Abstract
Background
Vitamin D is traditionally associated with bone metabolism. The immunologic effects of vitamin D have increasingly come into focus.
Aims
To review the evidence supporting a role of vitamin D in inflammatory bowel diseases.
Methods
A comprehensive search was performed on PubMed using the terms “crohn’s disease” “ulcerative colitis” and “vitamin D”
Results
Vitamin D deficiency is common in patients with IBD (16–95%) including those with recently diagnosed disease. Evidence supports immunologic role of vitamin D in IBD. In animal models, deficiency of vitamin D increases susceptibility to DSS colitis while 1,25(OH)2D3 ameliorates such colitis. One prospective cohort study found low predicted vitamin D levels to be associated with an increased risk of CD. Limited data also suggests an association between low vitamin D levels and increased disease activity, particularly in Crohn’s disease. In a large cohort, vitamin D deficiency (< 20ng/mL) was associated with increased risk of surgery (OR 1.8, 95% CI 1.2 – 2.5) in CD and hospitalizations in both CD (OR 2.1, 95% CI 1.6 – 2.7) and UC (OR 2.3, 95% CI 1.7 – 3.1). A single randomized controlled trial demonstrated that vitamin D supplementation may be associated with reduced frequency of relapses in patients with Crohn’s disease compared to placebo (13% vs. 29%, p = 0.06).
Conclusions
There is growing epidemiological evidence to suggest a role for vitamin D deficiency in the development of IBD and also its influence on disease severity. The possible therapeutic role of vitamin D in patients with IBD merits continued investigation.
Keywords: vitamin D, Crohn’s disease, ulcerative colitis, innate immunity, macrophage, autophagy
INTRODUCTION
Ulcerative colitis (UC) and Crohn’s disease (CD) constitute chronic idiopathic inflammatory bowel diseases (IBD). The key underlying pathogenic mechanisms for both diseases is a dysregulated host immune response to commensal intestinal flora in genetically susceptible individuals1, 2. Known genetic variants incompletely explain the variance in disease incidence suggesting a strong role for environmental factors, supported by epidemiologic evidence3, 4.
Vitamin D has long been recognized as a major regulator of calcium and phosphorus metabolism and key in maintaining bone health5–7. However, several recent studies have yielded new insights into the role of vitamin D in various other physiological processes. In particular, vitamin D appears to play important roles in immune regulation, particularly involving the innate immune system, cardiovascular and renal physiology, and development of cancer6. Importantly, an increasing body of literature supports an important role of vitamin D in the pathogenesis as well as potential therapy of IBD8–13. The current review examines the evidence linking vitamin D to IBD, both through its effect on bone health as well as association with pathogenesis and natural history of these diseases.
METHODS
A comprehensive literature search on Pubmed was conducted using the following search terms: “Crohn’s disease” “ulcerative colitis” and “vitamin D” to identify relevant English language articles published between 1966 and 2013. In addition, bibliographies of the retrieved articles were searched to identify additional relevant articles.
RESULTS
Vitamin D Synthesis
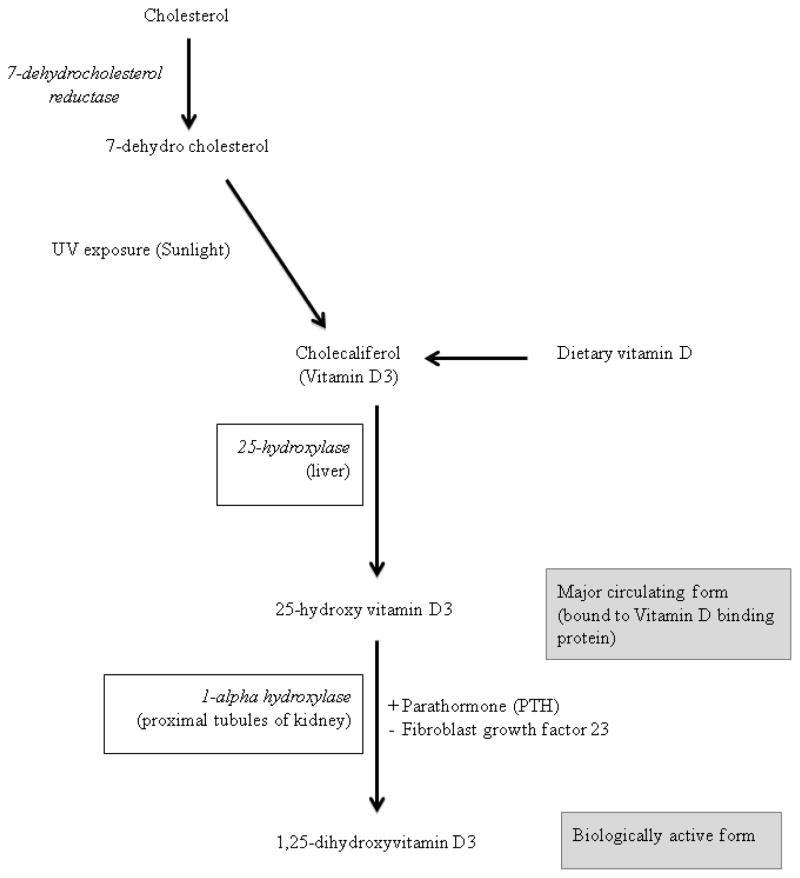
The main source of vitamin D is endogenous production in the skin where ultraviolet B energy in the sunlight converts 7-dehydrocholestrol to cholecalciferol (vitamin D3) (Figure 1)5, 14. Dietary contribution to vitamin D status includes foods such as egg yolk, beef liver, cod liver oil, fatty fish, fortified milk and milk products5. Vitamin D from the endogenous production on exposure to sunlight as well as that absorbed from diet is metabolized within the liver to 25-hydroxyvitamin D by the enzyme vitamin D 25-hydroxylase. 25-hydroxyvitamin D (25(OH)D) is the major circulating form of vitamin D and is also used to determine the status of vitamin D in clinical practice. 25(OH)D is biologically inactive and is activated within the proximal tubules of nephrons in the kidneys by the enzyme 25-hydroxyvitamin D-1alpha-hydroxylase (also known as CYP27B1) to 1,25-dihydroxyvitamin D (1,25(OH)2D). The renal synthesis of the active biological product of vitamin D (1,25(OH)2D) is regulated by various factors including serum calcium and phosphorus levels, parathormone and fibroblast growth factor 2315.
Figure 1.
Metabolism of vitamin D
Prevalence of vitamin D deficiency in IBD
While it is relatively easy to ascertain macronutrient deficiency clinically, micronutrient deficiency may not always be clinically evident and usually requires laboratory testing. The best measure of an individual’s vitamin D status is serum 25-hydroxyvitamin D [25(OH)D]5, 7, 16. Serum 25(OH)D levels of less than 20 ng/mL (50 nmol/L) indicate vitamin D deficiency. Serum 25(OH)D levels between 21 and 29 ng/mL (52.5 and 72.5 nmol/L) represent vitamin D insufficiency while levels between 30 and 100 ng/mL (75 and 250 nmol/L) represent normal values5, 7, 16. Several studies have reported a high prevalence of vitamin D deficiency in patients with IBD, though it has not been universally established that this rate is higher than in other chronic illnesses, inflammatory diseases, or even health individuals in that region (Table 1). Levin et al. reported vitamin D deficiency in 19% and insufficiency in 38% of children with IBD in a cohort predominantly consisting of patients with CD17. In contrast, Alkhouri et al. reported that the prevalence of vitamin D deficiency in children with IBD (62%) was lower than the rate in their controls (75%)18. In a large, retrospective study of adult patients with IBD from Wisconsin (101 UC, 403 CD), nearly 50% of the patients had vitamin D deficiency and about 11% of patients had severe vitamin D deficiency19, a frequency estimate that is consistent with other published IBD cohorts13. While most studies have examined prevalence in patients with well established IBD, deficiency of vitamin D does not appear to be consequent to long-standing disease alone. In a cohort of newly diagnosed IBD patients from Manitoba providence in Canada, only 22% were found to have sufficient levels of vitamin D20.
Table 1.
Prevalence of vitamin D deficiency in unselected cohorts of patients with inflammatory bowel diseases
| Author | Year | Cohort size | Vitamin D status |
|---|---|---|---|
| Driscoll84 | 1982 | 82 CD | 65% of patients had low serum 25(OH)D 25% had levels below 10ng/mL |
| Jahnsen44 | 2002 | 60 CD 60 UC |
27% of CD patients had 25(OH)D levels < 30nmol/L 15% of UC patients had 25(OH)D levels < 30nmol/L |
| Lamb85 | 2002 | 23 UC 11 CD 18 IBS controls |
Mean 25(OH)D was lower in IBD patients (18.7mcg/L) compared to controls (28.5 mcg/L) (p < 0.05) |
| Sentongo27 | 2002 | 112 CD† | 16% of CD patients had vitamin D < 38nmol/L |
| Siffledeen86 | 2003 | 242 CD | 22% had 25(OH)D levels < 40nmol/L 8% had 25(OH)D levels < 25nmol/L |
| Tajika25 | 2004 | 33 CD 15 controls |
27% of CD patients were deficient (25(OH)D < 10ng/mL) compared to 7% of controls |
| McCarthy22 | 2005 | 44 CD | During late-summer, 5% of controls and 18% of CD patients were deficient (<50nmol/L) During late-winter, 25% of controls and 50% of CD patients were deficient |
| Gilman21 | 2006 | 58 CD | 50% were vitamin D deficient during winter (< 50nmol/L) and 19% were deficient during summer |
| Pappa87 | 2006 | 94 CD† 36 UC† |
Prevalence of vitamin D deficiency (25(OH)D < 15ng/mL) was 35% |
| Leslie20 | 2008 | 56 CD 45 UC |
88% had serum 25(OH)D levels below 75nmol/L |
| Kuwabara88 | 2009 | 29 CD 41 UC |
Mean 25(OH) levels were lower in CD patients (11ng/mL) compared to those with UC (20ng/mL) (p < 0.001) |
| Joseph89 | 2009 | 34 CD 34 controls |
25(OH)D levels were lower in CD patients (16ng/mL) compared to controls (23ng/mL) |
| Ulitsky19 | 2011 | 403 CD 101 UC |
50% of patients had 25(OH)D < 30ng/dL, 11% had levels below 10ng/dL |
| Pappa90 | 2011 | 288 CD† 143 UC† 17 IC† |
Vitamin D insufficiency (< 20ng/mL) was seen in 31% of patients with CD and 28% of UC patients |
| Levin17 | 2011 | 70 CD 5 UC 3 IBDU |
19% of patients were deficient in vitamin D (< 51nmol/L) |
| Atia91 | 2011 | 125 IBD | 37% had vitamin D deficiency |
| Suibhne92 | 2012 | 81 CD | 63% of patients with CD were deficient (<50nmol/L) |
| Fu93 | 2012 | 60 UC 40 CD |
39% of the entire cohort had low vitamin D (< 50nmol/L); this was more frequent in 43% of CD and 37% of UC patients |
| Laakso94 | 2012 | 49 UC 28 CD |
30% of patients with CD had levels below 37.5nmol/L compared to 37% of controls |
| Hassan95 | 2013 | 60 IBD | 95% of patients had deficient vitamin D levels (25(OH)D < 30ng/ml) |
| Ananthakrishnan13 | 2013 | 1,763 CD 1,454 UC |
28% had insufficient (20–30ng/mL) and 32% had deficient (< 20ng/mL) levels |
| Alkhouri18 | 2013 | 61 IBD† | 62% of patients had low vitamin D levels compared to 75% of controls |
IBD – inflammatory bowel diseases; CD – Crohn’s disease; UC – ulcerative colitis, IC – indeterminate colitis
Pediatric cohorts
Causes of vitamin D deficiency in patients with IBD
There are several factors contributing to vitamin D deficiency in patients with IBD, some causes specifically related to the underlying bowel disease, while others are in common with the non-IBD population. These include inadequate exposure to sunlight either related to lifestyle or persistent symptoms of active disease restricting physical activity, inadequate dietary intake due to symptoms of bowel disease, impaired absorption, impaired conversion of vitamin D to its active products, increased catabolism and increased excretion5, 7. That inadequate exposure to sunlight is an important cause of vitamin D deficiency in patients with IBD is supported by evidence. Several studies, particularly from northern climates, have consistently demonstrated an association between vitamin D deficiency and winter season, a period of likely low sunlight and UVB exposure13, 21, 22. Insufficient dietary consumption also contributes to low vitamin D in some patients with IBD. In a detailed nutritional survey of 126 IBD patients, inadequate vitamin D consumption was found in 36% of patients and suboptimal serum vitamin D levels were found in 18% of patients23. Oral intake correlated significantly with serum levels in CD and with all IBD in remission23. While other small studies suggested no correlation between dietary vitamin D intake and serum 25(OH)D in CD patients, they may have been limited by lack of statistical power24.
Fats and fat-soluble vitamins are absorbed after emulsification by bile acids. The bile acid pool is maintained by an enterohepatic circulation occurring from the terminal ileum. Interruption of the enterohepatic circulation (for example, by terminal ileal resection) could theoretically contribute to vitamin D deficiency. However, clinical data in support of this is conflicting. Terminal ileal resection was associated with vitamin D deficiency in some studies25, 26. In a study of 12 CD patients who underwent terminal ileal resection, absorption of vitamin D was reduced with the decline in absorption correlating with the length of the resected segment. However, other studies failed to identify an effect of ileal resection or active disease19. Malabsorption may theoretically contribute to low vitamin D in CD patients as vitamin D is absorbed in the proximal part of small intestine. The prevalence of vitamin D deficiency is higher in CD patients with upper gastrointestinal tract involvement27. However when absorption of vitamin D was specifically tested, only 10% of patients with CD had decreased absorption of vitamin D compared to 50% of patients with pancreatic insufficiency28. There also appears to be a wide variation in absorption of vitamin D in patients with CD even in those with quiescent disease29. Protein losing enteropathy occurs in some patients with IBD. As vitamin D and its metabolites circulate predominantly as bound forms to plasma vitamin D binding protein (DBP), the loss of DBP along with the bound vitamin D could be an additional plausible mechanism of vitamin D deficiency, particularly in those with severe disease. Finally, recent studies have suggested that genetic variants contribute both to development of vitamin D insufficiency as well as response to supplementation. In a genome-wide association study of nearly 30,000 individuals of European descent, variants at three loci near the genes involved in cholesterol synthesis, vitamin D hydroxylation and vitamin D transport were associated with vitamin D insufficiency30. The contribution of such genetic variants to vitamin D status in patients with IBD has not yet been studied.
Role of Vitamin D in bone turnover and mineral metabolism
Vitamin D helps maintain calcium homeostasis by acting on the small intestine epithelium and osteoblasts. 1,25(OH)2D acts mainly through the nuclear vitamin D receptor (VDR) which forms a heterodimer with a retinoid X receptor, binds to the vitamin D response element (VDRE) and recruits co-activators and enzymes with histone acetylation activity, thereby regulating gene expression10, 31–33. 25(OH)D interacts with the VDR in the small intestinal epithelium and augments the absorption of calcium and phosphorus from the small intestine34. 1,25(OH)2D also interacts with the VDR on osteoblasts and increases the surface expression of Receptor Activator for Nuclear Factor κB ligand (RANKL) which after binding with RANK on pre-osteoclasts converts them into osteoclasts35, 36. Osteoclasts function in dissolution of bone matrix and mobilize calcium stores into circulation, thus helping in the maintenance of calcium homeostasis. Dissolution of bone matrix by osteoclasts is an essential part of bone remodelling.
Vitamin D deficiency leads to reduction in serum levels of ionized calcium leading to secondary hyperparathyroidism, resulting in osteoclastogenesis, a disproportionate increase in bone resorption, osteopenia and osteoporosis37. In children, vitamin D deficiency results in poor mineralization of the epiphyseal growth plates leading to bone deformities and stunted longitudinal growth, which are the typical features of rickets. In adults with vitamin D deficiency, there is defective mineralization of the newly formed bone collagen matrix resulting in osteomalacia which manifests as bone pain, fractures and proximal muscle weakness5, 7, 16.
There is a high prevalence of metabolic bone disease in patients with IBD. The prevalence of osteopenia ranges from 23% - 67% and osteoporosis from 7% - 35% among patients with CD or UC38–40. Active inflammatory disease is a strong risk factor for low bone mineral density (BMD) in patients with IBD, with bone mineral density improving with increasing duration of remission41. This is supported by the known effect of TNF-α and other pro-inflammatory cytokines like IL-1, IL-6, IL-17 in activating osteoclasts42, 43. In addition, glucocorticoids use is an important risk factor for bone loss in patients with IBD39. However, the data linking vitamin D deficiency and impaired bone mineral density in patients with IBD has been conflicting with some studies supporting such an association and others finding no effect20, 39, 44.
Vitamin D and innate immunity
Vitamin D receptor (VDR) is ubiquitously expressed in several human tissues including immune cells, keratinocytes, pancreatic beta-cells, cardiac myocytes, central nervous system, renal tubules, and the intestine. Many of these tissues also contain the enzymes for conversion of vitamin D to its active metabolites, supporting a widespread extraskeletal role of vitamin D45. Vitamin D appears to have an important role in innate immunity as well as adaptive immunity10, 33. It acts as a key link between toll-like receptor (TLR) activation and antibacterial responses in innate immunity. Activation of TLRs on macrophages by a Mycobacterium tuberculosis derived lipopeptide leads to upregulation of conversion of 25(OH)D to the active 1,25(OH)2D, upregulation of VDR expression and induction of downstream targets of VDR including cathelicidin, an antimicrobial peptide46. 1,25(OH)2D also acts synergistically with activated NF-κB to induce expression of β-defensin 4 gene47. Supplementation with vitamin D in individuals with insufficient serum levels of 25(OH)D leads to induction of cathelicidin, thus enhancing the innate immune defences against microbial agents48.
Autophagy plays an important role in the pathogenesis of CD, and several lines of evidence support the hypothesis that the effect of vitamin D on IBD pathogenesis may be through this pathway. 1,25 (OH)2D helps in autophagy in macrophages by enhancing the co-localization of pathogen harbouring phagosomes with autophagosomes in a cathelicidin-dependent manner49. Similar induction of autophagy by vitamin D has also been demonstrated in several models of cancer cell lines. Vitamin D3 has been hypothesized to regulate autophagy at several steps50. Increased calcium absorption mediated by the effect of vitamin D3 on the vitamin D receptor can activate autophagy through various calcium-dependent kinases and phosphates, while vitamin D3 can itself downregulated the expression of mTOR, a negative regulator of autophagy50, 51. Vitamin D3 can also induce autophagy through increasing beclin-1 expression, a regulatory of autophagy, and activating the PI3K signalling pathway50–52. Vitamin D has been long used to treat mycobacterial infections46, 53, 54 and vitamin D supplementation may reduce likelihood of tuberculin conversion55, 56. In a randomized controlled trial, vitamin D supplementation was associated with a reduced rate of development of a positive tuberculin reaction, suggesting a protective effect against tuberculosis infection in an endemic population56. Low serum vitamin D is also associated with reduced immunoreactivity to an anergy panel, and supplementation with vitamin D in anergic individuals with deficient levels restored delayed hypersensitivity response.57
Vitamin D also plays a role in preventing over-activation of pro-inflammatory responses. 1,25(OH)2D within the monocytes dose dependently inhibits lipopolysaccharide (LPS) induced p38 phosphorylation and production of IL-6 and TNF-α in LPS-stimulated monocytes58. Antigen presenting cells, including dendritic cells express VDR59. The action of 1,25(OH)2D on dendritic cells leads to a tolerogenic phenotype thus protecting against autoimmune type 1 diabetes in adult non-obese diabetic mice60. Maturation of dendritic cells is prevented by the interaction of 1,25(OH)2D with VDR on the dendritic cells61.
Vitamin D and adaptive immunity
VDR is expressed in mitotically active T and B lymphocytes62. 1,25(OH)2D acts on helper T cells (TH cells), inhibits production of IL-2 and immunoglobulin synthesis by TH cell regulated B lymphocytes63. Regulatory T cells (Treg) which are responsible for maintenance of tolerance to self-antigens are also modulated by 1,25(OH)2D10, 33. Though the effect of vitamin D on B cells in predominantly through modulation of T-cell function, recent evidence suggests that 1,25(OH)2 D may also acts directly on the B cells affecting the proliferation of activated B cells and inhibiting the generation of plasma cells and post-switch memory B cells64.
Role of vitamin D in the immunopathogenesis of IBD
Several lines of epidemiologic and laboratory evidence support a role for vitamin D in the pathogenesis of IBD. First, there is a north-south gradient in IBD incidence, a gradient that parallels UV exposure and consequently vitamin D levels. In a study by Khalili et al., residence in Southern latitudes of the United States, particularly at age 30 was associated with a significantly lower risk of CD (Hazard ratio (HR) 0.48, 95% CI 0.30 – 0.77) and UC (HR 0.62, 95% CI 0.42 – 0.90)65. This has been supported by other studies that have modelled residential UV exposure and shown an inverse correlation between UV exposure and IBD incidence66. Mice lacking VDR are more susceptible to dextran sodium sulphate (DSS) induced mucosal injury compared to the wild type mice67. The disruption in the epithelial junctions was severe in mice lacking VDR and 1,25(OH)2D preserved the integrity of the tight junctions in Caco-2 cells monolayers67. Genetic epidemiological studies have suggested a link between polymorphisms in the VDR gene region on chromosome 12 to development of IBD10, 68–70 though not all cohorts have yielded positive results. Variations in the vitamin D binding protein were also found to be associated with IBD71.
Few studies have been able to examine the association between vitamin D status and incident IBD directly. One such study was using the Nurses’ Health Study, a cohort of female registered nurses in the United States, followed prospectively using biennial questionnaires, and comprehensive assessment of diet and supplement intake and physical activity during the cohort follow-up timeline8. The vitamin D status of the participants was defined using a validated regression model incorporating race, diet, physical activity and region of residence. Over a 22 year follow-up, higher predicted plasma 25(OH)D leves was associated with a significant reduction in the risk of incident CD but not UC8. Compared to women with the lowest quartile of plasma vitamin D, those is highest quartile had a reduced risk of CD (HR 0.54, 95% CI 0.30 – 0.99)8. For each 1 ng/mL increase in the plasma level of 25(OH)D, there was a 6% relative risk reduction for CD. There was also an inverse association between vitamin D intake from dietary sources and supplement and the risk for incident UC; each 100 IU/day increase in total vitamin D intake was associated with a 10% relative reduction in the risk of UC8.
Relationship of vitamin D levels and IBD disease severity
In tune with its immune modulating effects, vitamin D may also influence severity of inflammation in IBD. Vitamin D deficiency causes more severe growth retardation and weight loss and also led to higher mortality in IL-10 KO mice colitis72. Disease severity correlated with vitamin D status in mice with DSS- induced colitis; both local as well as endocrine synthesis of 1,25 (OH)2D affect the disease severity73. TNFα plays a central role in inflammation. 1,25(OH)2D reduces the severity of colitis in IL-10 KO mice by downregulating several genes associated with TNFα74. When mice with tri-nitro-benzene sulphonic acid (TNBS) induced colitis were treated with a combination of corticosteroids and 1,25 (OH)2D, the improvement in disease activity paralleled downregulation of TH1 inflammatory cytokines profile as well as TH17 effector functions along with the promotion of TH2 and regulatory T cell profiles75.
Data supporting a clinical association between vitamin D deficiency and disease activity in IBD is conflicting (Table 2). Neither el-Matary et al. nor Levin et al. found a correlation between vitamin D levels and disease activity in cross-sectional studies of IBD cohorts17, 76. In contrast, a retrospective study by Ulitsky et al. concluded that vitamin D deficiency was associated with lower health-related quality of life and increased disease activity in patients with CD but not with UC19. Overcoming some of the limitations engendered by cross-sectional assessment of vitamin D and disease severity, we examined prospectively the association between vitamin D deficiency and need for IBD-related surgery or hospitalizations in a large cohort of 3,217 patients with at least one measurement of plasma 25(OH)D13. We found that plasma 25(OH)D <20 ng/mL was associated with an increased risk of surgery (Odds ratio (OR) 1.76; 95%CI 1.24–2.51) and hospitalization (OR 2.07; 95% CI 1.59–2.68) compared with those with sufficient levels13. Furthermore, CD patients who normalized their plasma 25(OH)D had a reduced likelihood of IBD-related surgery (OR 0.56; 95% CI 0.32–0.98) compared with those who remained deficient13.
Table 2.
Observational studies of the association between vitamin D levels and outcomes in Crohn’s disease and ulcerative colitis
| Author | Year | Study Design | Number of patients | Predictor variable | Outcome | Result |
|---|---|---|---|---|---|---|
| Joseph89 | 2009 | Cross-sectional | 34 CD | Serum 25(OH)D | HBI | Serum 25(OH)D negatively correlated with disease activity (correlation co-efficient −0.484) |
| El-Matary76 | 2011 | Cross-sectional | 60 IBD (39 CD, 21 UC) | Serum 25(OH)D | PCDAI or PUCAI | Vitamin D levels were not associated with disease activity |
| Ulitsky19 | 2011 | Retrospective | 504 IBD (403 CD, 101 UC) | Serum 25(OH)D | HBI or SCCAI Health-related quality of life | Vitamin D deficiency was associated with lower HRQoL (−2.2, 95% CI −4.1 to −0.3) and increased disease activity (1.1, 95% CI 0.4 – 1.7) in CD but not UC |
| Ananthakrishnan13 | 2013 | Prospective | 3217 IBD (1763 CD, 1454 UC) | Serum 25(OH)D | IBD-related surgery IBD-related hospitalizations |
Vitamin D deficiency (<20ng/mL) was associated with increased risk of surgery (OR 1.8, 95% CI 1.2 – 2.5) in CD and hospitalizations in both CD (OR 2.1, 95% CI 1.6 – 2.7) and UC (OR 2.3, 95% CI 1.7 – 3.1) |
| Zator79 | 2013 | Retrospective | 101 IBD (74 CD, 27 UC) patients initiating anti-TNF therapy | Serum 25(OH)D within 3 months of anti-TNF initiation | Cessation of anti-TNF therapy | Patients with low vitamin D had an increased risk of early cessation of anti-TNF therapy (HR 2.1, 95% CI 1.0 – 4.4) |
CD – Crohn’s disease; UC – ulcerative colitis; IBD – inflammatory bowel disease; HBI – Harvey Bradshaw index; PCDAI – Pediatric crohn’s disease activity index; PUCAI – pediatric ulcerative colitis activity index; SCCAI – simple clinical colitis activity index,
Does vitamin D have a role in the treatment of IBD
There have been several studies examining the role of vitamin D as a therapeutic agent for IBD in animal models77. Vitamin D deficient IL-10 KO mice spontaneously develop an accelerated and severe form of IBD. However, when such mice were fed with high calcium diet and 1,25(OH)2 D, they developed only mild disease72. Both in TNBS- and DSS-induced colitis models, administration of 1,25(OH)2 D led to an improvement in disease activity and addition of 1,25(OH)2D to a steroid regimen had a synergistic effect and this combination most effectively reduced the disease severity78. A novel vitamin D analog with anti-proliferative effects and limited calcemic activity was also found to alleviate disease activity in mice with DSS-induced colitis78.
There have been few human studies (Table 3). Jorgensen et al. conducted a multi-center, randomized, double blind, placebo controlled trial in Denmark evaluating the efficacy of 1,25(OH)2D as a maintenance therapy in CD patients in remission12. One hundred and eight patients were randomized to receive either 1200 IU of 1, 25(OH)2D with 1200 mg of calcium or 1200 mg of calcium alone daily over 1 year. Nearly one-third of the study population had vitamin D deficiency defined as serum 25(OH)D levels < 50 nmol/L. Only 13% of patients in the vitamin D group relapsed during the 1-year study period compared to 29% in the placebo group (p=0.06)12. A second study by Zator et al. examined the influence of vitamin D status on response to anti-TNF therapy. In a single center cohort of patients with CD and UC, plasma 25(OH)D levels measured within 3 months of initiation of anti-TNF therapy demonstrated a significant inverse association with durability of anti-TNF treatment, with a more pronounced effect on patients with CD79. Miheller et al. compared the therapeutic effects of 1,25(OH) 2D and 25(OH) D in patients with CD with respect to disease activity and bone health80. There was a significant improvement in disease activity as well as bone metabolism in the short term at 6 weeks with 1,25(OH)2D but not 25(OH)D80.
Table 3.
Interventional studies examining the effect of vitamin D supplementation on disease activity in Crohn’s disease and ulcerative colitis
| Author | Year | Study Design | Number of patients | Intervention | Outcome | Result |
|---|---|---|---|---|---|---|
| Miheller80 | 2009 | Open label | 37 patients with inactive CD | Daily 0.5mcg of alfacalcidiol or 1000 IU of cholecalciferol | CDAI | Mean CDAI decreased from 69 to 57 in patients treated with alfacalcidiol |
| Jorgensen12 | 2010 | Randomized placebo- controlled trial | 104 CD patients in clinical remission | Oral vitamin D3 1200 IU daily or placebo | Relapse | Relapse rate was lower in patients treated with vitamin D3 (13%) compared to placebo (29%) (p=0.06) |
| Yang81 | 2013 | Open label | 18 patients with mild-to-moderate CD | Vitamin D3 at 1,000 IU daily; dose increase after 2 weeks to achieved serum 25(OH)D of 40ng/mL | CDAI Quality of life | Vitamin D supplementation reduced CDAI scores from 230 to 118 (p < 0.0001), and improved health related quality of life |
CD – Crohn’s disease; CDAI –crohn’s disease activity index;
DISCUSSION
Limitations
Despite emerging promising data, there exist several limitations in the literature regarding the role of vitamin D in IBD pathogenesis. First, while consistently supported by experimental animal models, the association between low pre-diagnosis vitamin D and increased risk of CD has been examined in a single prospective cohort study that used a regression model to predict an individual’s vitamin D status. Ongoing analysis of pre-diagnosis banked specimens from ongoing prospective cohorts as well as additional high-risk IBD cohorts will provide a more definitive answer to this hypothesis as randomized controlled trials of vitamin D in prevention of IBD are unlikely to be feasible given relatively low incidence of disease in the general population, and need for large numbers of participants and long follow-up. The association between low vitamin D and increased disease activity, particularly in Crohn’s disease, is also supported primarily by observational data. While initial studies were cross-sectional and unable to differentiate effect of vitamin D on disease activity from that of disease course on vitamin D levels, more recent analyses of large cohorts have been able to prospectively demonstrate an association between low vitamin D levels and increased risk for surgery and hospitalizations, particularly in CD13. However, only one randomized controlled trial has examined the role of vitamin D in preventing relapse, but was also limited by small numbers12. Effect of vitamin D supplementation in ameliorating disease activity in CD has been examined only in two open label pilot studies, and no studies have evaluated this in UC. Consequently, there is a urgent need for high quality randomized intervention trials of vitamin D supplementation in both CD and UC with disease activity as a treatment endpoint80, 81.
Clinical Practice
Patients with IBD are at risk of developing vitamin D deficiency. Recommendations from the Endocrine Clinical Practice Guidelines Committee recommend screening of patients with IBD as well as patients who are on corticosteroids for vitamin D status16. While there is lack of professional guidelines regarding subsequent assessments of vitamin D status, we adopt the following in our practice. If the baseline vitamin D status is normal, it may be logical to consider rechecking the status annually or biennially if there is active disease, if there is documented metabolic bone disease or if there is continued use of systemic corticosteroids. The Institute of Medicine and the Endocrine Practice Guidelines Committee recommend a dietary intake of 400 IU of vitamin D per day for infants, 600 IU of vitamin D per day for children beyond 1 year of age and adults and 800 IU of vitamin D per day for the elderly aged above 70 years82. However, to consistently raise the level of 25(OH)D to more than 30 ng/mL, especially in patients who are at risk for vitamin D deficiency, the Endocrine Practice Guidelines Committee recommended that a maintenance dose of at least 1000 IU per day and would be required16. To treat documented vitamin D deficiency, it is recommended to use either vitamin D2 or vitamin D3 in a dosage of 2000 IU per day for 6 weeks, or 50,000 IU once a week for 6 weeks in case of children and vitamin D2 or vitamin D3 6000 IU per day for 8 weeks, or 50,000 IU once a week for 8 weeks for adults to achieve serum 25(OH)D levels of more than 30 ng/mL. The optimal therapeutic regimen in IBD patients was examined in a single clinical trial by Pappa et al. in which 71 patients with IBD aged 5–21 years with vitamin D deficiency were randomized to one of the following three regimens for 6 weeks: 2,000 IU daily of vitamin D2, 2,000 IU daily of vitamin D3, or 50,000 IU weekly of vitamin D283. It was found that the 6 week regimens of 50,000 IU of vitamin D2 per week and 2,000 IU of vitamin D3 daily were superior to vitamin D2 2,000 IU daily. Whereas the regimen of 50,000 IU per week of vitamin D2 improved the serum 25(OH)D levels to more than 32 ng/mL in 75% of patients, only 38% of patients who received 2,000 IU of vitamin D3 daily and 25% of patients who received 2,000 IU of vitamin D2 daily achieved serum 25 (OH)D levels of more 32 ng/mL after 6 weeks of therapy. All the three regimens were found to be safe and well tolerated.
Future directions
Several unanswered question remain regarding the role of vitamin D in IBD (Table 4). Further investigation is needed to understand the effects of dietary intake of vitamin D and vitamin D supplementation in relation to polymorphisms of DBP or VDR to identify if there are subgroups who may derive greater benefit from prophylaxis or who would require greater doses for treatment. With recent evidence pointing towards vitamin D deficiency as associated with IBD risk, confirmation of such findings in other cohorts would establish the vitamin D as one of the links in the gene-environment-gut microbiome-immune system interactions necessary for the development of IBD. It also merits investigation whether vitamin D deficiency leads causally to increased disease severity or is merely a consequence of severe disease. Furthermore, it needs to be identified if there are high risk groups who may need to be screened for vitamin D deficiency and pre-emptively treated to prevent the onset of IBD. Further high quality studies are needed to evaluate if correction of vitamin D deficiency or if vitamin D supplementation can prevent disease relapses, whether it can be used to induce remission in active disease, and whether it has a role in prevention of long-term disease related complications like colorectal cancer as has been identified in non-IBD patients. Continued and fertile interactions between biochemists, nutritional epidemiologists, laboratory scientists, and clinical researchers will help address many of these unanswered questions, improve our understanding of the role of the complex panoply of functions of vitamin D, and its application into clinical practice.
Table 4.
Unanswered clinical questions regarding the role of vitamin D in inflammatory bowel diseases
|
Acknowledgments
Source of funding: Ananthakrishnan is supported in part by a grant from the National Institutes of Health (K23 DK097142). This work is also supported by the National Institutes of Health (NIH) (P30 DK043351) to the Center for Study of Inflammatory Bowel Diseases.
Footnotes
Financial Conflicts of Interest: Dr. Ananthakrishnan has served on the scientific advisory boards for Janssen, Prometheus, and Cubist pharmaceuticals
Specific author contributions: Mouli and Ananthakrishnan performed literature review; Mouli wrote the first draft of the manuscript; Ananthakrishnan provided supervision and both authors approved the final version of the manuscript.
References
- 1.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–78. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–17. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, Essers J, Mitrovic M, Ning K, Cleynen I, Theatre E, Spain SL, Raychaudhuri S, Goyette P, Wei Z, Abraham C, Achkar JP, Ahmad T, Amininejad L, Ananthakrishnan AN, Andersen V, Andrews JM, Baidoo L, Balschun T, Bampton PA, Bitton A, Boucher G, Brand S, Buning C, Cohain A, Cichon S, D’Amato M, De Jong D, Devaney KL, Dubinsky M, Edwards C, Ellinghaus D, Ferguson LR, Franchimont D, Fransen K, Gearry R, Georges M, Gieger C, Glas J, Haritunians T, Hart A, Hawkey C, Hedl M, Hu X, Karlsen TH, Kupcinskas L, Kugathasan S, Latiano A, Laukens D, Lawrance IC, Lees CW, Louis E, Mahy G, Mansfield J, Morgan AR, Mowat C, Newman W, Palmieri O, Ponsioen CY, Potocnik U, Prescott NJ, Regueiro M, Rotter JI, Russell RK, Sanderson JD, Sans M, Satsangi J, Schreiber S, Simms LA, Sventoraityte J, Targan SR, Taylor KD, Tremelling M, Verspaget HW, De Vos M, Wijmenga C, Wilson DC, Winkelmann J, Xavier RJ, Zeissig S, Zhang B, Zhang CK, Zhao H, Silverberg MS, Annese V, Hakonarson H, Brant SR, Radford-Smith G, Mathew CG, Rioux JD, Schadt EE, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–24. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ananthakrishnan AN. Environmental triggers for inflammatory bowel disease. Curr Gastroenterol Rep. 2013;15:302. doi: 10.1007/s11894-012-0302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 6.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80:1678S–88S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 7.Rosen CJ. Clinical practice. Vitamin D insufficiency. N Engl J Med. 2011;364:248–54. doi: 10.1056/NEJMcp1009570. [DOI] [PubMed] [Google Scholar]
- 8.Ananthakrishnan AN, Khalili H, Higuchi LM, Bao Y, Korzenik JR, Giovannucci EL, Richter JM, Fuchs CS, Chan AT. Higher predicted vitamin d status is associated with reduced risk of Crohn’s disease. Gastroenterology. 2012;142:482–9. doi: 10.1053/j.gastro.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cantorna MT, Mahon BD. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med (Maywood) 2004;229:1136–42. doi: 10.1177/153537020422901108. [DOI] [PubMed] [Google Scholar]
- 10.Cantorna MT, Zhu Y, Froicu M, Wittke A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr. 2004;80:1717S–20S. doi: 10.1093/ajcn/80.6.1717S. [DOI] [PubMed] [Google Scholar]
- 11.Garg M, Lubel JS, Sparrow MP, Holt SG, Gibson PR. Review article: vitamin D and inflammatory bowel disease - established concepts and future directions. Aliment Pharmacol Ther. 2012;36:324–44. doi: 10.1111/j.1365-2036.2012.05181.x. [DOI] [PubMed] [Google Scholar]
- 12.Jorgensen SP, Agnholt J, Glerup H, Lyhne S, Villadsen GE, Hvas CL, Bartels LE, Kelsen J, Christensen LA, Dahlerup JF. Clinical trial: vitamin D3 treatment in Crohn’s disease - a randomized double-blind placebo-controlled study. Aliment Pharmacol Ther. 2010;32:377–83. doi: 10.1111/j.1365-2036.2010.04355.x. [DOI] [PubMed] [Google Scholar]
- 13.Ananthakrishnan AN, Cagan A, Gainer VS, Cai T, Cheng SC, Savova G, Chen P, Szolovits P, Xia Z, De Jager PL, Shaw SY, Churchill S, Karlson EW, Kohane I, Plenge RM, Murphy SN, Liao KP. Normalization of Plasma 25-Hydroxy Vitamin D Is Associated with Reduced Risk of Surgery in Crohn’s Disease. Inflamm Bowel Dis. 2013;19:1921–7. doi: 10.1097/MIB.0b013e3182902ad9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr. 2008;88:491S–499S. doi: 10.1093/ajcn/88.2.491S. [DOI] [PubMed] [Google Scholar]
- 15.Bienaime F, Prie D, Friedlander G, Souberbielle JC. Vitamin D metabolism and activity in the parathyroid gland. Mol Cell Endocrinol. 2011;347:30–41. doi: 10.1016/j.mce.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 16.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 17.Levin AD, Wadhera V, Leach ST, Woodhead HJ, Lemberg DA, Mendoza-Cruz AC, Day AS. Vitamin D deficiency in children with inflammatory bowel disease. Dig Dis Sci. 2011;56:830–6. doi: 10.1007/s10620-010-1544-3. [DOI] [PubMed] [Google Scholar]
- 18.Alkhouri RH, Hashmi H, Baker RD, Gelfond D, Baker SS. Vitamin and mineral status in patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2013;56:89–92. doi: 10.1097/MPG.0b013e31826a105d. [DOI] [PubMed] [Google Scholar]
- 19.Ulitsky A, Ananthakrishnan AN, Naik A, Skaros S, Zadvornova Y, Binion DG, Issa M. Vitamin D deficiency in patients with inflammatory bowel disease: association with disease activity and quality of life. JPEN J Parenter Enteral Nutr. 2011;35:308–16. doi: 10.1177/0148607110381267. [DOI] [PubMed] [Google Scholar]
- 20.Leslie WD, Miller N, Rogala L, Bernstein CN. Vitamin D status and bone density in recently diagnosed inflammatory bowel disease: the Manitoba IBD Cohort Study. Am J Gastroenterol. 2008;103:1451–9. doi: 10.1111/j.1572-0241.2007.01753.x. [DOI] [PubMed] [Google Scholar]
- 21.Gilman J, Shanahan F, Cashman KD. Determinants of vitamin D status in adult Crohn’s disease patients, with particular emphasis on supplemental vitamin D use. Eur J Clin Nutr. 2006;60:889–96. doi: 10.1038/sj.ejcn.1602395. [DOI] [PubMed] [Google Scholar]
- 22.McCarthy D, Duggan P, O’Brien M, Kiely M, McCarthy J, Shanahan F, Cashman KD. Seasonality of vitamin D status and bone turnover in patients with Crohn’s disease. Aliment Pharmacol Ther. 2005;21:1073–83. doi: 10.1111/j.1365-2036.2005.02446.x. [DOI] [PubMed] [Google Scholar]
- 23.Vagianos K, Bector S, McConnell J, Bernstein CN. Nutrition assessment of patients with inflammatory bowel disease. JPEN J Parenter Enteral Nutr. 2007;31:311–9. doi: 10.1177/0148607107031004311. [DOI] [PubMed] [Google Scholar]
- 24.Harries AD, Brown R, Heatley RV, Williams LA, Woodhead S, Rhodes J. Vitamin D status in Crohn’s disease: association with nutrition and disease activity. Gut. 1985;26:1197–203. doi: 10.1136/gut.26.11.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tajika M, Matsuura A, Nakamura T, Suzuki T, Sawaki A, Kato T, Hara K, Ookubo K, Yamao K, Kato M, Muto Y. Risk factors for vitamin D deficiency in patients with Crohn’s disease. J Gastroenterol. 2004;39:527–33. doi: 10.1007/s00535-003-1338-x. [DOI] [PubMed] [Google Scholar]
- 26.Leichtmann GA, Bengoa JM, Bolt MJ, Sitrin MD. Intestinal absorption of cholecalciferol and 25-hydroxycholecalciferol in patients with both Crohn’s disease and intestinal resection. Am J Clin Nutr. 1991;54:548–52. doi: 10.1093/ajcn/54.3.548. [DOI] [PubMed] [Google Scholar]
- 27.Sentongo TA, Semaeo EJ, Stettler N, Piccoli DA, Stallings VA, Zemel BS. Vitamin D status in children, adolescents, and young adults with Crohn disease. Am J Clin Nutr. 2002;76:1077–81. doi: 10.1093/ajcn/76.5.1077. [DOI] [PubMed] [Google Scholar]
- 28.Vogelsang H, Schofl R, Tillinger W, Ferenci P, Gangl A. 25-hydroxyvitamin D absorption in patients with Crohn’s disease and with pancreatic insufficiency. Wien Klin Wochenschr. 1997;109:678–82. [PubMed] [Google Scholar]
- 29.Farraye FA, Nimitphong H, Stucchi A, Dendrinos K, Boulanger AB, Vijjeswarapu A, Tanennbaum A, Biancuzzo R, Chen TC, Holick MF. Use of a novel vitamin D bioavailability test demonstrates that vitamin D absorption is decreased in patients with quiescent Crohn’s disease. Inflamm Bowel Dis. 2011;17:2116–21. doi: 10.1002/ibd.21595. [DOI] [PubMed] [Google Scholar]
- 30.Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Koller DL, Peltonen L, Cooper JD, O’Reilly PF, Houston DK, Glazer NL, Vandenput L, Peacock M, Shi J, Rivadeneira F, McCarthy MI, Anneli P, de Boer IH, Mangino M, Kato B, Smyth DJ, Booth SL, Jacques PF, Burke GL, Goodarzi M, Cheung CL, Wolf M, Rice K, Goltzman D, Hidiroglou N, Ladouceur M, Wareham NJ, Hocking LJ, Hart D, Arden NK, Cooper C, Malik S, Fraser WD, Hartikainen AL, Zhai G, Macdonald HM, Forouhi NG, Loos RJ, Reid DM, Hakim A, Dennison E, Liu Y, Power C, Stevens HE, Jaana L, Vasan RS, Soranzo N, Bojunga J, Psaty BM, Lorentzon M, Foroud T, Harris TB, Hofman A, Jansson JO, Cauley JA, Uitterlinden AG, Gibson Q, Jarvelin MR, Karasik D, Siscovick DS, Econs MJ, Kritchevsky SB, Florez JC, Todd JA, Dupuis J, Hypponen E, Spector TD. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376:180–8. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim S, Shevde NK, Pike JW. 1,25-Dihydroxyvitamin D3 stimulates cyclic vitamin D receptor/retinoid X receptor DNA-binding, co-activator recruitment, and histone acetylation in intact osteoblasts. J Bone Miner Res. 2005;20:305–17. doi: 10.1359/JBMR.041112. [DOI] [PubMed] [Google Scholar]
- 32.McDonnell DP, Pike JW, O’Malley BW. The vitamin D receptor: a primitive steroid receptor related to thyroid hormone receptor. J Steroid Biochem. 1988;30:41–6. doi: 10.1016/0022-4731(88)90074-x. [DOI] [PubMed] [Google Scholar]
- 33.Cantorna MT, Mahon BD. D-hormone and the immune system. J Rheumatol Suppl. 2005;76:11–20. [PubMed] [Google Scholar]
- 34.Christakos S. Recent advances in our understanding of 1,25-dihydroxyvitamin D(3) regulation of intestinal calcium absorption. Arch Biochem Biophys. 2011;523:73–6. doi: 10.1016/j.abb.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takeda S, Yoshizawa T, Nagai Y, Yamato H, Fukumoto S, Sekine K, Kato S, Matsumoto T, Fujita T. Stimulation of osteoclast formation by 1,25-dihydroxyvitamin D requires its binding to vitamin D receptor (VDR) in osteoblastic cells: studies using VDR knockout mice. Endocrinology. 1999;140:1005–8. doi: 10.1210/endo.140.2.6673. [DOI] [PubMed] [Google Scholar]
- 36.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95:3597–602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22:477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- 38.Ardizzone S, Bollani S, Bettica P, Bevilacqua M, Molteni P, Bianchi Porro G. Altered bone metabolism in inflammatory bowel disease: there is a difference between Crohn’s disease and ulcerative colitis. J Intern Med. 2000;247:63–70. doi: 10.1046/j.1365-2796.2000.00582.x. [DOI] [PubMed] [Google Scholar]
- 39.Bernstein CN, Leslie WD. Review article: Osteoporosis and inflammatory bowel disease. Aliment Pharmacol Ther. 2004;19:941–52. doi: 10.1111/j.1365-2036.2004.01876.x. [DOI] [PubMed] [Google Scholar]
- 40.Siffledeen JS, Fedorak RN, Siminoski K, Jen H, Vaudan E, Abraham N, Seinhart H, Greenberg G. Bones and Crohn’s: risk factors associated with low bone mineral density in patients with Crohn’s disease. Inflamm Bowel Dis. 2004;10:220–8. doi: 10.1097/00054725-200405000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Reffitt DM, Meenan J, Sanderson JD, Jugdaohsingh R, Powell JJ, Thompson RP. Bone density improves with disease remission in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2003;15:1267–73. doi: 10.1097/00042737-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S, Wong T, Campagnuolo G, Moran E, Bogoch ER, Van G, Nguyen LT, Ohashi PS, Lacey DL, Fish E, Boyle WJ, Penninger JM. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304–9. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- 43.Redlich K, Hayer S, Ricci R, David JP, Tohidast-Akrad M, Kollias G, Steiner G, Smolen JS, Wagner EF, Schett G. Osteoclasts are essential for TNF-alpha-mediated joint destruction. J Clin Invest. 2002;110:1419–27. doi: 10.1172/JCI15582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jahnsen J, Falch JA, Mowinckel P, Aadland E. Vitamin D status, parathyroid hormone and bone mineral density in patients with inflammatory bowel disease. Scand J Gastroenterol. 2002;37:192–9. doi: 10.1080/003655202753416876. [DOI] [PubMed] [Google Scholar]
- 45.Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, Hewison M. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab. 2001;86:888–94. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- 46.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 47.Liu PT, Schenk M, Walker VP, Dempsey PW, Kanchanapoomi M, Wheelwright M, Vazirnia A, Zhang X, Steinmeyer A, Zugel U, Hollis BW, Cheng G, Modlin RL. Convergence of IL-1beta and VDR activation pathways in human TLR2/1-induced antimicrobial responses. PLoS One. 2009;4:e5810. doi: 10.1371/journal.pone.0005810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adams JS, Ren S, Liu PT, Chun RF, Lagishetty V, Gombart AF, Borregaard N, Modlin RL, Hewison M. Vitamin d-directed rheostatic regulation of monocyte antibacterial responses. J Immunol. 2009;182:4289–95. doi: 10.4049/jimmunol.0803736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuk JM, Shin DM, Lee HM, Yang CS, Jin HS, Kim KK, Lee ZW, Lee SH, Kim JM, Jo EK. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. 2009;6:231–43. doi: 10.1016/j.chom.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 50.Wu S, Sun J. Vitamin D, vitamin D receptor, and macroautophagy in inflammation and infection. Discov Med. 2011;11:325–35. [PMC free article] [PubMed] [Google Scholar]
- 51.Wang J, Lian H, Zhao Y, Kauss MA, Spindel S. Vitamin D3 induces autophagy of human myeloid leukemia cells. J Biol Chem. 2008;283:25596–605. doi: 10.1074/jbc.M801716200. [DOI] [PubMed] [Google Scholar]
- 52.Sly LM, Lopez M, Nauseef WM, Reiner NE. 1alpha,25-Dihydroxyvitamin D3-induced monocyte antimycobacterial activity is regulated by phosphatidylinositol 3-kinase and mediated by the NADPH-dependent phagocyte oxidase. J Biol Chem. 2001;276:35482–93. doi: 10.1074/jbc.M102876200. [DOI] [PubMed] [Google Scholar]
- 53.Liu PT, Modlin RL. Human macrophage host defense against Mycobacterium tuberculosis. Curr Opin Immunol. 2008;20:371–6. doi: 10.1016/j.coi.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 54.Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179:2060–3. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- 55.Arnedo-Pena A, Juan-Cerdan JV, Romeu-Garcia A, Garcia-Ferrer D, Holguin-Gomez R, Iborra-Millet J, Herrero-Carot C, Pinana MJ, Bellido-Blasco J, Ferrero-Vega JA, Adsuara LS, Silvestre ES, Ferrer NM, Bartual VR. Latent tuberculosis infection, tuberculin skin test and vitamin D status in contacts of tuberculosis patients: a cross-sectional and case-control study. BMC Infect Dis. 2011;11:349. doi: 10.1186/1471-2334-11-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ganmaa D, Giovannucci E, Bloom BR, Fawzi W, Burr W, Batbaatar D, Sumberzul N, Holick MF, Willett WC. Vitamin D, tuberculin skin test conversion, and latent tuberculosis in Mongolian school-age children: a randomized, double-blind, placebo-controlled feasibility trial. Am J Clin Nutr. 2012;96:391–6. doi: 10.3945/ajcn.112.034967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toss G, Symreng T. Delayed hypersensitivity response and vitamin D deficiency. Int J Vitam Nutr Res. 1983;53:27–31. [PubMed] [Google Scholar]
- 58.Zhang Y, Leung DY, Richers BN, Liu Y, Remigio LK, Riches DW, Goleva E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012;188:2127–35. doi: 10.4049/jimmunol.1102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brennan A, Katz DR, Nunn JD, Barker S, Hewison M, Fraher LJ, O’Riordan JL. Dendritic cells from human tissues express receptors for the immunoregulatory vitamin D3 metabolite, dihydroxycholecalciferol. Immunology. 1987;61:457–61. [PMC free article] [PubMed] [Google Scholar]
- 60.Adorini L, Penna G, Giarratana N, Uskokovic M. Tolerogenic dendritic cells induced by vitamin D receptor ligands enhance regulatory T cells inhibiting allograft rejection and autoimmune diseases. J Cell Biochem. 2003;88:227–33. doi: 10.1002/jcb.10340. [DOI] [PubMed] [Google Scholar]
- 61.Griffin MD, Lutz W, Phan VA, Bachman LA, McKean DJ, Kumar R. Dendritic cell modulation by 1alpha,25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci U S A. 2001;98:6800–5. doi: 10.1073/pnas.121172198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221:1181–3. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- 63.Lemire JM, Adams JS, Kermani-Arab V, Bakke AC, Sakai R, Jordan SC. 1,25-Dihydroxyvitamin D3 suppresses human T helper/inducer lymphocyte activity in vitro. J Immunol. 1985;134:3032–5. [PubMed] [Google Scholar]
- 64.Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007;179:1634–47. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 65.Khalili H, Huang ES, Ananthakrishnan AN, Higuchi L, Richter JM, Fuchs CS, Chan AT. Geographical variation and incidence of inflammatory bowel disease among US women. Gut. 2012;61:1686–92. doi: 10.1136/gutjnl-2011-301574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nerich V, Jantchou P, Boutron-Ruault MC, Monnet E, Weill A, Vanbockstael V, Auleley GR, Balaire C, Dubost P, Rican S, Allemand H, Carbonnel F. Low exposure to sunlight is a risk factor for Crohn’s disease. Aliment Pharmacol Ther. 2011;33:940–5. doi: 10.1111/j.1365-2036.2011.04601.x. [DOI] [PubMed] [Google Scholar]
- 67.Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, Bissonnette M, Li YC. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. 2008;294:G208–16. doi: 10.1152/ajpgi.00398.2007. [DOI] [PubMed] [Google Scholar]
- 68.Dresner-Pollak R, Ackerman Z, Eliakim R, Karban A, Chowers Y, Fidder HH. The BsmI vitamin D receptor gene polymorphism is associated with ulcerative colitis in Jewish Ashkenazi patients. Genet Test. 2004;8:417–20. doi: 10.1089/gte.2004.8.417. [DOI] [PubMed] [Google Scholar]
- 69.Naderi N, Farnood A, Habibi M, Derakhshan F, Balaii H, Motahari Z, Agah MR, Firouzi F, Rad MG, Aghazadeh R, Zojaji H, Zali MR. Association of vitamin D receptor gene polymorphisms in Iranian patients with inflammatory bowel disease. J Gastroenterol Hepatol. 2008;23:1816–22. doi: 10.1111/j.1440-1746.2008.05525.x. [DOI] [PubMed] [Google Scholar]
- 70.Simmons JD, Mullighan C, Welsh KI, Jewell DP. Vitamin D receptor gene polymorphism: association with Crohn’s disease susceptibility. Gut. 2000;47:211–4. doi: 10.1136/gut.47.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eloranta JJ, Wenger C, Mwinyi J, Hiller C, Gubler C, Vavricka SR, Fried M, Kullak-Ublick GA. Association of a common vitamin D-binding protein polymorphism with inflammatory bowel disease. Pharmacogenet Genomics. 2011;21:559–64. doi: 10.1097/FPC.0b013e328348f70c. [DOI] [PubMed] [Google Scholar]
- 72.Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr. 2000;130:2648–52. doi: 10.1093/jn/130.11.2648. [DOI] [PubMed] [Google Scholar]
- 73.Liu N, Nguyen L, Chun RF, Lagishetty V, Ren S, Wu S, Hollis B, DeLuca HF, Adams JS, Hewison M. Altered endocrine and autocrine metabolism of vitamin D in a mouse model of gastrointestinal inflammation. Endocrinology. 2008;149:4799–808. doi: 10.1210/en.2008-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu Y, Mahon BD, Froicu M, Cantorna MT. Calcium and 1 alpha,25-dihydroxyvitamin D3 target the TNF-alpha pathway to suppress experimental inflammatory bowel disease. Eur J Immunol. 2005;35:217–24. doi: 10.1002/eji.200425491. [DOI] [PubMed] [Google Scholar]
- 75.Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther. 2008;324:23–33. doi: 10.1124/jpet.107.127209. [DOI] [PubMed] [Google Scholar]
- 76.El-Matary W, Sikora S, Spady D. Bone mineral density, vitamin D, and disease activity in children newly diagnosed with inflammatory bowel disease. Dig Dis Sci. 2011;56:825–9. doi: 10.1007/s10620-010-1380-5. [DOI] [PubMed] [Google Scholar]
- 77.Narula N, Marshall JK. Management of inflammatory bowel disease with vitamin D: beyond bone health. J Crohns Colitis. 2012;6:397–404. doi: 10.1016/j.crohns.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 78.Verlinden L, Leyssens C, Beullens I, Marcelis S, Mathieu C, De Clercq P, Verstuyf A. The vitamin D analog TX527 ameliorates disease symptoms in a chemically induced model of inflammatory bowel disease. J Steroid Biochem Mol Biol. 2013;136:107–11. doi: 10.1016/j.jsbmb.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 79.Zator ZA, Cantu SM, Konijeti GG, Nguyen DD, Sauk J, Yajnik V. Ananthakrishnan AN. Pre-treatment 25-hydroxy vitamin D levels and durability of anti-tumor necrosis factor α therapy in Inflammatory Bowel Diseases. Journal of Parenteral and Enteral Nutrition. doi: 10.1177/0148607113504002. (in press) [DOI] [PubMed] [Google Scholar]
- 80.Miheller P, Muzes G, Hritz I, Lakatos G, Pregun I, Lakatos PL, Herszenyi L, Tulassay Z. Comparison of the effects of 1,25 dihydroxyvitamin D and 25 hydroxyvitamin D on bone pathology and disease activity in Crohn’s disease patients. Inflamm Bowel Dis. 2009;15:1656–62. doi: 10.1002/ibd.20947. [DOI] [PubMed] [Google Scholar]
- 81.Yang L, Weaver V, Smith JP, Bingaman S, Hartman TJ, Cantorna MT. Therapeutic effect of vitamin d supplementation in a pilot study of Crohn’s patients. Clin Transl Gastroenterol. 2013;4:e33. doi: 10.1038/ctg.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–8. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pappa HM, Mitchell PD, Jiang H, Kassiff S, Filip-Dhima R, DiFabio D, Quinn N, Lawton RC, Varvaris M, Van Straaten S, Gordon CM. Treatment of vitamin D insufficiency in children and adolescents with inflammatory bowel disease: a randomized clinical trial comparing three regimens. J Clin Endocrinol Metab. 2012;97:2134–42. doi: 10.1210/jc.2011-3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Driscoll RH, Jr, Meredith SC, Sitrin M, Rosenberg IH. Vitamin D deficiency and bone disease in patients with Crohn’s disease. Gastroenterology. 1982;83:1252–8. [PubMed] [Google Scholar]
- 85.Lamb EJ, Wong T, Smith DJ, Simpson DE, Coakley AJ, Moniz C, Muller AF. Metabolic bone disease is present at diagnosis in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2002;16:1895–902. doi: 10.1046/j.1365-2036.2002.01363.x. [DOI] [PubMed] [Google Scholar]
- 86.Siffledeen JS, Siminoski K, Steinhart H, Greenberg G, Fedorak RN. The frequency of vitamin D deficiency in adults with Crohn’s disease. Can J Gastroenterol. 2003;17:473–8. doi: 10.1155/2003/391308. [DOI] [PubMed] [Google Scholar]
- 87.Pappa HM, Gordon CM, Saslowsky TM, Zholudev A, Horr B, Shih MC, Grand RJ. Vitamin D status in children and young adults with inflammatory bowel disease. Pediatrics. 2006;118:1950–61. doi: 10.1542/peds.2006-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kuwabara A, Tanaka K, Tsugawa N, Nakase H, Tsuji H, Shide K, Kamao M, Chiba T, Inagaki N, Okano T, Kido S. High prevalence of vitamin K and D deficiency and decreased BMD in inflammatory bowel disease. Osteoporos Int. 2009;20:935–42. doi: 10.1007/s00198-008-0764-2. [DOI] [PubMed] [Google Scholar]
- 89.Joseph AJ, George B, Pulimood AB, Seshadri MS, Chacko A. 25 (OH) vitamin D level in Crohn’s disease: association with sun exposure & disease activity. Indian J Med Res. 2009;130:133–7. [PubMed] [Google Scholar]
- 90.Pappa HM, Langereis EJ, Grand RJ, Gordon CM. Prevalence and risk factors for hypovitaminosis D in young patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2011;53:361–4. doi: 10.1097/MPG.0b013e3182250b3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Atia A, Murthy R, Bailey BA, Manning T, Garrett LL, Youssef D, Peiris AN. Vitamin D status in veterans with inflammatory bowel disease: relationship to health care costs and services. Mil Med. 2011;176:711–4. doi: 10.7205/milmed-d-10-00371. [DOI] [PubMed] [Google Scholar]
- 92.Suibhne TN, Cox G, Healy M, O’Morain C, O’Sullivan M. Vitamin D deficiency in Crohn’s disease: prevalence, risk factors and supplement use in an outpatient setting. J Crohns Colitis. 2012;6:182–8. doi: 10.1016/j.crohns.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 93.Fu YT, Chatur N, Cheong-Lee C, Salh B. Hypovitaminosis D in adults with inflammatory bowel disease: potential role of ethnicity. Dig Dis Sci. 2012;57:2144–8. doi: 10.1007/s10620-012-2130-7. [DOI] [PubMed] [Google Scholar]
- 94.Laakso S, Valta H, Verkasalo M, Toiviainen-Salo S, Viljakainen H, Makitie O. Impaired bone health in inflammatory bowel disease: a case-control study in 80 pediatric patients. Calcif Tissue Int. 2012;91:121–30. doi: 10.1007/s00223-012-9617-2. [DOI] [PubMed] [Google Scholar]
- 95.Hassan V, Hassan S, Seyed-Javad P, Ahmad K, Asieh H, Maryam S, Farid F, Siavash A. Association between Serum 25 (OH) Vitamin D Concentrations and Inflammatory Bowel Diseases (IBDs) Activity. Med J Malaysia. 2013;68:34–8. [PubMed] [Google Scholar]