Abstract
Background and Aim: Extracapillary proliferation (crescent) was not included in the Oxford classification, although previous attempts to correlate the crescent with clinical outcomes have produced conflicting results. In this study, we investigated the clinical and morphological significance of extracapillary proliferation in a group of IgA nephropathy (IgAN) patients with regard to the Oxford classification.
Patients and Methods: In an observational study conducted on IgAN patients, we collected a total of 114 biopsies. We diagnosed IgAN by light and immunofluorescence for all patients.
Results: Of the 114 patients, 70.2% were male. The mean age of the patients was 37.7 ± 13.6 years. The mean proteinuria was 1742 ± 1324 mg/day. The mean serum creatinine was 1.6 ± 1.5 mg/dL. Twenty-five (21.9%) patient kidney biopsies had extracapillary proliferation. We found a significant positive correlation between the number of crescents and serum creatinine (p<0.001). Furthermore, we found a positive association between the nephrotic syndrome and the total number of crescents (p<0.05). Additionally, we observed a significant positive correlation between the amount of sclerosed glomeruli and extracapillary proliferation (p=0.028).
Conclusion: Our findings confirm that extracapillary proliferation has a significant association with proteinuria and sclerotic glomeruli. We anticipate that extracapillary proliferation will be included in a revision of the Oxford classification of IgAN to widen the scope of the classification.
Keywords: IgA nephropathy, nephrotic syndrome, Oxford classification, endocapillary proliferation, crescent, end-stage renal disease, proteinuria
Introduction
The International IgA Nephropathy Network developed the Oxford classification, which identifies the four morphologic variables with the greatest prognostic importance (MEST score)1. These variables include mesangial hypercellularity (M), endocapillary hypercellularity (E), segmental glomerulosclerosis (S) and tubular atrophy/interstitial fibrosis (T). This classification provides a histopathologic grading system that is associated with kidney disease outcomes and is independent of clinical features; this classification demonstrates an improved capacity to predict the outcome of patients with IgA nephropathy (IgAN)1-3. All four variables of the Oxford classification have high reproducibility. However, low co-linearity and the lack of clinical factors cause the Oxford classification of IgAN to have some limitations4,5.
The IgAN is an immune-complex glomerulopathy that can result in extracapillary proliferation (crescent)5. Extracapillary proliferation was not included in the Oxford classification. However, previous attempts to correlate extracapillary proliferation with clinical outcomes have produced conflicting results5,6. IgAN is a highly heterogeneous disease with variable clinical patterns, pathologic features, long-term renal progression, and geographic prevalence5-14. The Oxford study comprised a small number of patients (n=265 patients). Therefore, we feel it is essential to conduct complementary studies using a larger number of patients from different geographic areas and ethnicities to identify important prognostic morphologic variables2,5,8,14. Moreover, studies have indicated that extracapillary proliferation is an important factor in IgAN2,3,5. According to the results of the Oxford cohort, extracapillary proliferation was not associated with kidney disease outcome. However, recent studies have demonstrated that extracapillary proliferation had prognostic significance5,15,16. Thus, it is important to re-evaluate crescents in IgAN patients. In our study, we investigated the clinical and morphological significance of extracapillary proliferation in a group of IgAN patients with regard to the Oxford classification.
Patients and Methods
We performed this study based on the Oxford classification of IgAN after it was published in July 2009 this classification1,17.
Definition of IgAN
The pathologic diagnosis of IgAN requires the observation of IgA-dominant mesangial or mesangial-capillary immune deposits through immunofluorescence (IF) microscopy because this technique can confirm the absence of C1q deposits. The immune deposits were semi-quantified from 0 to 3+ positive bright. The definition of IgAN requires the presence of diffuse and global IgA deposits of grade ≥2+ and the absence of C1q deposition1,3,17. All renal biopsies were performed at private or university medical centers in Isfahan, Iran, from July 2009 to July 2012; these biopsy specimens were sent to a reference laboratory. None of the patients underwent treatment before the biopsy. Those biopsies that were less than 8 glomeruli were excluded from this study. Based on the questionnaires completed at the time of the patients’ admission for biopsies, laboratory data in the patients’ records and brief histories provided by referring physicians, we found no patient diagnosed as secondary IgAN and no patient with a history of collagen vascular diseases, diabetes or liver cirrhosis.
Histologic data
All kidney biopsies were prepared for light and direct IF microscopy. The tissues were fixed in 10% formalin for histologic sectioning. Each renal biopsy was prepared by cutting paraffin blocks into 3-micron thick sections and staining 2 slides with periodic acid Schiff, 2 slides with hematoxylin and eosin, 1 slide with Jones methenamine silver and 1 slide with trichrome. Each slide contained 2 to 3 sections. Materials used for IF were snap-frozen in liquid nitrogen. For the IF study, sections (6 microns thick) were stained with fluorescein isothiocyanate-conjugated antibodies specific for human IgG, IgM, IgA, C1q, C3 and fibrin (DAKO, Produktionsvej 42, DK-2600 Glostrup, Denmark)18. Our nephropathologist (H.N.) classified the IF slides using a brightness scale ranging from 0 to +3. Unaware of patients’ data (blind), we performed an IF review before we evaluated the slides via light microscopy. After an IF diagnosis of IgAN, we reviewed the histopathology glass slides to define the morphologic variables, which were classified according to the Oxford-MEST method. We also assessed the presence of extracapillary proliferation (cellular, fibrocellular or fibrous crescents). After selecting those biopsies that had prominent IgA deposition according to IF study and did not meet any exclusion criteria, we classified the glass slides according to Oxford-MEST.
Definitions of morphologic variables of IgAN and MEST (Oxford classification)
We recorded the total number of glomeruli and the number of glomeruli with global sclerosis for each biopsy. We estimated the presence of mesangial hypercellularity (M), endocapillary proliferation (E), segmental glomerulosclerosis (S) and the proportion of tubular atrophy and interstitial fibrosis, IF/TA (T), as defined in the Oxford-MEST classification13,8,19.
Definitions of the extracapillary proliferation types
Extracapillary cell proliferation, or cellular crescent, was defined as “more than two cell layers, with >50% of the lesion occupied by cells”. Extracapillary fibrocellular proliferation, or fibrocellular crescent, was defined as “extracapillary lesion comprising cells and extracellular matrix, with < 50% cells and <90% matrix”. Extracapillary fibrosis, or fibrous crescent, was defined as “>10% of the circumference of Bowman’s capsule covered by a lesion composed of >90% matrix”1,8.
Clinical studies and laboratory data
We reviewed the patients’ medical records to obtain various demographic, clinical and laboratory data at the time of their biopsies and for follow-up. We gathered the following data at the time of biopsy: race, gender, age, serum creatinine and proteinuria (based on a 24-hour urine collection).
Statistical analysis
We determined the frequency, mean values and standard deviations and calculated the statistical significance of the differences between the male and female groups with regard to crescents using the Mann-Whitney U test. We used the Spearman’s correlation coefficient to check the correlations. We used a computer program (SPSS version 16.0, Chicago, IL) for statistical analysis. P<0.05 was considered statistically significant.
Results
Population Characteristics
In our observational study, we enrolled a total of 114 IgAN patient biopsies.
Prevalence
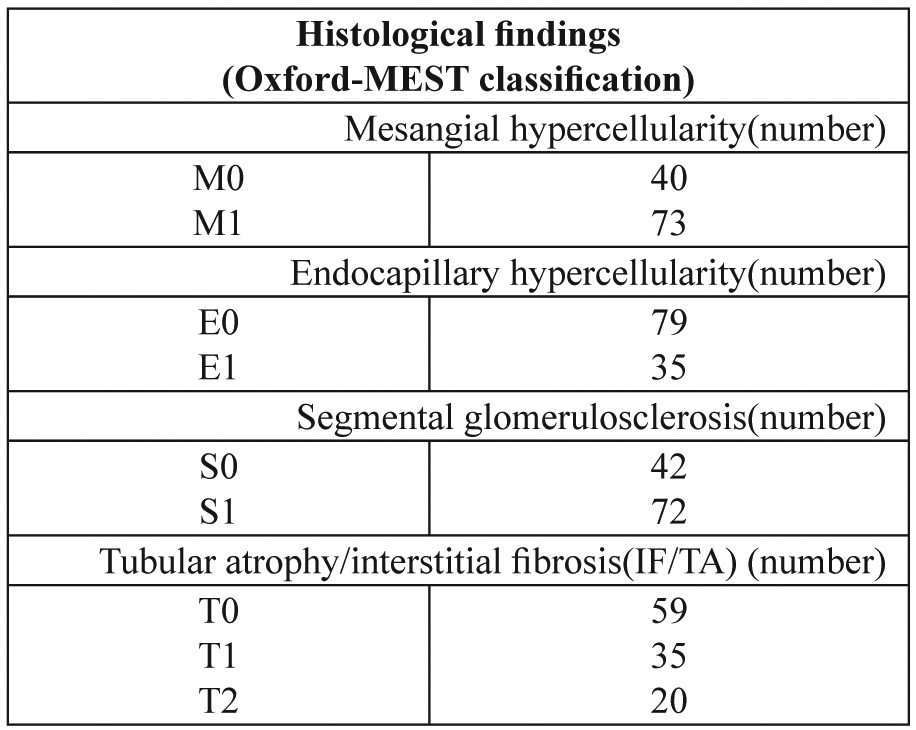
Of the 114 patients, 70.2 % were male. The mean age of the patients was 37.7 ± 13.6 years (39.0 ± 14.3 years and 35.0 ± 11.7 years for male patients and female patients, respectively). The morphologic variables of the Oxford-MEST classification are summarized in Table 1. The mean proteinuria was 1742 ± 1324 mg/day (median=1500 mg/day). The mean glomeruli number in all renal biopsies was 14.8 ± 7.2. In all biopsies, the mean totally sclerosed glomeruli number was 2.4 ± 2.9 (median=1). Also, the mean of serum creatinine was 1.6 ± 1.5 mg/dL (median=1.2 mg/dL). Of the 114 kidney biopsies, 25 (21.9%) had extracapillary proliferation. Of the 25 IgAN patients who had extracapillary proliferation, 13 patients had cellular crescent, 8 patients had fibrocellular crescent, and 9 patients had fibrous crescent.
Table 1. Histological findings of IgAN patients at the time of renal biopsy.
Gender differences
There were no significant differences in total crescents, fibrous crescent, cellular crescent or fibrocellular crescent between the male and female subjects (p>0.05).
Correlations
There was a significant positive correlation between the number of crescents and serum creatinine (p<0.001). Whereas there was a borderline correlation between the total crescents and the level of proteinuria (p=0.05), we found a significant positive correlation between fibrous crescent and proteinuria (p=0.028). Although 11 (9.6%) patients had nephrotic-range proteinuria, we found that our patients who had a nephrotic syndrome (proteinuria >3.5 g/day) had a positive association with total crescents (p<0.05). Additionally, there was a significant positive correlation between the amount of sclerosed glomeruli and extracapillary proliferation (p=0.028). There was no positive association between the patients’ age and the number of crescents (p>0.05). Except for the nonsignificant association of total crescents with the M variant of the Oxford classification (p=0.092), other variants of the Oxford classification, including E, S and T morphologic variables, had a significant association with total crescents (E: p=0.001; S: p=0.001; and T: p<0.001). A study on the association of antibody deposit scores with extracapillary proliferation showed a significant positive association of IgA score brightness with total crescents (r=0.263, p=0.005). There was a significant association between C3 scores and the number of crescents (p<0.05). There was also a significant positive correlation between extracapillary proliferation and tuft-capsule adhesion (synechiae) (p=0.003) or thickening of Bowman’s capsule (p=0.001).
Discussion
IgAN is one of the most common primary glomerular diseases in the world1-4. Accurately identifying patients who are at risk of developing a progressive disease is challenging2,3,5. The extent to which histopathologic features have prognostic importance is uncertain1-5. Although mesangial cell proliferation and matrix expansion are common in IgAN, glomerular pathology can also include extracapillary proliferation. However, the incidence and clinical significance of this lesion are unknown2-5.
Similar to our finding (21.9% crescents in 114 biopsies), in a clinical pathological review of 218 pediatric patients, Hogg et al. found that 20% of the patients with IgAN had crescents on their initial biopsy specimen20.They also found a positive association between the number of crescents and endocapillary proliferation. This finding is in accordance with our result20. We found a significant association between the E variant of the Oxford classification (endocapillary proliferation) and the number of crescents (p=0.001). Walsh et al. conducted a retrospective analysis of 146 cases with biopsy-proven IgAN. The prognostic significance of clinical and histopathologic parameters was determined using Cox proportional hazards models. In multivariable models adjusted for clinical characteristics, interstitial fibrosis, glomerular sclerosis and the number of crescents remained independent predictors of the primary outcome21. In our study, we found a significant positive correlation between extracapillary proliferation and the amount of totally sclerosed glomeruli, tuft-capsule adhesion (synechiae) or thickening of Bowman’s capsule. Totally sclerosed glomeruli, tuft-capsule adhesion and thickening of Bowman’s capsule are chronic lesions that affect the disease outcome2-30. In agreement with previous investigators, who have documented a higher incidence of nephrotic-range proteinuria in patients with the crescentic form of IgAN3,5, we found a significant positive correlation between fibrous crescent and proteinuria. We also found that the nephrotic syndrome had a positive association with total crescents, suggesting that patients with this variant of the disease may have a worse prognosis. This finding is in accordance with the findings of a study conducted by Nicholls et al31. Similarly, Abe et al. studied 205 patients with IgAN and found that patients with >25% crescents on initial biopsy had <50% renal survival after 4 years32.
To the best of our knowledge, our work is the first study that shows a positive association of the S (segmental sclerosis) and T (interstitial fibrosis/tubular atrophy) variants of the Oxford classification with total crescents. We also demonstrated a significant positive association between IgA score brightness and the number of crescents. Four variables of the Oxford classification, including mesangial hypercellularity, segmental glomerulosclerosis, endocapillary hypercellularity and tubular atrophy/interstitial fibrosis, have been shown to have independent values in predicting renal outcome in the original Oxford reports2,3,5,17. However, the significance of the crescents was not addressed in the Oxford classification because of low prevalence.
Our study suggests that crescents should be used as an important factor in IgAN. To determine which pathologic features have prognostic importance for the development of end-stage kidney disease in IgAN patients, Katafuchi et al, conducted a study on 702 patients with IgAN. The association of extracapillary proliferation with kidney survival was examined by a univariate analysis in 416 patients who met the Oxford criteria. In this analysis, these authors found that kidney survival was significantly lower in patients with extracapillary proliferation than in those patients without extracapillary proliferation. They concluded that extracapillary proliferation had prognostic significance in their cohort5. In agreement with this finding, Shima et al, analyzed 161 consecutive children with newly diagnosed IgA nephropathy from 1977 to 1989. In a multivariate analysis, only mesangial hypercellularity score, tubular atrophy and the number of crescents were significant predictors of renal outcome. These authors concluded that although the significance of crescents was not addressed in the Oxford classification, crescents were important outcome predictors15. Likewise, Kawamura et al. conducted a multicenter case-control study on 287 patients with IgAN to develop an evidence-based clinicopathologic classification of IgAN for predicting long-term renal outcome. They found that the independent pathological variables predicting progression to end-stage renal disease were global sclerosis, segmental sclerosis and fibrous crescent for early progressors and global sclerosis and cellular/fibrocellular crescents for late progressors16.
Our findings support the importance of extracapillary proliferation (crescent) due to its association with proteinuria and sclerotic glomeruli. We propose that extracapillary proliferation should be included in the revision of the Oxford classification of IgAN to widen the scope of the classification.
Conflict of interest
Authors have no conflicts of interest to declare.
References
- 1.Working Group of the International IgA Nephropathy Network and the Renal Pathology Society, Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, et al. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009;76:546–556. doi: 10.1038/ki.2009.168. [DOI] [PubMed] [Google Scholar]
- 2.Mubarak M. Oxford classification of IgA nephropathy: Broadening the scope of the classification. J Nephropathol. 2012;1:13–16. doi: 10.5812/jnp.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nasri H, Mortazavi M, Ghorbani A, Shahbazian H, Kheiri S, Baradaran A, et al. Oxford-MEST classification in IgA nephropathy patients: A report from Iran. J Nephropathol. 2012;1:31–42. doi: 10.5812/jnp.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Floege J, Feehally J. IgA nephropathy: recent developments. J Am Soc Nephrol. 2000;11:2395–2403. doi: 10.1681/ASN.V11122395. [DOI] [PubMed] [Google Scholar]
- 5.Katafuchi R, Ninomiya T, Nagata M, Mitsuiki K, Hirakata H. Validation study of oxford classification of IgA nephropathy: the significance of extracapillary proliferation. Clin J Am Soc Nephrol. 2011;6:2806–2813. doi: 10.2215/CJN.02890311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi SF, Wang SX, Jiang L, Lv JC, Liu LJ, Chen YQ, et al. Pathologic predictors of renal outcome and therapeutic efficacy in IgA nephropathy: validation of the oxford classification. Clin J Am Soc Nephrol. 2011;6:2175–2184. doi: 10.2215/CJN.11521210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Assadi F. The epidemic of pediatric chronic kidney disease: the danger of skepticism. J Nephropathol. 2012;1:61–64. doi: 10.5812/nephropathol.7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nasri H, Sajjadieh S, Mardani S, Momeni A, Merikhi A, Madihi Y, et al. Correlation of immunostaining findings with demographic data and variables of Oxford classificationin IgA nephropathy. J Nephropathol. 2013;2:190–195. doi: 10.12860/JNP.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sahni N, Gupta KL. Dietary antioxidents and oxidative stress in predialysis chronic kidney patients. J Nephropathol. 2012;1:134–142. doi: 10.5812/nephropathol.8108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kari J. Epidemiology of chronic kidney disease in children. J Nephropathol. 2012;1:162–163. doi: 10.5812/nephropathol.8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mubarak M. Collapsing focal segmental glomerulosclerosis: increasing the awareness. J Nephropathology. 2012;1:77–80. doi: 10.5812/nephropathol.7474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.TayebiKhosroshahi H. Short history about renal transplantation program in Iran and the world: Special focus on world kidney day 2012. J Nephropathol. 2012;1:5–10. doi: 10.5812/jnp.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sánchez-Niño MD, Ortiz A. Is it or is it not a pathogenic mutation? Is it or is it not the podocyte? J Nephropathol. 2012;1:152–154. doi: 10.5812/nephropathol.8110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohammadi Torbati P. Focal segmental glomerulosclerosis; collapsing variant. J Nephropathol. 2012;1:87–90. doi: 10.5812/nephropathol.7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mubarak M. Significance of immunohistochemical findings in Oxford classification of IgA nephropathy: The need for more validation studies. J Nephropathol. 2013;2:210–213. doi: 10.12860/JNP.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawamura T, Joh K, Okonogi H, Koike K, Utsunomiya Y, Miyazaki Y, et al. A histologic classification of IgA nephropathy for predicting long-term prognosis: emphasis on end-stage renal disease. J Nephrol. 2013;26:350–357. doi: 10.5301/jn.5000151. [DOI] [PubMed] [Google Scholar]
- 17.Working Group of the International IgA Nephropathy Network and the Renal Pathology Society, Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76:534–545. doi: 10.1038/ki.2009.243. [DOI] [PubMed] [Google Scholar]
- 18.Mubarak M, Kazi JI, Kulsoom U, Ishaque M. Detection of immunoglobulins and complement components in formalin fixed and paraffin embedded renal biopsy material by immunoflourescence technique. J Nephropathol. 2012;1:91–100. doi: 10.5812/nephropathol.7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barratt J, Feehally J. IgA Nephropathy. J Am Soc Nephrol. 2005;16:2088–2097. doi: 10.1681/ASN.2005020134. [DOI] [PubMed] [Google Scholar]
- 20.Hogg RJ, Silva FG, Wyatt RJ, Reisch JS, Argyle JC, Savino DA. Prognostic indicators in children with IgA nephropathy--report of the Southwest Pediatric Nephrology Study Group. Pediatr Nephrol. 1994;8:15–20. doi: 10.1007/BF00868251. [DOI] [PubMed] [Google Scholar]
- 21.Walsh M, Sar A, Lee D, Yilmaz S, Benediktsson H, Manns B, et al. Histopathologic features aid in predicting risk for progression of IgA nephropathy. Clin J Am Soc Nephrol. 2010;5:425–430. doi: 10.2215/CJN.06530909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koyama A, Igarashi M, Kobayashi M. Natural history and risk factors for immunoglobulin A nephropathy in Japan. Research Group on Progressive Renal Diseases. Am J Kidney Dis. 1997;29:526–532. doi: 10.1016/s0272-6386(97)90333-4. [DOI] [PubMed] [Google Scholar]
- 23.Tolou Ghamari Z. Nephro and neurotoxicity of calcineurin inhibitors and mechanisms of rejection: A review on tacrolimus and cyclosporin in organ transplantation. J Nephropathol. 2012;1:23–30. doi: 10.5812/jnp.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mortazavi M, Nasri H. Granulomatosis with polyangiitis (Wegener’s) presenting as the right ventricular masses: A case report and review of the literature. J Nephropathol. 2012;1:49–56. doi: 10.5812/jnp.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nasri H. Hypertension and renal failure with right arm pulse weakness in a 65 years old man. J Nephropathol. 2012;1:130–133. doi: 10.5812/nephropathol.8265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gheissari A, Hemmatzadeh S, Merrikhi A, Fadaei Tehrani S, Madihi Y. Chronic kidney disease in children: A report from a tertiary care center over 11 years. J Nephropathol. 2012;1:177–182. doi: 10.5812/nephropathol.8119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ardalan MR, Samadifar Z, Vahedi A. Creatine monohydrate supplement induced interstitial nephritis. J Nephropathol. 2012;1:117–120. doi: 10.5812/nephropathol.7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gheissari A, Mehrasa P, Merrikhi A, Madihi Y. Acute kidney injury: A pediatric experience over 10 years at a tertiary care center. J Nephropathol. 2012;1:101–108. doi: 10.5812/nephropathol.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haas M. IgA nephropathy histologically resembling focal-segmental glomerulosclerosis: a clinicopathologic study of 18 cases. Am J Kidney Dis. 1996;28:365–371. doi: 10.1016/s0272-6386(96)90493-x. [DOI] [PubMed] [Google Scholar]
- 30.Ferrario F, Napodano P, Rastaldi M, D’Amico G. Capillaritis in IgA nephropathy. Contrib Nephrol. 1995;111:8–12. doi: 10.1159/000423869. [DOI] [PubMed] [Google Scholar]
- 31.Nicholls K, Walker RG, Dowling JP, Kincaid-Smith P. “Malignant” IgA nephropathy. Am J Kidney Dis. 1985;5:42–46. doi: 10.1016/s0272-6386(85)80134-7. [DOI] [PubMed] [Google Scholar]
- 32.Abe T, Kida H, Yoshimura M, Yoshimura H, Koshino Y, Tomosugi N, et al. Participation of extracapillary lesions (ECL) in progression of IgA nephropathy. Clin Nephrol. 1986;25:37–41. [PubMed] [Google Scholar]


