Abstract
Previous linkage studies in Mexican-Americans localized a major susceptibility locus for type 2 diabetes, NIDDM1, to chromosome 2q. This evidence for linkage to type 2 diabetes was recently found to be associated with a common G→A polymorphism (UCSNP-43) within the CAPN10 gene. The at-risk genotype was homozygous for the UCSNP-43 G allele. In the present study among Pima Indians, the UCSNP-43 G/G genotype was not associated with an increased prevalence of type 2 diabetes. However, Pima Indians with normal glucose tolerance, who have a G/G genotype at UCSNP-43, were found to have decreased rates of postabsorptive and insulin-stimulated glucose turnover that appear to result from decreased rates of glucose oxidation. In addition, G/G homozygotes were found to have reduced CAPN10 mRNA expression in their skeletal muscle. A decreased rate of insulin-mediated glucose turnover, or insulin resistance, is one mechanism by which the polymorphism in CAPN10 may increase susceptibility to type 2 diabetes mellitus in older persons.
Introduction
Type 2 diabetes mellitus is a metabolically complex disease with major genetic determinants (1). Linkage studies in Mexican-American affected sib pairs living in Starr County, Texas, localized a major susceptibility locus for type 2 diabetes, NIDDM1, to chromosome band 2q37.3 (2). The CAPN10 gene, which encodes calpain-10, a nonlysosomal cysteine protease expressed in many tissues, including skeletal muscle, liver, and pancreas, was positionally cloned within the NIDDM1 region (3). UCSNP-43, a common G→A transition within intron 3 of the CAPN10 gene, was associated with the evidence for linkage in the NIDDM1 region in Mexican-American sib pairs concordant for the at-risk genotype (G/G)(3).
The Pima Indians of Arizona have the world’s highest reported incidence and prevalence of type 2 diabetes mellitus (4). Their diabetes is prototypic of this disease and is characterized by obesity, insulin resistance, insulin secretory dysfunction, and increased rates of endogenous glucose production (5). In prospective studies of prediabetic Pima Indians, insulin resistance and insulin secretory dysfunction are major predictors of the disease, whereas increased rates of endogenous glucose production are not (6). Insulin resistance results, in part, from obesity, but some determinants of impaired insulin action are independent of fatness (7). These determinants aggregate in families (8), indicative of genetic effects. Variation in insulin secretion, independent of body fatness and insulin action, also aggregates in families (9).
In this study we investigated whether these familial prediabetic metabolic abnormalities are associated with, and therefore may result from, variation at UCSNP-43. Results of clinical studies on full-heritage Pima Indians, or closely related Tohono O’Odham Indians, who have been part of our ongoing longitudinal and prospective studies of the etiology of type 2 diabetes mellitus, were analyzed. Tests included measures of body composition, a 75-g oral glucose-tolerance test, an intravenous glucose-tolerance test to determine the acute insulin response as a measure of insulin secretory function, and measures of postabsorptive glucose appearance and insulin-stimulated glucose-disappearance rates using the euglycemic insulin clamp technique with simultaneous indirect calorimetry to estimate insulin action on total glucose disposal and oxidation. Most individuals were also studied in a respiratory chamber for 24 hours to measure sleeping and 24-hour rates of energy expenditure, as well as substrate oxidation rates, while eating a weight-maintaining diet. Only data from people with normal glucose tolerance (10) were analyzed since deterioration to impaired glucose tolerance or diabetes is associated with secondary changes in insulin secretory function and glucose metabolism due to increases in plasma glucose concentration (1) and therefore may not reflect primary or genetic determinants.
Methods
Subjects.
Volunteers were recruited from the Gila River Indian Community and written informed consent was obtained before participation. All studies were approved by the Tribal Council and the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases. For detailed metabolic testing, individuals were admitted to our clinical research ward for 7–10 days, and only persons found to be healthy by medical history, physical examination, and routine laboratory tests, and not taking medications, were studied. The association analysis of metabolic characteristics (Table 1) included data from 158 Pima Indians with normal glucose tolerance (2-hour glucose < 7.8 mM). If data on an individual was available from more than one yearly study, only the data from the first study was used in these analyses. The association analysis of type 2 diabetes (Table 2) included data on 720 Pima Indians selected randomly from participants in our ongoing longitudinal epidemiologic study (4). Individuals were classified into groups according to sex and six decades of age, and 60 individuals were selected for genotyping from each group. Diabetes was defined according to the criteria of the World Health Organization (10).
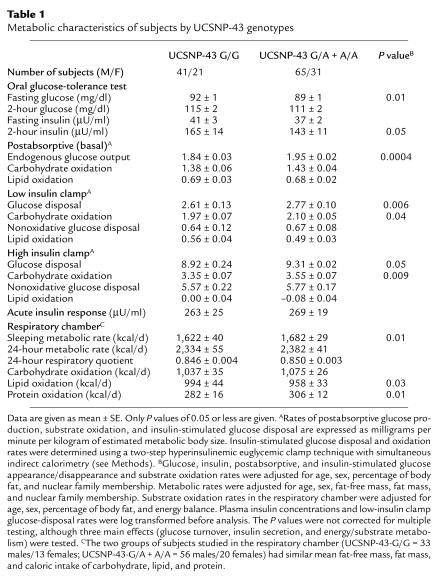
Table 1.
Metabolic characteristics of subjects by UCSNP-43 genotypes
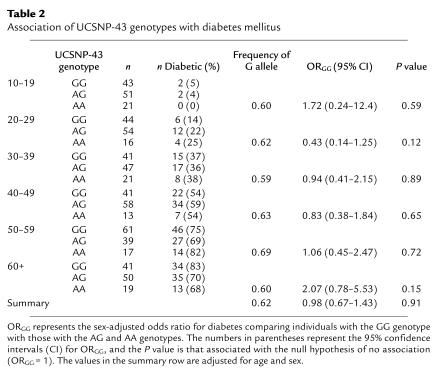
Table 2.
Association of UCSNP-43 genotypes with diabetes mellitus
Clinical tests.
Until January, 1996, body composition was estimated by underwater weighing and by dual-energy x-ray absorptiometry (DPX-1; Lunar Radiation Corp., Madison, Wisconsin, USA) thereafter. A conversion equation derived from comparative analyses was used to make estimates of body composition comparable between methods (11). Oral glucose tolerance was measured after 2–3 days on a weight-maintaining diet of mixed composition. Subjects ingested 75 g of glucose, and blood for plasma glucose and insulin concentrations was drawn before ingesting the glucose and at 30, 60, 120, and 180 minutes. Glucose tolerance was classified according to the criteria of the World Health Organization (10). Subjects also received a 25-g intravenous injection of glucose over 3 minutes to measure the acute insulin response. Blood samples were collected before infusion and at 3, 4, 5, 6, 8, and 10 minutes after infusion to determine plasma glucose and insulin concentrations. The acute insulin response was calculated as half the mean increment in plasma insulin concentrations from 3–5 minutes.
For the insulin clamp studies, both basal glucose appearance and insulin-stimulated glucose disappearance (uptake) rates were determined, as described in detail elsewhere (6, 8). Briefly, insulin was infused to achieve physiologic and maximally stimulating plasma insulin concentrations (137 ± 3 and 2,394 ± 68 μU/ml, respectively) for 100 minutes for each step. Plasma glucose concentrations were held constant at ∼100 mg/dl by a variable 20%-glucose infusion. Tritiated glucose was infused for 2 hours before the insulin infusion to calculate rates of postabsorptive glucose appearance rates and to calculate glucose disappearance rates during the lower dose of insulin infusion. Ventilated-hood indirect calorimetry was used to estimate rates of glucose and lipid oxidation before and during the insulin infusions (7). Glucose appearance, disappearance, oxidation, and lipid oxidation rates were normalized to estimated metabolic body size (fat-free mass + 17.7), as described (6–8).
The measurement of energy expenditure and substrate oxidation in the respiratory chamber has been described previously (11). Briefly, volunteers entered the chamber after an overnight fast and remained in the chamber for 23 hours. Subjects were fed calories to maintain energy balance according to previously determined equations, and the rate of energy expenditure was measured continuously, calculated for each 15-minute interval within the chamber, and then extrapolated to 24 hours (24-EE). The sleeping metabolic rate was calculated between 2300 hours and 0500 hours as the mean metabolic rate of all 15-minute periods during which spontaneous physical activity was detected by radar less than 1.5% of the time. Carbon dioxide production (VCO2) and oxygen consumption (VO2) were calculated for every 15-minute interval. The 24-hour respiratory quotient (24-RQ) was calculated as the ratio of 24-hour VCO2 and 24-hour VO2. Based upon 24-RQ, 24-EE, and 24-hour urinary nitrogen excretion, the 24-hour oxidation rates of fat, carbohydrate, and protein were determined (11).
Genotyping.
Genomic DNA extracted from peripheral blood lymphocytes was genotyped for UCSNP-43 (CAPN10-g.4852G/A) (3) by sequencing a PCR-amplified fragment. PCR primers were forward 5′-GCTGGCTGGTGACATCAGTG-3′ and reverse 5′-TCAGGTTCCATCTTTCTGCCAG-3′. PCR was performed with 60 ng of genomic DNA in a buffer containing 1.5 mM MgC12, 0.25 mM dNTPs, and 0.15 μl of AmpliTaq Gold (Applied Biosystems Inc., Foster City, California, USA) DNA polymerase. PCR conditions were 94°C for 10 minutes, followed by 33 cycles of 94°C for 30 seconds, 60° for 30 seconds, and 72° for 30 seconds, followed by a final extension at 72° for 10 minutes. DNA was sequenced with the primer 5′-AGCAGGGTTGGAGCTTGAGAG-3′. DNA-cycle sequencing was carried out using the Big Dye Terminator on an automated DNA sequencer (model 377; Applied Biosystems Inc.).
Preparation of total RNA from human skeletal muscle biopsies and real-time quantitative RT-PCR.
Percutaneous muscle biopsies were obtained from the quadriceps femoris muscle after local anesthesia of skin and fascia with 2% lidocaine. Biopsies were immediately frozen in liquid nitrogen, and RNA was extracted using ToTALLY RNA kit (Ambion, Austin, Texas, USA). The cDNA was synthesized using the Advantage RT-for-PCR kit (CLONTECH Laboratories, Palo Alto, California, USA). Quantitation of reverse-transcribed CAPN10 mRNA (and β-actin mRNA as an endogenous reference) was done as a real-time measurement in an ABI PRISM 7700 Sequence Detection System (Applied Biosystems Inc.), according to the manufacturer’s instructions. The sequences of the PCR primers and probe for CAPN10 detection were forward: 5′-CACCTACCTGCCGGACACA-3′, reverse: 5′-TGCCATGACGGAGACCTCTT-3′, and probe: 5′(Fam)-CAACCAGGATTGACAGGCCATCCATT-(Tamra)p-3′. This CAPN10 probe recognized seven of the eight isoforms of CAPN10 (excluding the g isoform). The sequences of PCR primers and probe for β-actin detection were forward: 5′-TCACCCACACTGTGCCCATCTACGA-3′, reverse: 5′-CAGCGGAACCGCTCATTGCCAATGG-3′, and probe: 5′(Vic)-ATGCCCCCCCCATGCCATCCTGCGT(Tamra)p-3′. For each probe and primer set, a standard curve was generated by serial dilution (done in triplicate) of cDNA from a skeletal muscle biopsy of a healthy Pima subject. All samples were run in duplicate. The data were calculated using the standard curve method and expressed as a ratio to the β-actin reference.
Statistical analyses.
Statistical analyses were performed using the statistical analysis system of the SAS Institute (Cary, North Carolina, USA). For continuous variables, the general estimating equation procedure (GEE) was used to adjust for covariates — age, sex, percentage of body fat, energy balance, fat-free mass, fat mass, and nuclear family membership, as indicated. Plasma insulin concentration and rates of glucose disappearance during the low-dose insulin infusion were log-transformed before analyses to approximate a normal distribution. The association of CAPN10 genotype with diabetes was assessed by analysis of contingency tables. The Mantel-Haenszel procedure was used to calculate the age- and sex-adjusted odds ratio (OR) comparing individuals with the UCSNP-43 G/G genotype with those with the A/G or the A/A genotype.
Results
UCSNP-43 has allelic frequencies of G = 0.62 and A = 0.38 in Pima Indians. Since the evidence for linkage in the NIDDM1 region in Mexican-Americans was attributable to the affected sib pairs homozygous for the high-frequency G allele (3), the data for Pima Indians were analyzed similarly. Individuals homozygous for the UCSNP-43 G allele (GG) were compared with individuals not homozygous for the UCSNP-43 G allele (i.e., A/A and G/A). The two groups had similar ages (mean ± SE = 26 ± 1, 27 ± 1 years) and percentage of body fat (mean ± SE = 31 ± 1 for both groups). Metabolic characteristics of the two groups were compared after adjusting for age, sex, percentage of body fat, and nuclear family membership, since many of the subjects were siblings (Table 1). People homozygous for the G allele had a higher mean fasting plasma glucose (P = 0.01). In contrast, the mean fasting rate of glucose appearance, or endogenous glucose production, was lower in the G/G group (P = 0.0004). The mean plasma insulin concentration 2 hours after ingesting 75 g glucose was higher in the G/G group (P = 0.05), indicative of insulin resistance. The results of the euglycemic clamp studies confirmed this with lower mean glucose-disposal rates in the G/G group during the low-dose (physiologic) insulin infusion (P = 0.006) and the high-dose (maximal) insulin infusion (P = 0.05). The lower rate of glucose disposal appeared to be the result of a lower glucose-oxidation rate, since differences in carbohydrate oxidation were more significant (P = 0.04 and P = 0.009 for the low- and high-dose insulin infusion, respectively) than differences in nonoxidative glucose disposal (P = 0.26 and P = 0.23 for the low- and high-dose insulin infusion, respectively).
Differences in substrate oxidation rates between the genotype groups were also observed in data from the respiratory chamber (Table 1). People homozygous for the UCSNP-43 G allele oxidized more lipid (P = 0.03) and less protein (P = 0.01) over 24 hours than people with the G/A or A/A allelic combinations (after adjusting for age, sex, percentage of body fat, and energy balance). The G/G group also oxidized less carbohydrate, but this difference (P = 0.81) did not reach statistical significance. The differences in substrate oxidation rates occurred despite all individuals having eaten a diet of similar energy and substrate content during the indirect calorimetric measurements. These results indicate significant differences in nutrient partitioning between the groups. In addition, the mean sleeping metabolic rate was lower in the G/G group (P = 0.01, after adjusting for age, sex, fat-free mass, fat mass, and nuclear family membership), most likely as a result of the lower rate of endogenous glucose production, which is an energy-costly process to the extent that it is due to gluconeogenesis. Note that whenever multiple phenotypes are analyzed, some may differ significantly by chance. The P values in this study were not adjusted for multiple testing, although three main effects were tested (glucose turnover, insulin secretion, and energy/substrate metabolism). To examine whether the effects of the UCSNP-43 G/G genotype on glucose turnover and nutrient partitioning might lead to an increased risk of type 2 diabetes, a random sample of 720 full-heritage Pima Indians was genotyped. Overall, there was no statistically significant association between the UCSNP-43 genotype and prevalence of diabetes (Table 2).
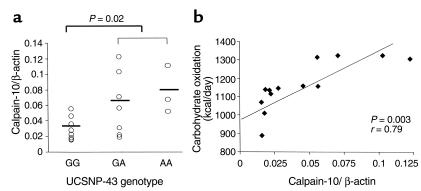
In vitro evidence suggests that the nucleotide sequence encompassing UCSNP-43 may be involved in regulating CAPN10 expression (3). Therefore, we investigated the in vivo expression of CAPN10 mRNA in skeletal muscle, a major target for insulin action, in 18 male nondiabetic Pima subjects. Individuals homozygous for the G allele had 53% lower mean CAPN10 transcript levels compared with heterozygotes, whereas A/A homozygotes had the highest mean levels (Figure 1a). As predicted from the larger data set, individuals with lower CAPN10 transcription (G/G genotype) had reduced basal and insulin-stimulated carbohydrate oxidation rates, but perhaps due to small sample size (n = 18), this association did not reach statistical significance. However, a significant correlation (r = 0.79; P = 0.003) was observed between the level of CAPN10 expression in skeletal muscle and the rate of carbohydrate oxidation measured in the respiratory chamber over 24 hours (Figure 1b).
Figure 1.
Association between CAPN10 mRNA expression and UCSNP-43 genotype and 24-hour carbohydrate oxidation rates. (a) CAPN10 mRNA expression, as measured by real-time PCR, in skeletal muscle of nondiabetic males representing G/G (n = 8), G/A (n = 7), and A/A (n = 3) UCSNP-43 genotypes. The levels of CAPN10 transcripts are normalized to the levels of endogenous β-actin. The mean transcript level was significantly lower in the G/G homozygotes when compared with the G/A and A/A groups (P = 0.02). (b) The relationship between CAPN10 transcript levels in skeletal muscle and 24-hour carbohydrate oxidation rates (r = 0.79). Carbohydrate oxidation rates are adjusted for age, percentage of body fat, and energy balance.
Discussion
The results from our metabolic tests show that Pima Indians with a UCSNP-43 G/G genotype have reduced rates of endogenous glucose production, which, in the presence of an increased fasting plasma glucose concentration, is indicative of a decreased rate of peripheral glucose disappearance. The lower rate of glucose disappearance in the presence of both physiologic and maximally stimulated plasma insulin concentrations apparently results from decreased glucose oxidation. When provided with exogenous substrate, including lipid and protein in addition to carbohydrate, G/G homozygotes preferentially oxidize lipids. No difference was observed between the groups in insulin secretory function as measured by the acute insulin response; however, since CAPN10 is expressed in pancreatic islets and insulinoma cell lines, a deficit in glucose oxidation would be predicted to affect insulin secretion since it depends on glucose metabolism. Therefore, additional studies of insulin secretory function using graded glucose infusions to measure insulin responses will be needed to more accurately assess the consequences of the UCSNP-43 genotype on insulin secretion.
The UCSNP-43 G/G phenotype in Pima Indians is strikingly similar to the phenotype proposed to aid in the survival of famines in early humans, originally described by Cahill (12) and recently revisited by Reaven (13). In times of famine, Cahill hypothesized, “…if tissues are better able to exclude glucose… then gluconeogenesis and, in turn, body protein should be spared...” (12). Preservation of body protein stores maintains the skeletal muscle mass essential for flight responses and hunting (12). We would extend Cahill’s proposed phenotype based on our present data. The tissues of UCSNP-43 G/G individuals “exclude” glucose oxidation, but not glucose storage. This preserves not only skeletal muscle protein, but also skeletal muscle glycogen, which is necessary to fuel high-energy muscle contraction for flight or hunting. A decreased rate of gluconeogenesis, evidenced by a decreased sleeping metabolic rate, further serves to reduce caloric requirements during food deprivation. Preferential oxidation of lipid, as is also observed among UCSNP-43 G/G individuals, enables an easier switch to ketone production to fuel the brain when carbohydrate supply is limited. However, in times of ample food supply, the UCSNP-43 G/G phenotype would favor positive carbohydrate balance, which may lead to expansion of glycogen stores and secondary insulin resistance, as we have reported previously in subjects overeating 1,000 kcal/day for only 1 week (14).
In conclusion, these studies indicate that people homozygous for the UCSNP-43 G allele have reduced muscle CAPN10 mRNA and insulin resistance, apparently due to lower rates of insulin-stimulated glucose oxidation. When eating a diet of mixed composition they preferentially oxidize fat. These phenotypic differences are indicative of altered nutrient partitioning. Postabsorptively, there is also a lower rate of glucose turnover and endogenous glucose production, resulting in a lower metabolic rate. The magnitude of the difference in the prevalence of diabetes in the older Pima Indians (60+ years) is consistent with what would be predicted by the small but significantly lower rate of glucose turnover among nondiabetic UCSNP-43 G/G homozygotes. The phenotypic effects of UCSNP-43 are detectable even though little evidence for linkage between type 2 diabetes (and prediabetic/preobese phenotypes) and markers from this region of chromosome 2 was observed in our genomic scan in Pima Indians (11, 15, 16). This discordance is not surprising, since a number of factors may affect the power to detect linkage differently than they affect the power to detect association and suggests that phenotypic effects of UCSNP-43 may be detected in other populations, as well. Indeed, in two European populations, as well as in Mexican-Americans, an increased risk of diabetes was observed in individuals with a specific CAPN10 haplotype, which included UCSNP-43 as well as two additional polymorphisms within this gene (3), although this haplotype did not increase the risk of diabetes in the Pima population. These combined molecular genetic and physiologic studies are the early stages of unraveling the complex, oligogenic basis of this common metabolic disease.
Acknowledgments
We thank the members of the Gila River Indian Community without whose continuous cooperation these studies would not be possible. We gratefully acknowledge the technical assistance of Christopher Wiedrich, Kim Cray, Michael Traurig, Jeff Sutherland, Bonnie Cooper, Pam Thuillez, Angie Dobberfuhl, Pam O’Connor, and Jeff Bunkelmann. We also thank Linda Phillips and other members of the Diabetes and Arthritis Epidemiology Section for obtaining DNA samples. Finally, we thank Michael Milner, Carol Massengill, and the nurses of the Clinical Research Ward, and Arline Salbe and the staff of the Metabolic Kitchen for the care of the research volunteers.
References
- 1.DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care. 1992;15:318–368. doi: 10.2337/diacare.15.3.318. [DOI] [PubMed] [Google Scholar]
- 2.Hanis CL, et al. A genome-wide search for human non-insulin-dependent (type 2) diabetes genes reveals a major susceptibility locus in chromosome 2. Nat Genet. 1996;13:161–166. doi: 10.1038/ng0696-161. [DOI] [PubMed] [Google Scholar]
- 3.Horikawa Y, et al. Genetic variation in the gene encoding calpain-10 is associated with type 2 diabetes mellitus. Nat Genet. 2000;26:163–175. doi: 10.1038/79876. [DOI] [PubMed] [Google Scholar]
- 4.Knowler WC, Bennett PH, Hamman RF, Miller M. Diabetes incidence and prevalence in Pima Indians: a 19-fold greater incidence than in Rochester, Minnesota. Am J Epidemiol. 1978;108:497–505. doi: 10.1093/oxfordjournals.aje.a112648. [DOI] [PubMed] [Google Scholar]
- 5.Bogardus, C. 1996. Metabolic abnormalities in the development of non-insulin dependent diabetes mellitus. In Diabetes mellitus: a fundamental and clinical text. D. LeRoith, S.I. Taylor, and J.M. Olefsky, editors. Lippincott-Raven. Philadelphia, Pennsylvania, USA. 459–467.
- 6.Lillioja S, et al. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N Engl J Med. 1993;329:1988–1992. doi: 10.1056/NEJM199312303292703. [DOI] [PubMed] [Google Scholar]
- 7.Lillioja S, Bogardus C. Obesity and insulin resistance: lessons learned from the Pima Indians. Diabetes Metab Rev. 1988;4:517–540. doi: 10.1002/dmr.5610040508. [DOI] [PubMed] [Google Scholar]
- 8.Lillioja S, et al. In vivo insulin action is a familial characteristic in non-diabetic Pima Indians. Diabetes. 1987;36:1329–1335. doi: 10.2337/diab.36.11.1329. [DOI] [PubMed] [Google Scholar]
- 9.Sakul H, Pratley R, Ravussin E, Mott D, Bogardus C. Familiality of physical and metabolic characteristics that predict the development of non-insulin dependent diabetes mellitus in Pima Indians. Am J Hum Genet. 1997;60:651–656. [PMC free article] [PubMed] [Google Scholar]
- 10.1985. Diabetes mellitus. World Health Organization. Geneva, Switzerland. Technical Report Series, No. 727.
- 11.Norman RA, et al. Autosomal genomic scan for loci linked to obesity and energy metabolism in Pima Indians. Am J Hum Genet. 1998;62:659–668. doi: 10.1086/301758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cahill GF, Owen OE. Starvation and survival. Trans Am Clin Climatol Assoc. 1967;79:13–20. [PMC free article] [PubMed] [Google Scholar]
- 13.Reaven GM. Hypothesis: muscle insulin resistance is the (“not-so”) thrifty genotype. Diabetologia. 1998;41:482–484. doi: 10.1007/s001250050933. [DOI] [PubMed] [Google Scholar]
- 14.Mott DM, Lillioja S, Bogardus C. Overnutrition induced decrease in insulin action for glucose storage: in vivo and in vitro in man. Metabolism. 1996;35:160–165. doi: 10.1016/0026-0495(86)90118-6. [DOI] [PubMed] [Google Scholar]
- 15.Hanson RL, et al. An autosomal genomic scan for loci linked to type II diabetes mellitus and body-mass index in Pima Indians. Am J Hum Genet. 1998;63:1130–1138. doi: 10.1086/302061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pratley RE, et al. An autosomal genomic scan for loci linked to pre-diabetic phenotypes in Pima Indians. J Clin Invest. 1998;101:1757–1764. doi: 10.1172/JCI1850. [DOI] [PMC free article] [PubMed] [Google Scholar]