In stage 1 there were 6.8% vs 11.3 %; in stage 2, 55.3% vs 64.1%, in stage 3, 26.2% vs 18.9% and in stage 4, 11.7% vs 5.7% (p=0.332).
Thirteen patients were untreated and the rest were receiving levodopa or/and a dopaminergic agonist. The mean daily levodopa dose was 506.19 ± 250.2 mg and the mean duration of treatment 5.7 ± 4.66 years.
B. NMSQuest data
All subjects completed the NMSQuest successfully.Reliability analysis of NMSQuest items revealed a satisfactory Cronbach’s alpha of 0.778. Half of NMSQuest items showed an optimal item to total correlation coefficient, only item No 6 (fecal incontinence) and No 30 (delusions) showed a very weak correlation and the rest a moderate one. Intraclass correlation coefficient of the test /retest total NMSQuest score was 0.74 (p=0.000), slightly above standard level. Therefore, scale reliability reached acceptable levels9.
PD patients obtained a mean NMSQuest total score of 6.76 ± 4.22 (95% confidence interval of the mean ranged from 6.11 to 7.4). The median value was 6 and the interquartile range was 6. Only 3% reported absence of nonmotor symptoms. The NC group yielded a total NMSQuest score of 5.44 ± 4.45 (95% confidence interval of the mean ranged from 4.34 to 6.53). Their median value was 5 and the interquartile range was 6. Absence of nonmotor symptoms was reported by 12.1% of NC. Figure 1 presents the difference of mean NMSQuest total scores between PD patients and NC which reached statistical significance (t = -2.117, p=0.035). There was no difference in the performance in the NMSQuest between men and women in both groups. Mean total NMS score for PD men was 6.71 ± 4.24 and 6.46 ± 3.74 for PD women (p=0.714), while mean total NMS score for NC men was 5.12 ± 4.13 and 5.71 ± 3.64 for NC women (p=0.08).
Figure 1. Mean values of total Non-Motor Symptoms Questionnaire (NMSQuest) score of Parkinson’s disease patients and controls (p=0.035).
Comparisons of the differences regarding the frequencies of positive answers, more specifically the presence of each one of the nonmotor symptoms, in PD and controls are shown in Table 1. Nine symptoms were found significantly different with drooling, taste / smellloss, constipation and urination related problems yielded the lowest p values.
Table 1. Percentage (%) of positive answers in all Non-Motor Symptoms Questionnaire (NMSQuest) items of Parkinson’s disease patients and Normal controls.
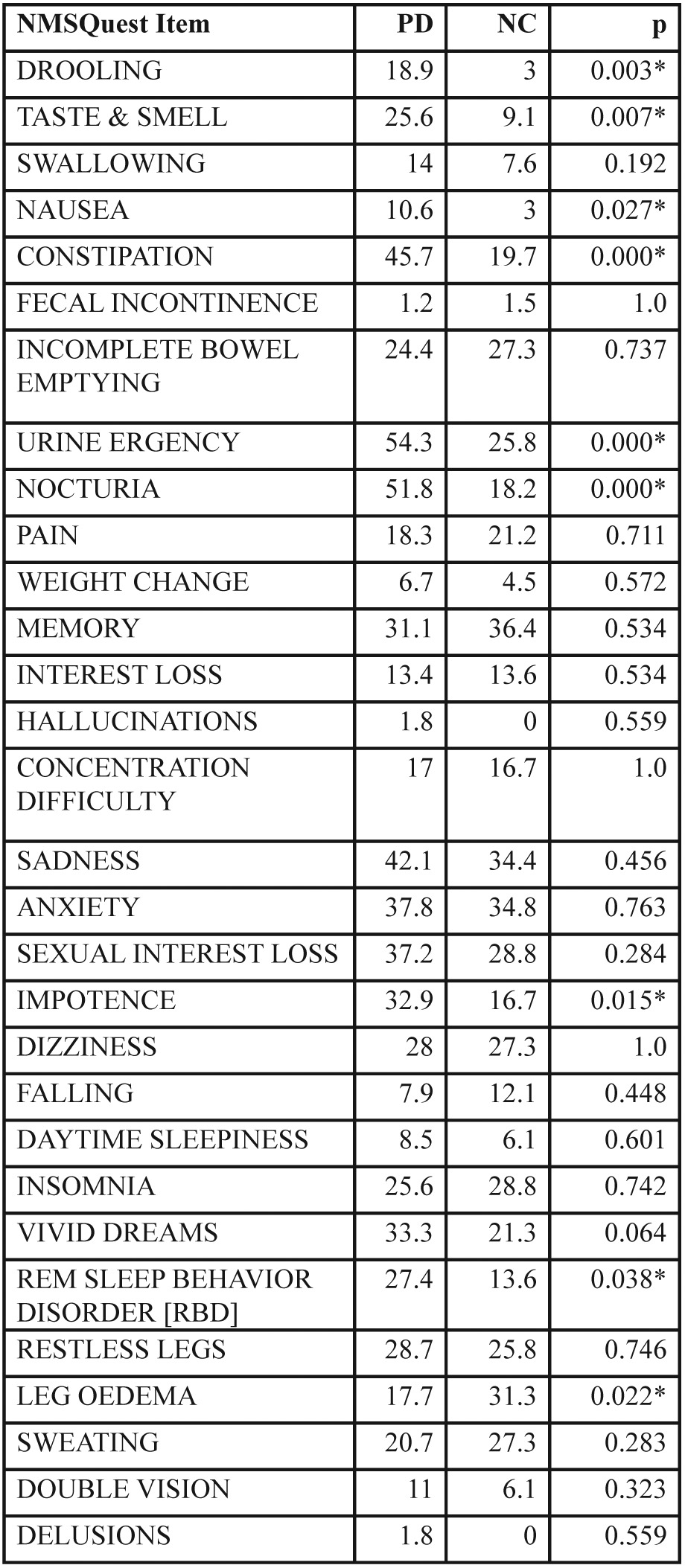
NMSQuest: Non-Motor Symptoms Questionnaire. PD: Parkinson’s disease patients. NC: Normal controls. *: significant difference
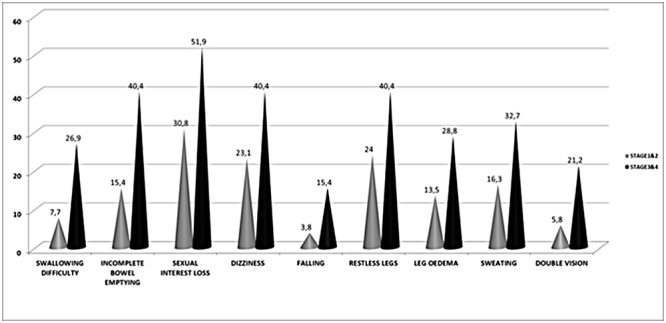
The impact of disease severity on NMSQuest total scale is presented in Figure 2.There is a gradual significant increase of mean NMSQuest score as the disease progresses (p=0.001). For a detailed exploration of differences between patients in relation to disease severity, the PD patient population was divided in two subgroups according to stage .One group comprised of patients with mild form of the disease (stage 1 & 2; 66.7%) and the other of patients with a moderate to severe disability (stage 3 & 4; 33.3%). In Table 2 differences between the two groups regarding the positive answers in all items of the NMSQuest are presented. Significant difference in relation to frequency of non-motor symptom appearance was observed in the following symptoms between the two groups: swallowing difficulties, incomplete bowel emptying, sexual interest loss, dizziness, falling, restless legs, leg oedema, sweating and double vision. Figure 3 highlights the items with significantly high proportion of positive answers in relation to stage of the disease.
Figure 2. Non-Motor Symptoms Questionnaire (NMSQuest) mean total score of Parkinson’s disease patients showing significant increase as disease progresses (p=0.001).
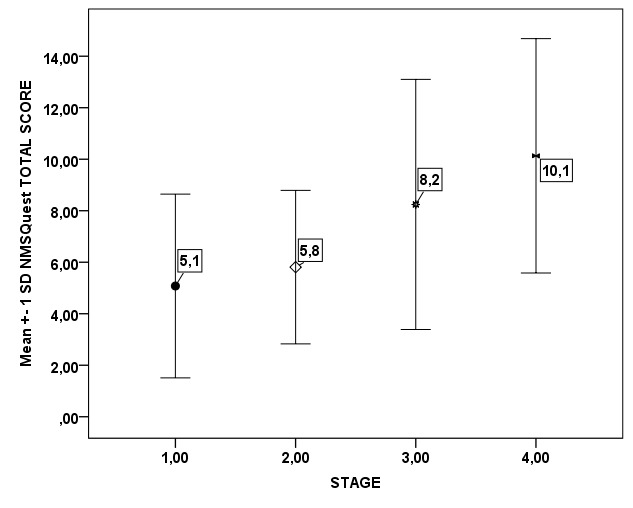
Table 2. Absolute resistivity (AbsR) and normalized relative impedance change (Z) values obtained before (A) and after (B and C) surfactant administration.
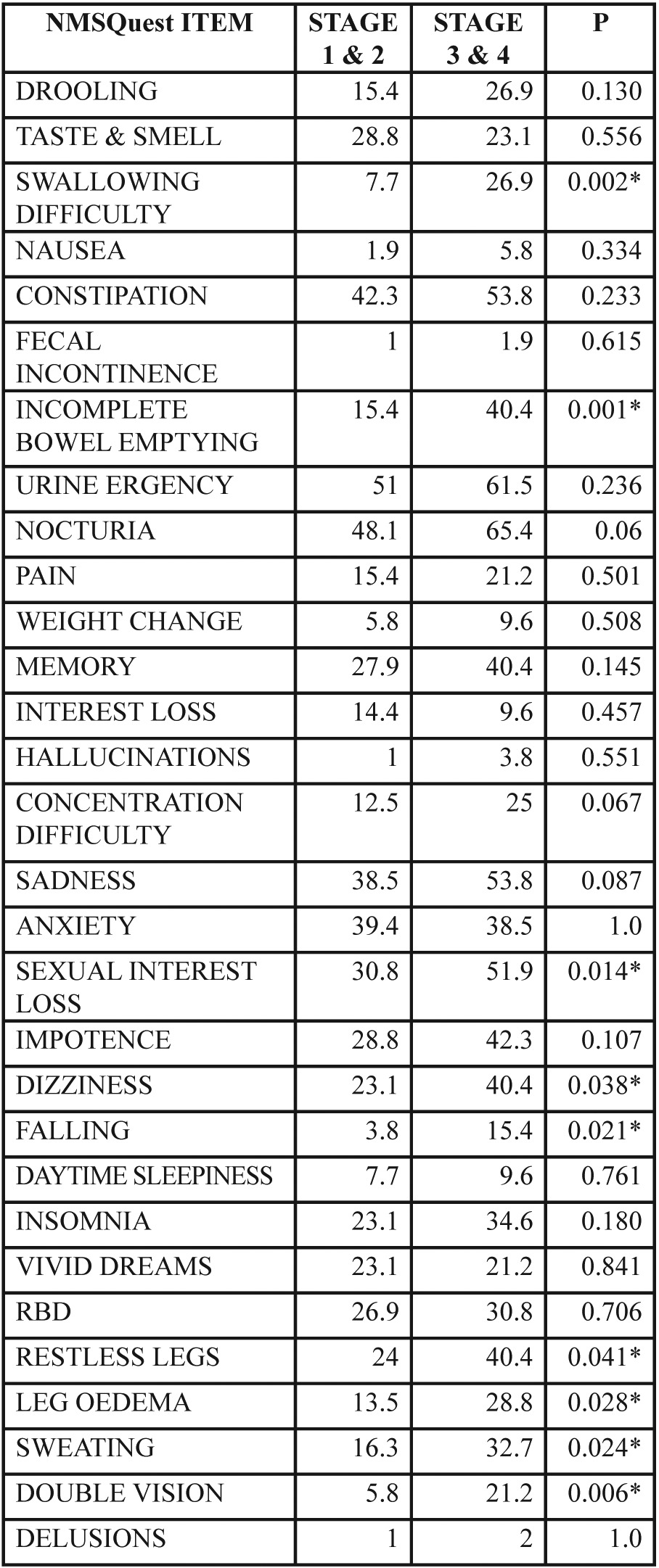
NMSQuest: Non-Motor Symptoms Questionnaire, *: significant difference
Figure 3. Selected Non-Motor Symptoms Questionnaire (NMSQuest) items showing significant difference in relation to disease severity in Parkinson’s disease patients.
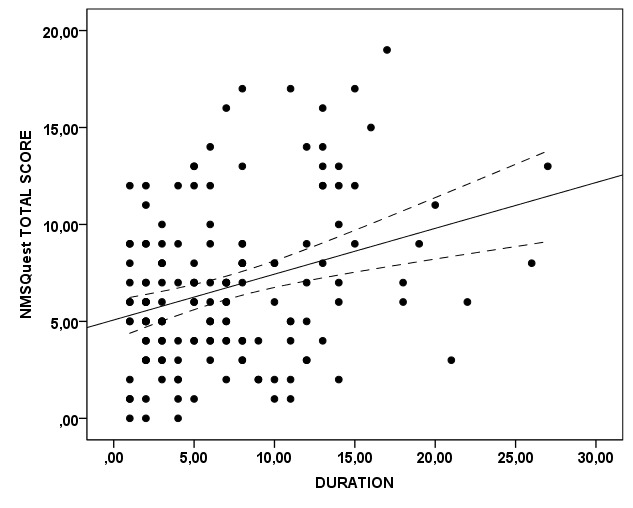
Correlation between NMSQuest total score and duration of the disease was significant (r=0.282; p=0.000). This relationship is graphically depicted in Figure 4. Correlations between parameters of levodopa treatment, such as dose or duration of treatment and NMSQuest total score, after controlling for disease duration, were not significant.
Figure 4. Scatter plot depicting the correlation between Non-Motor Symptoms Questionnaire (NMSQuest) total score and duration of the disease (years) in Parkinson’s disease patients (p=0.000).
Discussion
Non-motor symptoms are an important aspect of PD but remain under-recognized and often undeclared. This under-recognition of the non-motor symptoms may reflect the tendency of neurologists, patients and families to focus on the motor symptoms and to ignore the non-motor symptoms. Shulman et al3found that the diagnostic accuracy for the caring neurologist was low for depression, anxiety, fatigue and sleep disturbance. Chaudhuri et al10reported that the frequency of the undeclared nonmotor symptoms range from 33.2% (diplopia) to 65.2% (delusions). The most frequently undeclared symptoms were delusions, daytime sleepiness, vivid dreams and dizziness. Finally, according to Gallagher et al11 many symptoms that PD patients possibly perceive as embarrassing or unrelated to the disease remain under-reported.Cheon et al12 examined the patient awareness of non-motor symptoms associated with PD. The average number of symptoms that patients knew to be associated with the disease was 5.2 ± 6.8. This low patient awareness may account for the low detection rates of NMS. The least known non-motor symptoms that the patients were unaware to be related to PD were delusions, bowel incontinence and parasomnias.
In our study the median NMSQuest total score was 6 and the mean ± SD was 6.76 ± 4.22. Three complete prevalence studies of non-motor symptoms have been reported in the literature4,13,14. In the NMSQuest validation study4the median NMSQuest total score was 9,while the mean ± SD NMSQuest total score in a large international multicenter study13was 10.3 ± 5.4, ranging from 9.28 ± 4.3 in Italy to 12.71 ± 5.7 in Israel. In the PRIAMO study the mean (SD) number of non-motor symptoms per patient was 7.8 (4.9). Compared with the aforementioned studies our patients’ MNSQuest score was lower. This finding of our study can be explained by differences in the study populations, since in our study the majority of our PD patients wasat comparatively milder stages of the disease compared with the patients of the aforementioned studies.
The most common non-motor symptoms reported in our study were urinary urgency (54.3%), nocturia (51.8%), constipation (45.7%) and sadness (42.1%).These results are consistent with an International study13 where nocturia (61.9%), urinary urgency (55.81%), constipation (52.48%) and sadness(50.10%) were the most frequently reported. However, in the large Italian study14, the most frequent non-motors symptoms were fatigue, anxiety, leg pain and insomnia. In the study of Cheon et al12the most frequent non-motor symptom was nocturia followed by restless legs, constipation, sadness, orthostatic dizziness and memory disturbances. Similarly nocturia, urinary urgency, memory difficulties and restless legs are the most frequent non-motor symptoms reported by Gallagher et al11. Based on Hwynn et al15data, the frequency of non-motor symptoms in patients with advanced Parkinson disease varied with instrument used. That is, in the NMSQuest the most frequent non-motor symptoms were gastrointestinal, sleep-related and urinary while in the NMSS the most frequent ones were sleep-related, gastrointestinal and mood-related.
Nine symptoms were found significantly different between our patients and controls, but among them drooling, taste / smell loss, constipation and urination related problems yielded the lowest p values. Krishnan et al16comparing non-motor symptoms in PD and age-matched controls reported that these symptoms were more frequent in PD patients and cardiovascular, mood/cognition and perceptual/hallucinations problems were more related to PD than aging.
We found that the total NMSQuest score significantly increased with disease severity and duration meaning that the number of individual non-motor symptoms reported by our patients increases as the disease progresses. This correlation with disease severity was reported also in the NMSQuest validation study4 and in a large International study13where patients with disease duration of ≥ 15 years had the highest total NMSQuest score. Characteristically, Hely et al17 in the Sidney Multicenter Study of PD reported that non-motor symptoms dominate the clinical picture of patients who survive 15 years from the diagnosis. Especially cognitive decline, hallucinations, depression, symptomatic postural hypotension and urinary incontinence were the most frequent ones. The frequency and severity of the non-motor symptoms with the progression of the disease is consistent with the current neuropathological studies of motor and non-motor symptoms in PD according to the Braak’s hypothesis18. However, the neuropathological and neurochemical basis for many non-motor symptoms remain unknown. Many neurotransmitters and lesions in different anatomical regions have been implicated reflecting the multisystem nature of the disease. The pathological hallmarks of the disease are the loss of the dopaminergic neurons in the substantia nigra, causing dopamine depletion in the striatum, and the presence of Lewy bodies in the surviving neurons. However, a-synuclein deposition, neuronal loss and Lewy bodies are found not only in substantia nigra but also in other brain regions (locus coeruleus, raphe nucleus, pedunculopontine nucleus, olfactory bulb, dorsal motor nucleus of the vagal nerve, nucleus of Meynert and cerebral cortex) and as well as in the parasympathetic and sympathetic post-ganglionic neurons19,20.
Non-motor symptoms in PD result in significant disability and worsen the health and quality of life of the patient and their family. Gallagher et al11 reported that nonmotor symptoms are important determinants of patients’ quality of life and autonomic dysfunction, psychiatric complications, pain, fatigue and sleep problems are major correlates of poor quality of life. In two large international studies scores from the NMS scale were associated with those from PDQ-8 and PDQ-395,21. In a more recent study, Martinez-Martin et al22evaluated the impact of non-motor symptoms on health-related quality of life of parkinsonian patients. They found that the non-motor symptoms, especially sleep, fatigue, mood and apathy, have great impact on the quality of life and also that non-motor symptom progression contributes to the decline of quality of life. These data confirm that the total burden of NMS is likely to be more important than the motor symptoms in determining the quality of life across all stages of PD2.
In conclusion, non-motor symptoms in PD are too important to remain undetected. By incorporating into every day practice the use of suitable, reliable questionnaires, we will be able to facilitate detection and management of these symptoms. Furthermore, since a considerable number of the non-motor symptoms involve other organ systems e.g. genito-urinary, circulatory or digestive, it is noticeable that their presence might act as an inviting point for other specialties to join forces with neurology for a more multidisciplinary approach in the treatment of PD patients.
Conflict of Interest
None.
Acknowledgement
The authors would like to thank Prof. Pablo Martinez-Martin for his cooperation in this project.
References
- 1.Chauhuri KR, Healy DG, Schapira AH. National Institute for Clinical Excellence. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 2006;5:235–245. doi: 10.1016/S1474-4422(06)70373-8. [DOI] [PubMed] [Google Scholar]
- 2.Chaudhuri KR, Odin P, Antonini A, Martinez-Martin P. Parkinson’s disease: The non-motor issues. Parkinsonism Relat Disord. 2011;17:717–723. doi: 10.1016/j.parkreldis.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 3.Shulman LM, Taback RL, Rabinstein AA, Weiner WJ. Non-recognition of depression and other non-motor symptoms in Parkinson’s disease. Parkinsonism Relat Disord. 2002;8:193–197. doi: 10.1016/s1353-8020(01)00015-3. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhuri KR, Martinez-Martin P, Schapira AH, Stocchi F, Sethi K, Odin P, et al. International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson’s disease: the NMSQuest study. Mov Disord. 2006;21:916–923. doi: 10.1002/mds.20844. [DOI] [PubMed] [Google Scholar]
- 5.Chaudhuri KR, Martinez-Martin P, Brown RG, Sethi K, Stocchi F, Odin P, et al. The metric properties of a novel non-motor symptoms scale for Parkinson’s disease: Results from an international pilot study. Mov Disord. 2007;22:1901–1911. doi: 10.1002/mds.21596. [DOI] [PubMed] [Google Scholar]
- 6.Gibb WR, Lees AJ. The relevance of the Lewy body to the patrhogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1988;51:745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 8.Bland JM, Altman DG. Cronbach’s alpha. BMJ. 1997;314:572. doi: 10.1136/bmj.314.7080.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41:582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 10.Chaudhuri KR, Prieto-Jurcynska C, Naidu Y, Mitra T, Frades-Payo B, Tluk S, et al. The nondeclaration of nonmotor symptoms of Parkinson’s disease to health care professionals: an international study using the nonmotor symptoms questionnaire. Mov Disord. 2010;25:704–709. doi: 10.1002/mds.22868. [DOI] [PubMed] [Google Scholar]
- 11.Gallagher DA, Lees AJ, Schrag A. What are the most important nonmotor symptoms in patients with Parkinson’s disease and are we missing them? Mov Disord. 2010;25:2493–2500. doi: 10.1002/mds.23394. [DOI] [PubMed] [Google Scholar]
- 12.Cheon SM, Ha MS, Park MJ, Kim JW. Nonmotor symptoms of Parkinson’s disease: prevalence and awareness of patients and families. Parkinsonism Relat Disord. 2008;14:286–290. doi: 10.1016/j.parkreldis.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Martinez-Martin P, Schapira AH, Stocchi F, Sethi K, Odin P, MacPhee G, et al. Prevalence of nonmotor symptoms in Parkinson’s disease in an international setting; study using nonmotor symptoms questionnaire in 545 patients. Mov Disord. 2007;22:1623–1629. doi: 10.1002/mds.21586. [DOI] [PubMed] [Google Scholar]
- 14.Barone P, Antonini A, Colosimo C, Marconi R, Morgante L, Avarello TP, et al. The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord. 2009;24:1641–1649. doi: 10.1002/mds.22643. [DOI] [PubMed] [Google Scholar]
- 15.Hwynn N, Haq IU, Malaty IA, Resnick AS, Okun MS, Carew DS, et al. The frequency of nonmotor symptoms among advanced Parkinson patients may depend on instrument used for assessment. Parkinsons Dis. 2011;2011: 290195 doi: 10.4061/2011/290195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krishnan S, Sarma G, Sarma S, Kishore A. Do nonmotor symptoms in Parkinson’s disease differ from normal aging? Mov Disord. 2011;26:2110–2113. doi: 10.1002/mds.23826. [DOI] [PubMed] [Google Scholar]
- 17.Hely MA, Morris JG, Reid WG, Trafficante R. Sydney multicenter study of Parkinson’s disease: non-L-dopa-responsive problems dominate at 15 years. Mov Disord. 2005;20:190–199. doi: 10.1002/mds.20324. [DOI] [PubMed] [Google Scholar]
- 18.Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 19.Lim SY, Fox SH, Lang AE. Overview of the extranigral aspects of Parkinson disease. Arch Neurol. 2009;66:167–172. doi: 10.1001/archneurol.2008.561. [DOI] [PubMed] [Google Scholar]
- 20.Jellinger KA. Synuclein deposition and non-motor symptoms in Parkinson disease. J Neurol Sci. 2011;310:107–111. doi: 10.1016/j.jns.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 21.Martinez-Martin P, Rodriguez-Blazquez C, Abe K, Bhattacharyya K, Bloem BR, Carod-Artal FJ, et al. International study on the psychometric attributes of the non-motor symptoms scale in Parkinson disease. Neurology. 2009;73:1584–1591. doi: 10.1212/WNL.0b013e3181c0d416. [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Martin P, Rodriguez-Blazquez C, Kurtis MM, Chaudhuri KR. NMSS Validation Group. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov Disord. 2011;26:399–406. doi: 10.1002/mds.23462. [DOI] [PubMed] [Google Scholar]


