Abstract
Innate and adaptive immune responses can speed nigrostriatal neurodegeneration in Parkinson’s disease (PD). We posit that GM-CSF can attenuate such responses. In 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) intoxicated mice, GM-CSF given prior to MPTP protected nigral dopaminergic neurons coincident with altered microglial morphologies and regulatory T cell (Treg) induction. Adoptive transfer of GM-CSF-induced Treg to MPTP mice protected nigral neurons and their striatal termini. Gene expression analyses revealed novel immune-based neuronal protection pathways. The results provide evidence that GM-CSF modulation of immunity could be of clinical benefit for PD.
Keywords: Parkinson’s disease; neuroprotection; regulatory T cells; innate immunity; microglia; 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; neuroinflammation
1. Introduction
Parkinson’s disease (PD) is second only to Alzheimer’s disease (AD) as the most common neurodegenerative condition. There are upwards of 1 million cases in the United States alone (Olanow et al., 2009). Thus, therapies that affect neural repair have commanded significant current attention in recent years in the face of significant rises in disease prevalence (Benner et al., 2004, Laurie et al., 2007, Reynolds et al., 2007, Nie et al., 2013, Shrivastava et al., 2013). A therapeutic strategy gaining increasing interest is one that interdicts PD-associated immunity, a pathobiologic disease component linked to neuronal injury that governs the tempo of progressive movement and clinical posture deterioration. This idea is supported by both animal and human studies that have uncovered substantive changes in both innate and adaptive immunity during disease (Kurkowska-Jastrzebska et al., 1999, Reynolds et al., 2008, Brochard et al., 2009, Reynolds et al., 2010, Iannaccone et al., 2013). Notably, innate immunity leads to the release of neurotoxic products, including reactive oxygen species, pro-inflammatory cytokines and chemokines, excitotoxins and arachidonic acid metabolites; all possessing the potential to singularly affect dopaminergic neuronal function and death (McGeer et al., 1988, Damier et al., 1993, Liberatore et al., 1999, Benner et al., 2008, Reynolds et al., 2009b). Among adaptive immune changes, increased presence of T effector memory cells (Tem) and dysfunctional regulatory T cells (Treg) are seen during PD progression (Hutter Saunders et al., 2012). Similar altered profiles of effector T cell (Teff) and dysregulated Treg are seen in a range of disorders that include multiple sclerosis, stroke, Crohn’s disease, type 1 diabetes, rheumatoid arthritis, and myasthenia gravis (Balandina et al., 2005, Fletcher et al., 2009, Ishikawa et al., 2013, Nie et al., 2013, Schwarz et al., 2013). Based on these data, we reasoned that the induction of Treg could correct such immune aberrations. To this end, we sought to restore a homeostatic immune environment by administration of a drug targeted to expand Treg and positively affect PD pathobiology (Li et al., 2012, Marek-Trzonkowska et al., 2013, Mayne and Williams, 2013, Safinia et al., 2013).
Anti-inflammatory agents have been used to test the idea that altered immunity in PD, if properly controlled, could reduce signs and symptoms of disease. For example, in a recent clinical study, ibuprofen elicited decreased risks for PD (Gao et al., 2011). Based on such clinical outcomes, we reasoned that increasing Treg numbers and function could have similar and perhaps even stronger clinical potential through the cell’s immune modulatory activities. With this directive in mind, we tested granulocyte macrophage colony stimulating factor (GM-CSF, termed Leukine or sargramostim) for its potential to halt the progression of nigrostriatal degeneration. GM-CSF has been safely used for decades in the clinic to mobilize myeloid progenitor cells in immunosuppressed patients following chemotherapy or transplant (Buchsel et al., 2002). However, between its initial description in the 1960s and the present, it has become increasingly clear that this agent has more far-reaching activity. Aside from its immune stimulatory activities, it has been shown to induce Treg (Sheng et al., 2011, Rowin et al., 2012). It has also shown efficacy in trials for Crohn’s disease in which GM-CSF was able to reduce disease severity (Roth et al., 2009, Wong 2011). In the CNS, GM-CSF is produced by resident astroglia and T cells, and can also cross the blood-brain and blood-spinal cord barriers where it engages receptors on both immune and neural cells (McLay et al., 1997). GM-CSF affects neuronal plasticity, learning, and memory, and has neurotrophic and anti-apoptotic activities for photoreceptors (Huang et al., 2007, Kim et al., 2012, Krieger et al., 2012, Schallenberg et al., 2012, Kim et al., 2013). In CNS disease states, it is involved in recovery and control of cell death following spinal cord injury (Ha et al., 2005). Interestingly, GM-CSF is implicated in the prevention of Alzheimer’s disease in rheumatoid arthritis patients as well as animal models of AD and is currently approved in a clinical trial for its potential to reduce dementia (McGeer et al. 1990, Boyd et al., 2010).
Importantly, GM-CSF has been shown to be neuroprotective in PD models, including MPTP, 6-hydroxydopamine (OHDA), and paraquat, by decreasing the proteins associated with neuronal apoptosis, Bcl-2 and Bax, and inducing brain derived neurotrophic factor (BDNF) (Kim et al., 2009, Choudhury et al., 2011, Mangano et al., 2011). These findings benefit PD patients administered GM-CSF and may provide an additional mechanism to interdict disease progression, but they provide a poor clinically measurable outcome. PD is not diagnosed until 50–60% of dopaminergic neurons are already lost, from diagnosis the disease progresses differently in each individual, and there are no clinical tests designed to measure neuronal loss. Therefore, we sought to find a measurable signature of GM-CSF treatment that is correlated to PD progression.
Thus, we investigated the link between the neuroprotective potential of GM-CSF and its immune modulatory capability in a PD model. We demonstrate that GM-CSF protects dopaminergic neurons from 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced death, and that this protection is facilitated by modulation of both innate and adaptive immunity that includes the increased Treg regulation of neuroinflammation. Our data identifies GM-CSF as a candidate for clinical translation for PD neuroprotection based on its broad immune regulatory activities.
2. Materials and Methods
Animals, drug treatment, and MPTP intoxication
Male C57Bl6/J mice, 5 wk old (The Jackson Laboratory, Bar Harbor, ME) were used as donor mice in all studies with the exception of laser capture microdissection (LCM) studies. Donor and pretreated mice were administered recombinant mouse GM-CSF (Peprotech, Rocky Hill, NJ) reconstituted in sterile Dulbecco’s PBS (DPBS), at a dosage of 50 μg/kg body weight, i.p., daily for 5 days unless otherwise specified. Recipient mice, C57Bl6/J mice, 5 wk old (The Jackson Laboratory), received four s.c. injections of either vehicle (DPBS, 10 ml/kg body weight) or MPTP-HCl (Sigma-Aldrich, St. Louis, MO) at 16 mg MPTP (free base)/kg body weight in DPBS; each injection was given at 2-h intervals. Twelve hours after the last injection of MPTP, isolated CD4+ T cells or Treg from donors were adoptively transferred to MPTP-intoxicated recipient mice (n = 5–8 mice per group per time point). On days 2 and 6 after MPTP, mice were sacrificed and brains processed for analysis. In vivo experiments were repeated 3 times. All animal procedures were in accordance with National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center. MPTP safety measures were in accordance of published guidelines (Jackson-Lewis and Przedborski, 2007).
Isolation and adoptive transfer of CD4+ T cells
On the sixth day following GM-CSF administration, donor mice were sacrificed and single-cell suspensions obtained from spleen and lymph nodes (brachial, axillary, inguinal) by passing tissues through a 70 μm cell strainer (Fisher Scientific). CD4+ T cells were negatively selected using CD4+ T cell Isolation Kit II, mouse, as per manufacturer’s instructions (Miltenyi Biotech, Auburn, CA). Treg were isolated using the CD4+CD25+ Regulatory T cell Isolation kit, mouse (Miltenyi Biotech). Following isolation, samples were labeled with anti-mouse CD4 PE-Cyanine 7 and anti-mouse CD25 PE (eBioscience, San Diego, CA). Cells were permeabilized and labeled with anti-mouse FoxP3 APC using Anti-Mouse/Rat FoxP3 staining kit APC (eBioscience). CD4+ populations were >94% CD4+ and Treg were >90% CD4+CD25+FoxP3+. These populations were resuspended in DBPS at 40 × 106 cells/ml for CD4+ cells and 4 × 106 cells/ml for Treg. Recipient mice received i.v. tail injections of 0.25 ml from the freshly isolated and appropriate cell suspension (10 × 106 CD4+ cells or 1 × 106 Treg) within 12 h of the final MPTP dose.
Flow cytometry
Fluorescently labeled cell fractions were analyzed by a FACSCalibur flow cytometer and FACSDiva software v6.0 (BD Biosciences, San Jose, CA) as previously reported (Reynolds et al.).
Immunohistochemistry
Under terminal anesthesia, mice were transcardially perfused with DPBS followed by 4% paraformaldehyde (Sigma-Aldrich) in DPBS. To assess dopaminergic neurons in the SN, frozen midbrain sections (30 μm) were immunostained for tyrosine hydroxylase (TH) (anti-TH, 1:2000, Calbiochem, San Diego, CA) and counterstained for Nissl substance by thionin staining (Benner et al., 2004). To assess microglia within the SN, midbrain sections were immunostained for Mac-1 (anti-CD11b, 1:1000, Abd Serotech, Raleigh, NC). To assess dopaminergic termini, striatal sections (30 μm) were labeled with anti-tyrosine hydroxylase (anti-TH, 1:1000, Calbiochem). To visualize antibody-labeled tissues, sections were incubated in streptavidin-HRP solution (ABC Elite Vector Kit, Vector Laboratories, Burlingame, CA) and color developed using an H2O2 generation system and diaminobenzidine (DAB) chromogen (Sigma-Aldrich) as described (Benner et al., 2008). Within the SN, total numbers of Mac-1+ cells, TH+Nissl+ (dopaminergic neurons), and TH−Nissl+ (non-dopaminergic neurons) were estimated by stereological analysis with Stereo Investigator software (MBF Bioscicence, Williston, VT) using the optical fractionator module. Density of dopaminergic neuron termini in the striatum were determined from scanned TH+ sections by digital densitometry using Image J software (National Institutes of Health, Bethesda, MD).
Laser capture microdissection, RNA isolations and polymerase chain reaction
Transgenic TH-GFP/21-31 recipient mice (7 week old males) were obtained from Dr. Kazuto Kobayashi, Fukushima Medical University School of Medicine, Fukushima, Japan and were maintained on the C57Bl6/J background at our facility. These mice express GFP under the control of the rat TH promoter (Matsushita et al., 2002); thus all TH-expressing dopaminergic neurons also expressed GFP. MPTP-intoxicated mice were treated with PBS, GM-CSF, or with T cells by adoptive transfer. All procedures were carried out under RNAse-free conditions. Two days after MPTP-intoxication, brains were removed and immediately snap-frozen in dry ice-cooled 2-methylbutane. Midbrain sections (30 μm) were collected onto polyethylene terephthalate (PET) steel frame slides (Leica Microsystems, Wetzlar, Germany). Sections were excited with the GFP/Cy3 filter; the resulting fluorescence was used to demarcate, cut, and capture the SN, using a laser microdissection system (LMD6500, Leica Microsystems). Laser dissected tissues were collected by gravity into Buffer RLT lysis buffer (Qiagen, Germantown, MD) containing 10% β-mercaptoethanol. For each midbrain, 8–10 sections were collected and RNA was isolated immediately using RNEasy Plus Micro kit (Qiagen). RNA integrity numbers (RIN) for usable RNA samples were >8 as determined by the RNA 6000 Pico kit and evaluated on the 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). cDNA was generated from high-quality RNA using the RT2 PreAMP cDNA Synthesis Kit (Qiagen) and pre-amplification was performed using the appropriate primer mixes for each array used: Mouse Inflammatory Cytokines and Receptors and Mouse Th1-Th2-Th3 (Qiagen). Quantitative RT-PCR was performed on an Eppendorf Mastercycler realplex ep following the manufacturer’s instructions (Eppendorf, Hamburg, Germany). Data analysis was performed using RT2 Profiler PCR Array web-based data analysis software (Qiagen).
Statistical Analyses
All values are expressed as mean ± SD. Differences in between-group means were analyzed by one-way ANOVA, followed by the Bonferroni correction post-hoc test for multiple comparisons (GraphPad Software, Inc., La Jolla, CA).
3. Results
Neuroprotective responses of GM-CSF
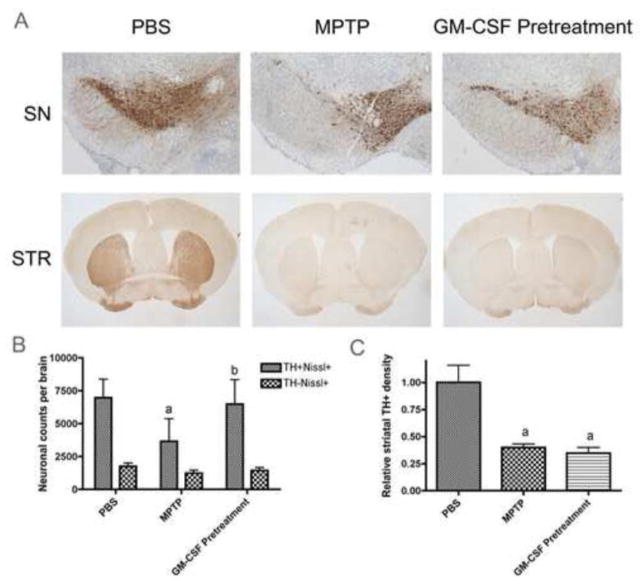
GM-CSF is a known inducer of both innate and adaptive immunity, and has also shown neuroprotective abilities in MPTP-induced dopaminergic neuronal injury (Henze et al., 2005, Kim et al., 2009, Sheng et al., 2011, Zou et al., 2011). To confirm and extend these observations, we first administered GM-CSF daily for 5 days prior to acute MPTP intoxication. Immunohistochemical (IHC) examination for TH expression in the midbrain and striatum of animals six days after MPTP exposure were assessed to determine nigral dopaminergic neuronal cell density and striatal fiber density (dopaminergic termini) (Fig. 1A). Following MPTP treatment, the total number of dopaminergic neurons (TH+Nissl+) in the SN as measured by blinded stereological analysis were decreased from 6912 ± 1940 to 3653 ± 1725 (Fig. 1B). This MPTP-induced neuronal loss was attenuated, in part, by GM-CSF pretreatment, as 6169 ± 2085 total TH+Nissl+ neurons were counted in GM-CSF- and MPTP-intoxicated animals. Numbers of non-dopaminergic neurons (TH−Nissl+) were unaffected by either MPTP or GM-CSF treatment. The presence of striatal fiber density assessed by TH densitometry showed no change following GM-CSF treatment (Fig. 1C).
Figure 1.
GM-CSF pretreatment is neuroprotective in vivo. Mice were treated with PBS, MPTP (16 mg/kg × 4 doses), or pretreated with 50 μg GM-CSF/kg for 5 days followed by MPTP and assessed by IHC on 6 d after MPTP intoxication. Sections were stained with anti-TH Ab and HRP-conjugated secondary Ab and visualized with DAB. SN sections were counterstained with thionin. A. Photomicrographs of TH+Nissl+ neurons in the substantia nigra (SN) and TH+ termini in the striatum (STR). B. Total numbers of dopaminergic neurons (TH+Nissl+) and non-dopaminergic neurons (TH−Nissl+) in the SN. C. TH densitometry of total dopaminergic termini within the striatum. B and C, Differences in mean ± SD (n = 8 per group) were determined where p < 0.05 compared with groups treated with aPBS, bMPTP, or cGM-CSF pretreatment and MPTP (GMCSF Pretreatment).
GM-CSF and MPTP metabolism
Since GM-CSF readily crosses the BBB where the pro-toxin MPTP is converted to the active toxin MPP+, we first sought to rule out that the neuroprotective effects of GM-CSF found here and in other studies (Henze et al., 2005, Kim et al., 2009, Sheng et al., 2011, Zou et al., 2011) were due to interference with the conversion of MPTP to MPP+. Thus, we compared levels of MPTP and MPP+ in the midbrains and striata of animals treated with MPTP and those pretreated with GM-CSF prior to MPTP. Reverse phase-HPLC analysis with UV detection showed no significant differences in the levels of MPTP or MPP+ in either midbrain (Fig, 2A) or striatum (Fig. 2B) of animals treated with MPTP alone or GM-CSF and MPTP. These data strengthen our and others’ results describing the neuroprotective potential of GM-CSF since those effects can be accurately ascribed to mechanisms outside the possibility of MPTP metabolism interference.
Figure 2.
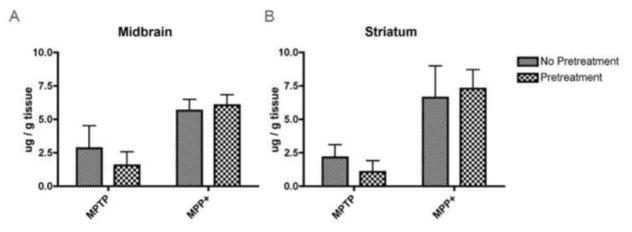
GM-CSF administration does not inhibit the metabolism of MPTP to MPP+. RP-HPLC and UV detection were used to determine the levels of MPTP and MPP+ in the midbrains (A) and striata (B) of mice pretreated with 50 μg GM-CSF/kg for 5 days (Pretreatment) or not (No Pretreatment). All mice were treated with 4 doses of 16 mg/kg MPTP and midbrains and striata were dissected within 90 min of the last MPTP injection. Differences in mean ± SD (n = 5 per group) were determined where p < 0.05 compared with groups treated with MPTP alone or pretreated with GM-CSF and MPTP.
Microglial morphology and GM-CSF neuroprotection
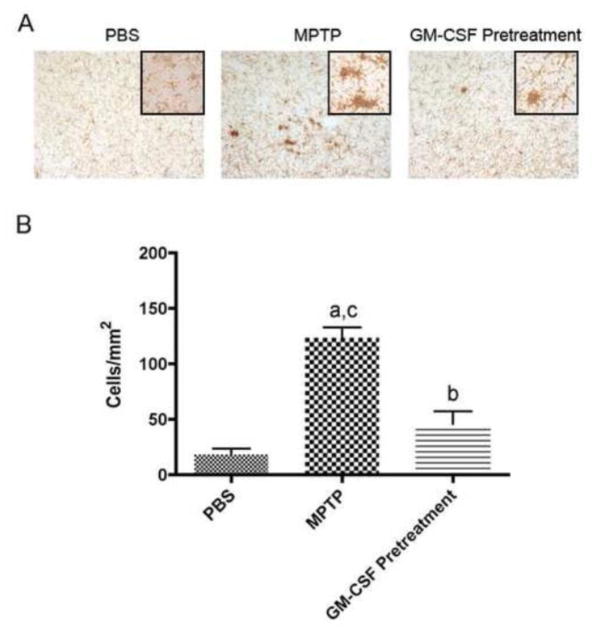
Correlations between microglial morphology/activation and dopaminergic neuronal integrity are well-known (Kurkowska-Jastrzebska et al., 1999, Reynolds et al., 2010). We next sought to assess potential immune mechanisms that underlie the observed neuroprotective effects. To this end, we investigated whether GM-CSF-induced neuroprotective responses were linked to changes in microglial responses. In animals pretreated with GM-CSF, microglial responses were evaluated two days following MPTP intoxication. This time point was chosen as it represented the time of maximal MPTP-induced inflammatory responses (Kurkowska-Jastrzebska et al., 1999). Anti-Mac-1 stained microglia, and changes in cell morphology, namely larger cell bodies and reductions in ramified processes, reflect cell activation (Fig. 3A). Notably, MPTP treatment increased the density of activated microglia (microgliosis) in the SN to 123.5 ± 21.2 cells/mm2 compared to 18.3 ± 10.8 cells/mm2 for PBS-treated controls (Fig. 3B). GM-CSF pretreatment reduced microgliosis to 45.8 ± 22.9 cells/mm2, indicating that GM-CSF pretreatment reduced microglial activation and this response may also affect neuronal survival even though the protective mechanism(s) originate in the periphery.
Figure 3.
GM-CSF attenuates innate immune activation. Photomicrographs and enumeration of anti-CD11b+ microglia in the SN of mice treated with PBS, MPTP (16 mg/kg × 4 doses), or pretreated with 50 μg GM-CSF/kg followed by MPTP A. Midbrains from mice 2 d after MPTP treatment stained with anti-Mac-1 Ab, HRP-conjugated secondary Ab, and visualized with DAB. Activated microglia are identified as those stained cells exhibiting large amoeboid cell bodies and shortened processes. B. Differences in mean ± SD (n = 5 per group) were determined where p < 0.05 compared with groups treated with aPBS, bMPTP, or cGM-CSF pretreated and MPTP (GMCSF Pretreatment).
GM-CSF modulates adaptive immune responses
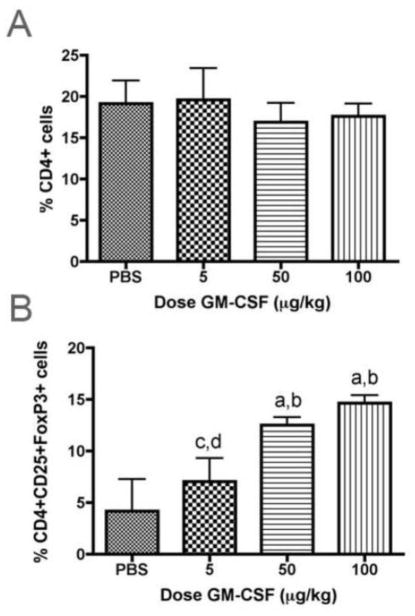
We next sought to assess the pathways involved in GM-CSF neuroprotective activities. Based on a wealth of prior data from our laboratory implicating specific T cell subsets, namely Treg, with attenuation of microgliosis and subsequent neuroprotective responses following MPTP intoxication (Reynolds et al., 2007, Reynolds et al., 2009b, a, Reynolds et al., 2010), we examined whether these were also operative in the anti-inflammatory and neuroprotective responses induced by GM-CSF. Thus, we first assessed the ability of GM-CSF to induce Treg in response to escalating doses (5, 50, and 100 μg/kg) administered daily for 5 days. We initially determined that GM-CSF at any dose did not significantly affect the frequencies of CD4+ spleen cells compared to PBS-treated controls (Fig 4A). In contrast, frequencies of CD25+FoxP3+ Treg within the CD4+ splenocyte population increased with the dose of GM-CSF (r2 = 0.91, p = 0.04) (Fig 4B). Moreover, we observed significant increases in the proportion of Treg at the 50 and 100 μg/kg doses of GM-CSF compared to PBS controls and those treated with the lowest dose of GM-CSF (Fig. 4B). Based on these results, we posited that GM-CSF adaptive immune modulation could be the bridge between decreased microgliosis and reduced neurodegeneration.
Figure 4.
GM-CSF expands Treg upon in vivo administration. Mice were treated for 5 days with PBS or escalating doses of GM-CSF and spleen cells assayed by flow cytometric analysis. A. Mean percentages of CD4+ cells within the lymphocyte gate. B. Mean percentages of Treg, defined as cells within the CD4+ gate also co-expressing CD25 and FoxP3 as analyzed via flow cytometry. Linear regression analysis of percentage of Treg as a function of dose indicated r2 = 0.9146 and p = 0.0437. A and B. Differences in mean ± SD (n = 5 mice per group) were determined where p < 0.05 compared with groups treated with aPBS, b5 μg/kg GM-CSF, c50 μg/kg GM-CSF, or d100 μg/kg GM-CSF.
GM-CSF induced Treg and neuroprotection
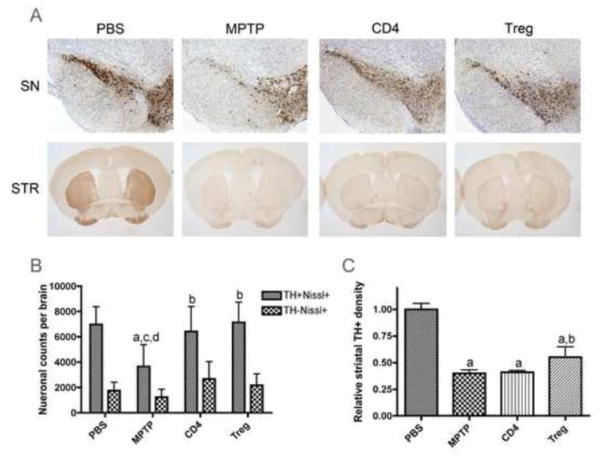
We next investigated the potential neuroprotective activities of T cell subsets from GM-CSF-treated animals after adoptive transfer to MPTP-intoxicated recipients. T cells were isolated from donor mice treated with GM-CSF at 50 μg/kg every day for 5 days, magnetically sorted into CD4+ and CD4+CD25+FoxP3+ Treg populations, and adoptively transferred into MPTP-intoxicated mice within 8–12 h after MPTP-intoxication. The number of surviving nigral neurons and striatal termini were determined 6 days after MPTP intoxication (Fig. 5A). CD4+ T cells were enriched to 94% CD4+ and contained 12% CD4+CD25+FoxP3+ cells, while Treg were enriched to 91% CD4+CD25+FoxP3+ cells (data not shown). These experiments show both CD4+ and Treg subsets provided virtually total neuroprotection for dopaminergic neurons (TH+Nissl+), resulting in numbers of neurons in the SN that were not significantly different than those of PBS-treated controls (Fig. 5B). Additionally, numbers of non-dopaminergic neurons (TH−Nissl+) were unaffected by either MPTP or T cell transfer (Fig. 5B). Interestingly, the group of MPTP mice receiving Treg by adoptive transfer also displayed significant, albeit slight protection of neuronal termini within the striata as assessed by densitometric analysis (Fig. 5C). Together, these results suggest that GM-CSF affects both innate and adaptive immunity to afford neuroprotective responses in this model of PD.
Figure 5.
GM-CSF neuroprotection is mediated via adaptive immunity. CD4+ spleen cells and Treg for adoptive transfer were enriched from spleens of donor mice treated with 5 daily doses of 50 μg GM-CSF/kg. Recipient mice were treated with PBS, 4 doses of 16 mg/kg MPTP alone, MPTP and CD4+ T cells (CD4), or MPTP and Treg. Tissues were obtained on day 7 after MPTP intoxication. Sections were stained with anti-TH Ab and HRP-conjugated secondary Ab, and were visualized with DAB. SN sections were counterstained with thionin. A. TH+ neurons in the SN and striatal (STR) termini of mice. B. Total numbers of dopaminergic neurons (TH+Nissl+) and non-dopaminergic neurons (TH−Nissl+) in the SN. C. TH densitometry of dopaminergic termini within the striata. B and C, Differences in mean ± SD (n = 8 per group) were determined where p < 0.05 compared with groups treated with aPBS, bMPTP, cMPTP and CD4+ cells (CD4), or dMPTP and Treg.
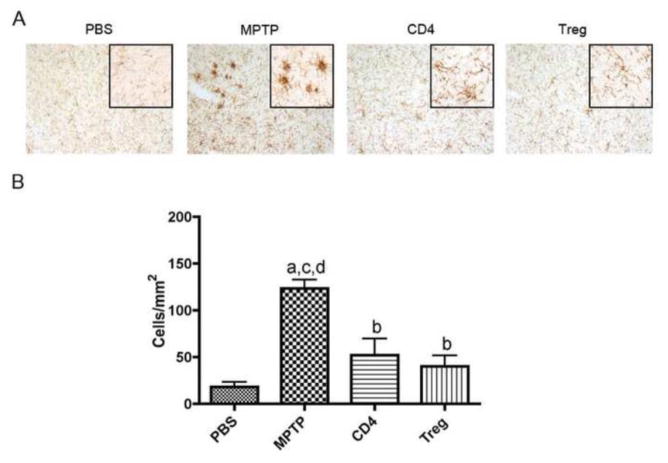
Microglial morphology and adaptive immunity
We next determined the capability of GM-CSF induced Treg to control innate immunity. Following MPTP intoxication, Mac-1+ microglia exhibited an activated and inflammatory cell morphology by IHC, while adoptive transfer of CD4+ T cells or Treg from GM-CSF treated mice attenuated much of the inflammatory response (Fig. 6A). Indeed, analysis of activated microglia densities showed that MPTP induced a 6.7-fold increase in numbers of activated microglia compared to PBS-treated control, while adoptive transfer of CD4+ T cells or Treg, both from GM-CSF treated mice, diminished reactive microglia densities by 58% and 67%, respectively (Fig. 6B). These results suggest complimentary mechanisms between drug administration and adaptive immune modulation following GM-CSF treatment.
Figure 6.
GM-CSF adaptive immune modulation attenuates innate immune activation. Photomicrographs and enumeration of anti-CD11b+ microglia in the SN of mice treated with PBS, 4 doses of 16 mg MPTP/kg alone, and MPTP mice receiving donor spleen cells enriched for either CD4+ T cells or Treg from mice treated with 5 daily GM-CSF doses of 50 μg/kg. Midbrain tissues were obtained 2 days after MPTP-intoxication. A. Midbrain sections stained with anti-CD11b Ab, HRP-conjugated secondary Ab, and visualized with DAB. Activated microglia are identified as those stained cells exhibiting large amoeboid cell bodies and shortened processes. B. Differences in mean ± SD (n = 5 per group) were determined where p < 0.05 compared with groups treated with aPBS, bMPTP alone, and MPTP intoxicated receiving either cCD4+ T cells (CD4) or dTreg.
Mechanisms of GM-CSF neuroprotective activities
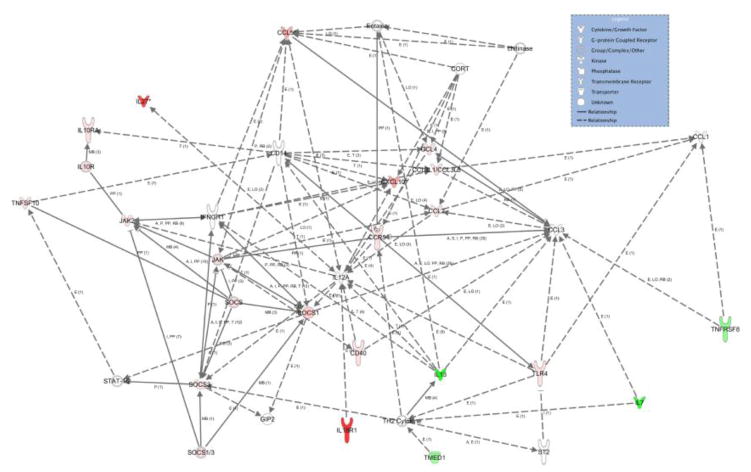
To examine possible mechanisms by which GM-CSF or GM-CSF induced Treg affect neuroprotection, we investigated inflammatory gene expression changes within the SN that may be reflective of innate and adaptive immune modulation. For these experiments, we treated male TH-GFP/21-31 mice with MPTP alone, with 50 μg/kg GM-CSF 5 days before MPTP, or with GM-CSF induced Treg 8–12 hours after MPTP. Midbrains were removed 2 days after MPTP-intoxication, snap frozen, and sectioned. Since all dopaminergic neurons express GFP, the SN was dissected and collected by LCM (Fig. 7A), mRNA was isolated and cDNA was synthesized and pre-amplified for 40 cycles. Quantitative PCR analyses were performed using arrays for the expression of genes associated with both inflammation and T cell subsets. Expression of genes from animals treated with GM-CSF prior to MPTP or treated with MPTP followed by adoptive transfer of GM-CSF induced Treg were compared to those from animals treated with MPTP alone. All significant fold changes in mRNA expression are listed in Figure 7B. Genes that were highly changed (fold change > 5) in both groups included Il18r1, IL-27, Cxcl10, Ccl5, and Socs1. Genes that were moderately changed (4.99 > fold change > 2) included Cxcl10, Ccl5, Il13, Il7, Ccl4, Ccr5, Ccl3, and Ccl12. Genes that showed low, but significant changes in gene expression (fold change < 1.99) included Socs3, Il10ra, Cd40, Tlr4, Tnfsf8, Jak2, and Tmed1. Because the samples were from the entire SN, we chose to include all fold changes > 1.1 in our model since there are many potential sources from which these mRNA could be derived (e.g., neurons, glial cells and immunocytes). Therefore, even small changes in message within this microenvironment may have large implications for neuronal injury/protection. The search for functional relationships between genes with significant differences in expression compared to those from MPTP-treated mice (Ingenuity Pathway Analysis, IPA, Ingenuity Systems, Redwood City, CA) suggests GM-CSF administration and immune modulation induce pro- and anti-inflammatory responses of both innate and adaptive immune origin (Fig 8). Pro-inflammatory responses include increases in expression of mRNA for Il18r1, Tlr4, Ccl5, Cxcl10, Ccl12, Jak2, Cd40, Ccr5, Ccl4, Ccl3 and a decrease in Il13 and Il7, while anti-inflammatory responses were signaled by the increase of Il27, Socs1, Socs3, and Il10ra and a decrease in Tnfsfr8 and Tnfsf10. Changes in mRNA expression suggested that innate mechanisms include changes in Il13, Il27, Ccl5, Cxcl10, Ccl12, and Il10ra, while adaptive immune changes include Il18r1, Tlr4, Il7, Tnfrsf8, Ccl5, Jak2, Socs1, Tmed1, Cd40, Ccr5, and Il10ra. Taken together, these results reveal a potential for GM-CSF as a neuroprotective agent via the interaction between innate and adaptive immuninty.
Figure 7.
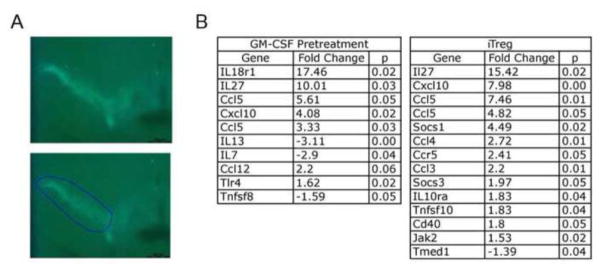
GM-CSF induces both pro- and anti-inflammatory gene expression changes. All TH-GFP/21-31 mice that express GFP under control of the TH promoter were treated with MPTP. Controls received no other treatment, one group was pretreated with 50 μg GM-CSF/kg 5 days before MPTP intoxication (4 doses of 16 mg/kg), and another group received GM-CSF-induced Treg by adoptive transfer within 12 hours after MPTP intoxication. A. Laser capture microdissection was used to isolate SN tissues harvested 2 days after MPTP intoxication and mRNA from each animal was isolated. Shown is the demarcation of the SN by the presence of GFP-TH neurons (top panel) and a representative section to be dissected (bottom panel). B. qRT-PCR data showing gene expression changes within the SN of mice pretreated with GM-CSF followed by MPTP (GM-CSF Pretreatment) or GM-CSF-induced Treg compared to those of MPTP controls (n = 3 for each group). Fold changes and p value determined by SABioscience RT2 Profiler PCR Array Data Analysis, version 3.5.
Figure 8.
GM-CSF induces both pro- and anti-inflammatory responses. Shown is a pathway connecting inflammatory and T cell genes that are significantly changed from MPTP-treatment group after either GM-CSF pretreatment or GM-CSF induced Treg adoptive transfer. Genes were submitted to IPA for analyses to evaluate known interactions and are indicated with a connecting line. Red coloration indicates an increase in expression as compared to MPTP control, and green indicates a decrease in expression. Color intensity indicates degree of increase or decrease. Lack of color indicates a molecule involved, but not identified in the data set. Differences in fold change (n = 3 per group) were determined where p < 0.05. The gene interaction network was generated using Ingenuity Pathways Analysis (Winter 2012 release; version 14400082) and the Path Explorer tool.
4. Discussion
Herein we demonstrate a link between the neuroprotective effects of peripheral GM-CSF administration for dopaminergic neurons in the SN and the modulation of innate and adaptive neuroimmunity. Not only does GM-CSF pretreatment prevent MPTP-induced neurodegeneration, but it also decreases the microglial activation responses associated with neuronal destruction along the nigrostriatal axis. Given the potential of GM-CSF to modulate adaptive immunity, we hypothesized that this effect was related to interactions between the innate and adaptive immune arms. We found GM-CSF induces CD4+CD25+FoxP3+ Treg in a dose-dependent manner, and these immune cells, when administered to MPTP-intoxicated mice, elicited robust neuroprotective responses. Importantly, Treg diminished microgliosis as observed with GM-CSF pretreatment. Prior works demonstrated that GM-CSF modulates Treg and microglial numbers and function (Kared et al., 2008, Parajuli et al., 2012). The regulatory role of GM-CSF and resulting immune consequences likely result from a cross-talk between innate and adaptive immunity inside and outside the brain, and this phenomenon is further explored via gene expression analysis.
The neuroprotective activities of GM-CSF are thought linked to its effects on both pro- and anti-inflammatory responses. These results have parallels with other agents that include rapamycin, which induces Treg while, at the same time, leads to Teff apoptosis (Pan et al., 2009, Malagelada et al., 2010, Peter et al., 2010, Ogino et al., 2011). Histone deacetylase inhibitors (HDACi), such as trichostatin A (TSA) or suberoylanilide hydroxamic acid (SAHA) can also induce Treg and expand natural Treg while enhancing immune regulatory FoxP3-related functions (Tao et al., 2007, Liu et al., 2010). However, the targeting of HDAC9- or class IIa-linked Treg development deserves further investigation as an approach for clinical applications. Vitamin D has been studied for potential clinical application as it positively affects Treg function and correlates negatively with autoimmunity, suggesting a role for vitamin D in Treg regulation (Smolders et al., 2009). Similarly, bee venom increases both numbers and function of Treg and decreases neurotoxicity associated with MPTP-intoxication (Pereira-Santos et al., 2008, Chung et al., 2012).
In the current study, we observed increases in both SOCS1 and SOCS3 transcript by treatment with GM-CSF before MPTP-intoxication. Not only do these negatively regulate cytokine expression, but brain responses to infections also accompany the binding of SOCS1 and SOCS3 to JAK, which is also upregulated, as a compensatory mechanism to limit neuroinflammation (Tamiya et al., 2011). The most significant changes within the SN include the 10- and 15-fold increases in the message for IL-27 after GM-CSF pretreatment and transfer of Treg to MPTP-treated mice. IL-27 is a member of the pro-inflammatory IL-12 family and regulates these effects by balancing Th17 and Treg function via manipulation of their plasticity to favor the predominance of Treg and the diminution of Th17 (Trinchieri, 2003). Directly, IL-27 inhibits the expression of ROR-γt, thereby inhibiting the commitment of Th17 development, and consequently, reduces neurodestructive Th17 cells (Zhang et al., 2008, Diveu et al., 2009). Indirectly, IL-27 upregulates SOCS1, which suppresses IL-17 (Liu and Rohowsky-Kochan, 2011). Also, IL-27 promotes the production of regulatory-like IL-10 producing cells, and although IL-10 was not upregulated in our study, IL-10 receptor gene expression was increased 1.8-fold (Carrier et al., 2012). Thus, the functional relationships presented warrant further study, as they suggest the mechanism of GM-CSF lies in restoring the ability of the neuroimmune environment to self-regulate. Additionally, noting that the peripheral administration of GM-CSF induces robust changes within the SN is quite remarkable and lends credibility to its clinical use.
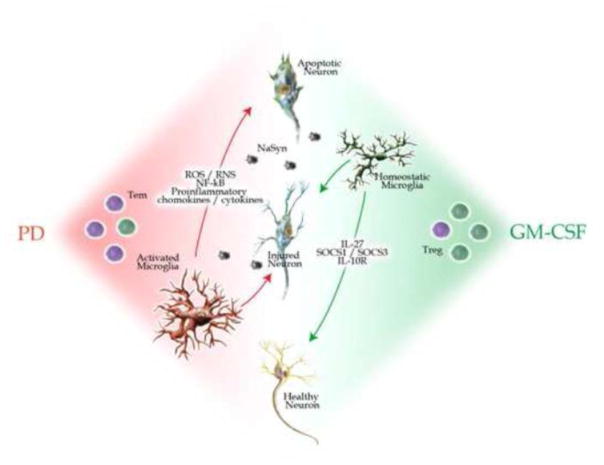
PD is characterized by a dysregulated inflammatory environment, involving an emergence of Tem and a dysfunctional Treg, as seen in PD patients (Fig 9). These aberrancies incite microgliosis and exacerbate neurodegeneration due to release of N-α-syn from dying neurons, which in turn supports chronic activation of immune responses. Previous studies have shown microglia activated via contact with N-α-syn upregulates NF-κB and downstream pathways as well as proinflammatory cytokines, chemokines, and reactive oxygen and nitrogen species (ROS and RNS, respectively) (Zhang et al., 2005, Reynolds et al., 2009b). This activation results in not only the morphological changes cited herein, but also causes the differential expression of regulatory, structural, and redox-active proteins and functions including protein processing, trafficking, and degradation (Reynolds et al., 2009b). We hypothesize that GM-CSF could restore balance to each of these immune arms through Treg-inducing neurotrophic capabilities. GM-CSF indeed induces Treg, reduces microgliosis, and changes the neuroimmune environment within the SN such that it promotes disease resolution through anti-inflammatory responses. It has been previously shown that pretreatment of Treg prior to the N-α-syn-induced activation of microglial cells in vivo results in enhanced proteasomal systems and decreased inflammatory signals, suggesting Treg help the chronically activated and deleterious microglia to perform functions conducive to neural repair thereby alleviating neuroinflammation. Here, we show that Treg induction and the resulting reduction in microgliosis can be achieved via the peripheral administration of GM-CSF. Additionally, we hypothesize these beneficial effects are achieved via both innate and adaptive immune mechanisms, such as the upregulation of SOCS1, SOCS3, and IL-27.
Figure 9.
Proposed mechanism for GM-CSF neuroprotection. Neuroinflammation in PD is characterized by increased numbers of Tem (purple) and of the presence of activated microglia. Such chronic inflammation can exacerbate dopaminergic neuronal death and accelerates disease progression with limited balances. Administration of GM-CSF restores the balance to the brain inflammatory microenvironment through a number of innate and adaptive immune mechanisms leading to modulation of microgliosis, induction of Treg numbers (green) or function leading to neuroprotection of damaged or disease susceptible dopaminergic neurons.
Thus, we will use this strategy as a translational platform for the clinic. Since immune biomarkers that correlate to disease severity have been identified in PD and include increased frequencies in CD4+ T cells, CD4+ Tem expressing CD45RO and FAS, and dysfunctional Treg with deficiencies in CD31 expression, the targeting of such aberrancies provide the opportunity to monitor changes in disease progression or therapy in the peripheral blood (Saunders et al., 2012). This is particularly useful by constructing objective data that can be correlated to clinical observations, such as the Unified Parkinson’s Disease Rating Scale Part III, thereby improving the success of short-term trials in which patients are likely to exhibit stable scores regardless of drug or placebo treatments. Further, this study establishes a platform for testing other anti-inflammatory agents with the potential to alter PD immunity.
Taken together, we believe this study serves to identify GM-CSF as a potential agent to halt PD progression, and this is a strategy that can be applied in clinic immediately for patient benefit. In addition, it sets up a platform in which GM-CSF and other agents may be applied to clinic or to curative PD research. Altogether, we provide convincing data bridging neuroprotective responses with immunomodulation of GM-CSF supporting its role as a potential PD therapy.
Highlights.
GM-CSF elicits neuroprotective activities in MPTP intoxicated mice
GM-CSF Treg elicit anti-inflammatory and neuroprotective responses
GM-CSF modulates innate microglial immunity
GM-CSF protects dopaminergic neurons against MPTP intoxication
GM-CSF can be developed for Parkinson’s disease
Acknowledgments
We thank Kristi Anderson, Max Kuenstling, Matthew Follett and Rebekah Priluck for critical technical assistance. We thank Janice A. Taylor and James R. Talaska of the Confocal Laser Scanning Microscope Core Facility at the University of Nebraska Medical Center for providing assistance with confocal microscopy and the Nebraska Research Initiative and the Eppley Cancer Center for their support of the Core Facility. We thank Tony Bilek for graphical assistance.
Footnotes
This work was supported, in part, by the Carol Swarts Neuroscience Research Laboratory, the Frances and Louie Blumkin Foundation and National Institutes of Health grants P01 DA028555, R01 NS36126, P01 NS31492, 2R01 NS034239, P01 MH64570, P01 NS43985 (HEG) and R01NS070190 (RLM).
Conflict of Interest: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balandina A, Lecart S, Dartevelle P, Saoudi A, Berrih-Aknin S. Functional defect of regulatory CD4(+)CD25+ T cells in the thymus of patients with autoimmune myasthenia gravis. Blood. 2005;105:735–741. doi: 10.1182/blood-2003-11-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner EJ, Banerjee R, Reynolds AD, Sherman S, Pisarev VM, Tsiperson V, Nemachek C, Ciborowski P, Przedborski S, Mosley RL, Gendelman HE. Nitrated alpha-synuclein immunity accelerates degeneration of nigral dopaminergic neurons. PLoS One. 2008;3:e1376. doi: 10.1371/journal.pone.0001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner EJ, Mosley RL, Destache CJ, Lewis TB, Jackson-Lewis V, Gorantla S, Nemachek C, Green SR, Przedborski S, Gendelman HE. Therapeutic immunization protects dopaminergic neurons in a mouse model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2004;101:9435–9440. doi: 10.1073/pnas.0400569101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd TD, Bennett SP, Mori T, Governatori N, Runfeldt M, Norden M, Padmanabhan J, Neame P, Wefes I, Sanchez-Ramos J, Arendash GW, Potter H. GM-CSF upregulated in rheumatoid arthritis reverses cognitive impairment and amyloidosis in Alzheimer mice. J Alzheimers Dis. 2010;2:507–18. doi: 10.3233/JAD-2010-091471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochard V, Combadiere B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay JM, Duyckaerts C, Flavell RA, Hirsch EC, Hunot S. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest. 2009;119:182–192. doi: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsel PC, Forgey A, Grape FB, Hamann SS. Granulocyte macrophage colony-stimulating factor: current practice and novel approaches. Clin J Oncol Nurs. 2002;6:198–205. doi: 10.1188/02.CJON.198-205. [DOI] [PubMed] [Google Scholar]
- Carrier Y, Whitters MJ, Miyashiro JS, LaBranche TP, Ramon HE, Benoit SE, Ryan MS, Keegan SP, Guay H, Douhan J, Collins M, Dunussi-Joannopoulos K, Medley QG. Enhanced GITR/GITRL interactions augment IL-27 expression and induce IL-10-producing Tr-1 like cells. Eur J Immunol. 2012;42:1393–1404. doi: 10.1002/eji.201142162. [DOI] [PubMed] [Google Scholar]
- Choudhury ME, Sugimoto K, Kubo M, Nagai M, Nomoto M, Takahashi H, Yano H, Tanaka J. A cytokine mixture of GM-CSF and IL-3 that induces a neuroprotective phenotype of microglia leading to amelioration of (6-OHDA)-induced Parkinsonism of rats. Brain Behav. 2011;1:26–43. doi: 10.1002/brb3.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung ES, Kim H, Lee G, Park S, Bae H. Neuro-protective effects of bee venom by suppression of neuroinflammatory responses in a mouse model of Parkinson’s disease: role of regulatory T cells. Brain Behav Immun. 2012;26:1322–1330. doi: 10.1016/j.bbi.2012.08.013. [DOI] [PubMed] [Google Scholar]
- Damier P, Hirsch EC, Zhang P, Agid Y, Javoy-Agid F. Glutathione peroxidase, glial cells and Parkinson’s disease. Neuroscience. 1993;52:1–6. doi: 10.1016/0306-4522(93)90175-f. [DOI] [PubMed] [Google Scholar]
- Diveu C, McGeachy MJ, Boniface K, Stumhofer JS, Sathe M, Joyce-Shaikh B, Chen Y, Tato CM, McClanahan TK, de Waal Malefyt R, Hunter CA, Cua DJ, Kastelein RA. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol. 2009;182:5748–5756. doi: 10.4049/jimmunol.0801162. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Lonergan R, Costelloe L, Kinsella K, Moran B, O’Farrelly C, Tubridy N, Mills KH. CD39+Foxp3+ regulatory T Cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J Immunol. 2009;183:7602–7610. doi: 10.4049/jimmunol.0901881. [DOI] [PubMed] [Google Scholar]
- Gao X, Chen H, Schwarzschild MA, Ascherio A. Use of ibuprofen and risk of Parkinson disease. Neurology. 2011;76:863–869. doi: 10.1212/WNL.0b013e31820f2d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha Y, Park HS, Park CW, Yoon SH, Park SR, Hyun DK, Kim EY, Park HC. Synthes Award for Resident Research on Spinal Cord and Spinal Column Injury: granulocyte macrophage colony stimulating factor (GM-CSF) prevents apoptosis and improves functional outcome in experimental spinal cord contusion injury. Clin Neurosurg. 2005;52:341–347. [PubMed] [Google Scholar]
- Henze C, Hartmann A, Lescot T, Hirsch EC, Michel PP. Proliferation of microglial cells induced by 1-methyl-4-phenylpyridinium in mesencephalic cultures results from an astrocyte-dependent mechanism: role of granulocyte macrophage colony-stimulating factor. J Neurochem. 2005;95:1069–1077. doi: 10.1111/j.1471-4159.2005.03416.x. [DOI] [PubMed] [Google Scholar]
- Huang X, Choi JK, Park SR, Ha Y, Park H, Yoon SH, Park HC, Park JO, Choi BH. GM-CSF inhibits apoptosis of neural cells via regulating the expression of apoptosis-related proteins. Neurosci Res. 2007;58:50–57. doi: 10.1016/j.neures.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Iannaccone S, Cerami C, Alessio M, Garibotto V, Panzacchi A, Olivieri S, Gelsomino G, Moresco RM, Perani D. In vivo microglia activation in very early dementia with Lewy bodies, comparison with Parkinson’s disease. Parkinsonism Relat Disord. 2013;19:47–52. doi: 10.1016/j.parkreldis.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Ishikawa D, Okazawa A, Corridoni D, Jia LG, Wang XM, Guanzon M, Xin W, Arseneau KO, Pizarro TT, Cominelli F. Tregs are dysfunctional in vivo in a spontaneous murine model of Crohn’s disease. Mucosal Immunol. 2013;6:267–275. doi: 10.1038/mi.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat Protoc. 2007;2:141–151. doi: 10.1038/nprot.2006.342. [DOI] [PubMed] [Google Scholar]
- Hutter Saunders JAL, Estes K, Kosloski L, Allen H, Dempsey KM, Torres D, Meza J, Santamaria P, Bertoni J, Murman D, Ali HH, Standaert DG, Mosley RL, Gendelman HE. CD4+ T cell subsets profile motor dysfunction in Parkinson’s disease. J Neuroimmune Pharmacol. 2012;7(4):927–38. doi: 10.1007/s11481-012-9402-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kared H, Leforban B, Montandon R, Renand A, Layseca Espinosa E, Chatenoud L, Rosenstein Y, Schneider E, Dy M, Zavala F. Role of GM-CSF in tolerance induction by mobilized hematopoietic progenitors. Blood. 2008;112:2575–2578. doi: 10.1182/blood-2008-02-140681. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Oh JS, An SS, Pennant WA, Gwak SJ, Kim AN, Han PK, Yoon DH, Kim KN, Ha Y. Hypoxia-specific GM-CSF-overexpressing neural stem cells improve graft survival and functional recovery in spinal cord injury. Gene Ther. 2012;19:513–521. doi: 10.1038/gt.2011.137. [DOI] [PubMed] [Google Scholar]
- Kim JY, Oh CH, Huang X, Kim MH, Yoon SH, Kim KH, Park H, Park HC, Park SR, Choi BH. Improvement in sensory function via granulocyte-macrophage colony-stimulating factor in rat spinal cord injury models. J Neurosurg Spine. 2013;18:69–75. doi: 10.3171/2012.9.SPINE1235. [DOI] [PubMed] [Google Scholar]
- Kim NK, Choi BH, Huang X, Snyder BJ, Bukhari S, Kong TH, Park H, Park HC, Park SR, Ha Y. Granulocyte-macrophage colony-stimulating factor promotes survival of dopaminergic neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced murine Parkinson’s disease model. Eur J Neurosci. 2009;29:891–900. doi: 10.1111/j.1460-9568.2009.06653.x. [DOI] [PubMed] [Google Scholar]
- Krieger M, Both M, Kranig SA, Pitzer C, Klugmann M, Vogt G, Draguhn A, Schneider A. The hematopoietic cytokine granulocyte-macrophage colony stimulating factor is important for cognitive functions. Sci Rep. 2012;2:697. doi: 10.1038/srep00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurkowska-Jastrzebska I, Wronska A, Kohutnicka M, Czlonkowski A, Czlonkowska A. The inflammatory reaction following 1-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine intoxication in mouse. Exp Neurol. 1999;156:50–61. doi: 10.1006/exnr.1998.6993. [DOI] [PubMed] [Google Scholar]
- Laurie C, Reynolds A, Coskun O, Bowman E, Gendelman HE, Mosley RL. CD4+ T cells from Copolymer-1 immunized mice protect dopaminergic neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. J Neuroimmunol. 2007;183:60–68. doi: 10.1016/j.jneuroim.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Li P, Gan Y, Sun BL, Zhang F, Lu B, Gao Y, Liang W, Thomson AW, Chen J, Hu X. Adoptive regulatory T-cell therapy protects against cerebral ischemia. Ann Neurol. 2012 doi: 10.1002/ana.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, Dawson VL, Dawson TM, Przedborski S. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med. 1999;5:1403–1409. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- Liu H, Rohowsky-Kochan C. Interleukin-27-mediated suppression of human Th17 cells is associated with activation of STAT1 and suppressor of cytokine signaling protein 1. J Interferon Cytokine Res. 2011;31:459–469. doi: 10.1089/jir.2010.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zhang C, Sun J. Deacetylase inhibitor trichostatin A down-regulates Foxp3 expression and reduces CD4+CD25+ regulatory T cells. Biochem Biophys Res Commun. 2010;400:409–412. doi: 10.1016/j.bbrc.2010.08.090. [DOI] [PubMed] [Google Scholar]
- Malagelada C, Jin ZH, Jackson-Lewis V, Przedborski S, Greene LA. Rapamycin protects against neuron death in in vitro and in vivo models of Parkinson’s disease. J Neurosci. 2010;30:1166–1175. doi: 10.1523/JNEUROSCI.3944-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangano EN, Peters S, Litteljohn D, So R, Bethune C, Bobyn J, Clarke M, Hayley S. Granulocyte macrophage-colony stimulating factor protects against substantia nigra dopaminergic cell loss in an environmental toxin model of Parkinson’s disease. Neurobiol Dis. 2011;43:99–112. doi: 10.1016/j.nbd.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Marek-Trzonkowska N, Mysliwec M, Siebert J, Trzonkowski P. Clinical application of regulatory T cells in type 1 diabetes. Pediatr Diabetes. 2013 doi: 10.1111/pedi.12029. [DOI] [PubMed] [Google Scholar]
- Matsushita N, Okada H, Yasoshima Y, Takahashi K, Kiuchi K, Kobayashi K. Dynamics of tyrosine hydroxylase promoter activity during midbrain dopaminergic neuron development. J Neurochem. 2002;82:295–304. doi: 10.1046/j.1471-4159.2002.00972.x. [DOI] [PubMed] [Google Scholar]
- Mayne CG, Williams CB. Induced and Natural Regulatory T Cells in the Development of Inflammatory Bowel Disease. Inflamm Bowel Dis. 2013;19(8):1772–88. doi: 10.1097/MIB.0b013e318281f5a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer E, Rogers J, Sibley J. Anti-inflammatory drugs and Alzheimer’s disease. Lancet. 1990;335:1037–1047. doi: 10.1016/0140-6736(90)91101-f. [DOI] [PubMed] [Google Scholar]
- McLay RN, Kimura M, Banks WA, Kastin AJ. Granulocyte-macrophage colony-stimulating factor crosses the blood--brain and blood--spinal cord barriers. Brain. 1997;120 (Pt 11):2083–2091. doi: 10.1093/brain/120.11.2083. [DOI] [PubMed] [Google Scholar]
- Nie H, Zheng Y, Li R, Guo TB, He D, Fang L, Liu X, Xiao L, Chen X, Wan B, Chin YE, Zhang JZ. Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-alpha in rheumatoid arthritis. Nat Med. 2013;19:322–328. doi: 10.1038/nm.3085. [DOI] [PubMed] [Google Scholar]
- Ogino H, Nakamura K, Iwasa T, Ihara E, Akiho H, Motomura Y, Akahoshi K, Igarashi H, Kato M, Kotoh K, Ito T, Takayanagi R. Regulatory T cells expanded by rapamycin in vitro suppress colitis in an experimental mouse model. J Gastroenterol. 2011 doi: 10.1007/s00535-011-0502-y. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Stern MB, Sethi K. The scientific and clinical basis for the treatment of Parkinson disease (2009) Neurology. 2009;72:S1–136. doi: 10.1212/WNL.0b013e3181a1d44c. [DOI] [PubMed] [Google Scholar]
- Pan T, Rawal P, Wu Y, Xie W, Jankovic J, Le W. Rapamycin protects against rotenone-induced apoptosis through autophagy induction. Neuroscience. 2009;164:541–551. doi: 10.1016/j.neuroscience.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Parajuli B, Sonobe Y, Kawanokuchi J, Doi Y, Noda M, Takeuchi H, Mizuno T, Suzumura A. GM-CSF increases LPS-induced production of proinflammatory mediators via upregulation of TLR4 and CD14 in murine microglia. J Neuroinflammation. 2012;9:268. doi: 10.1186/1742-2094-9-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira-Santos MC, Baptista AP, Melo A, Alves RR, Soares RS, Pedro E, Pereira-Barbosa M, Victorino RM, Sousa AE. Expansion of circulating Foxp3+)D25bright CD4+ T cells during specific venom immunotherapy. Clin Exp Allergy. 2008;38:291–297. doi: 10.1111/j.1365-2222.2007.02887.x. [DOI] [PubMed] [Google Scholar]
- Peter C, Waldmann H, Cobbold SP. mTOR signalling and metabolic regulation of T cell differentiation. Curr Opin Immunol. 2010;22:655–661. doi: 10.1016/j.coi.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Reynolds AD, Banerjee R, Liu J, Gendelman HE, Mosley RL. Neuroprotective activities of CD4+CD25+ regulatory T cells in an animal model of Parkinson’s disease. J Leukoc Biol. 2007;82:1083–1094. doi: 10.1189/jlb.0507296. [DOI] [PubMed] [Google Scholar]
- Reynolds AD, Glanzer JG, Kadiu I, Ricardo-Dukelow M, Chaudhuri A, Ciborowski P, Cerny R, Gelman B, Thomas MP, Mosley RL, Gendelman HE. Nitrated alpha-synuclein-activated microglial profiling for Parkinson’s disease. J Neurochem. 2008;104:1504–1525. doi: 10.1111/j.1471-4159.2007.05087.x. [DOI] [PubMed] [Google Scholar]
- Reynolds AD, Stone DK, Hutter JA, Benner EJ, Mosley RL, Gendelman HE. Regulatory T cells attenuate th17 cell-mediated nigrostriatal dopaminergic neurodegeneration in a model of Parkinson’s disease. J Immunol. 2010;184:2261–2271. doi: 10.4049/jimmunol.0901852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AD, Stone DK, Mosley RL, Gendelman HE. Nitrated {alpha}-synuclein-induced alterations in microglial immunity are regulated by CD4+ T cell subsets. J Immunol. 2009a;182:4137–4149. doi: 10.4049/jimmunol.0803982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AD, Stone DK, Mosley RL, Gendelman HE. Proteomic studies of nitrated alpha-synuclein microglia regulation by CD4+CD25+ T cells. J Proteome Res. 2009b;8:3497–3511. doi: 10.1021/pr9001614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth L, Macdonald JK, McDonald JW, Chande N. Sargramostim (GM-CSF for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2011;11:CD008538. doi: 10.1002/14651858.CD008538.pub2. [DOI] [PubMed] [Google Scholar]
- Rowin J, Thiruppathi M, Arhebamen E, Sheng J, Prabhakar BS, Meriggioli MN. Granulocyte macrophage colony-stimulating factor treatment of a patient in myasthenic crisis: effects on regulatory T cells. Muscle Nerve. 2012;46:449–453. doi: 10.1002/mus.23488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safinia N, Leech J, Hernandez-Fuentes M, Lechler R, Lombardi G. Promoting transplantation tolerance; adoptive regulatory T cell therapy. Clin Exp Immunol. 2013;172:158–168. doi: 10.1111/cei.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallenberg M, Charalambous P, Thanos S. GM-CSF protects rat photoreceptors from death by activating the SRC-dependent signalling and elevating anti-apoptotic factors and neurotrophins. Graefes Arch Clin Exp Ophthalmol. 2012;250:699–712. doi: 10.1007/s00417-012-1932-9. [DOI] [PubMed] [Google Scholar]
- Schwarz A, Schumacher M, Pfaff D, Schumacher K, Jarius S, Balint B, Wiendl H, Haas J, Wildemann B. Fine-tuning of regulatory T cell function: the role of calcium signals and naive regulatory T cells for regulatory T cell deficiency in multiple sclerosis. J Immunol. 2013;190:4965–4970. doi: 10.4049/jimmunol.1203224. [DOI] [PubMed] [Google Scholar]
- Sheng JR, Muthusamy T, Prabhakar BS, Meriggioli MN. GM-CSF-induced regulatory T cells selectively inhibit anti-acetylcholine receptor-specific immune responses in experimental myasthenia gravis. J Neuroimmunol. 2011;240–241:65–73. doi: 10.1016/j.jneuroim.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava P, Vaibhav K, Tabassum R, Khan A, Ishrat T, Khan MM, Ahmad A, Islam F, Safhi MM. Anti-apoptotic and anti-inflammatory effect of Piperine on 6-OHDA induced Parkinson’s rat model. J Nutr Biochem. 2013;24:680–687. doi: 10.1016/j.jnutbio.2012.03.018. [DOI] [PubMed] [Google Scholar]
- Smolders J, Thewissen M, Peelen E, Menheere P, Tervaert JW, Damoiseaux J, Hupperts R. Vitamin D status is positively correlated with regulatory T cell function in patients with multiple sclerosis. PLoS One. 2009;4:e6635. doi: 10.1371/journal.pone.0006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamiya T, Kashiwagi I, Takahashi R, Yasukawa H, Yoshimura A. Suppressors of cytokine signaling (SOCS) proteins and JAK/STAT pathways: regulation of T-cell inflammation by SOCS1 and SOCS3. Arterioscler Thromb Vasc Biol. 2011;31:980–985. doi: 10.1161/ATVBAHA.110.207464. [DOI] [PubMed] [Google Scholar]
- Tao R, de Zoeten EF, Ozkaynak E, Chen C, Wang L, Porrett PM, Li B, Turka LA, Olson EN, Greene MI, Wells AD, Hancock WW. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13:1299–1307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- Wong AC, Lee SD. Long-term therapy with sargramostim in a patient with Crohn’s disease. Inflamm Bowel Dis. 2011;6:1447–8. doi: 10.1002/ibd.21542. [DOI] [PubMed] [Google Scholar]
- Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhang W, Zhou Y, Hong JS, Zhang J. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- Zhang X, Jin J, Peng X, Ramgolam VS, Markovic-Plese S. Simvastatin inhibits IL-17 secretion by targeting multiple IL-17-regulatory cytokines and by inhibiting the expression of IL-17 transcription factor RORC in CD4+ lymphocytes. J Immunol. 2008;180:6988–6996. doi: 10.4049/jimmunol.180.10.6988. [DOI] [PubMed] [Google Scholar]
- Zou T, Satake A, Ojha P, Kambayashi T. Cellular therapies supplement: the role of granulocyte macrophage colony-stimulating factor and dendritic cells in regulatory T-cell homeostasis and expansion. Transfusion. 2011;51(Suppl 4):160S–168S. doi: 10.1111/j.1537-2995.2011.03379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]