Abstract
Background
Mild disturbances of higher order activities of daily living are present in people diagnosed with mild cognitive impairment (MCI). These deficits may be difficult to detect among those still living independently. Unobtrusive continuous assessment of a complex activity such as home computer use may detect mild functional changes and identify MCI. We sought to determine whether long-term changes in remotely monitored computer use differ in persons with MCI in comparison to cognitively intact volunteers.
Methods
Participants enrolled in a longitudinal cohort study of unobtrusive in-home technologies to detect cognitive and motor decline in independently living seniors were assessed for computer usage (number of days with use, mean daily usage and coefficient of variation of use) measured by remotely monitoring computer session start and end times.
Results
Over 230,000 computer sessions from 113 computer users (mean age, 85; 38 with MCI) were acquired during a mean of 36 months. In mixed effects models there was no difference in computer usage at baseline between MCI and intact participants controlling for age, sex, education, race and computer experience. However, over time, between MCI and intact participants, there was a significant decrease in number of days with use (p=0.01), mean daily usage (~1% greater decrease/month; p=0.009) and an increase in day-to-day use variability (p=0.002).
Conclusions
Computer use change can be unobtrusively monitored and indicate individuals with MCI. With 79% of those 55–64 years old now online, this may be an ecologically valid and efficient approach to track subtle clinically meaningful change with aging.
Keywords: Mild Cognitive Impairment, Assessment of cognitive disorders/dementia, Cohort studies, Activities of daily living, Computer use
1. Introduction
The use of technology and in particular computer-based devices continues to proliferate in everyday life. This phenomenon although commonly thought to be characteristic of younger populations is also seen among our aging population. Almost eighty percent of adults age 55–64 who represent the next generation of seniors are on-line in the US [1]. This generation has among the most rapid rates of adoption of smartphone [2] and social media technologies [1]. They are perceived as driving the digital fitness industry with their interest in playing on-line games and participating in brain fitness programs to stave off cognitive decline. The shift to adoption of computer technologies may also come of necessity. For example, last year the Social Security Administration announced that they would no long mail annual statements, but only post them on-line. The increasing use and incorporation of these technologies into the flow of the day has in effect created a new higher order or “instrumental” activity of daily living (IADL) increasingly important to the senior community, achieving in many cases the status of a near necessity for contemporary life.
In the context of considering computer activity as an emerging IADL, it is important to consider computer use itself as a complex task taxing multiple cognitive domains (attention, working memory, episodic memory, executive function, etc.). As a complex task, computer use is likely to be sensitive to cognitive change and thus might be a bellwether of brain health, aging cognition or the onset of mild cognitive impairment (MCI). This is especially important for the growing community of adults age 55–64.
People with MCI are by definition not demented. However, definitions of MCI are not specific with regard to criteria for determining the functional status of the person with MCI, leaving uncertainty as to what degree of function qualifies as having “essentially normal functional activities”[3]. This is at least in part a reflection of the difficulty of clinically assessing the gradual and subtle affect that mildly degraded cognition may have on day-to-day function. People with MCI may continue to work and independently engage in community and household affairs and to operate electronic devices. Nevertheless, a large body of evidence suggests that mild cognitive decline is accompanied by decrements in the ability to perform complex IADLs such as medication taking, telephone use and meal preparation, even years before frank dementia or symptomatic MCI is evident[4–16]. Speaking to the subtlety of this functional disturbance, several studies have shown that self-report may not be adequate to detect this change. Thus, direct functional performance tasks simulating IADLs and performed in front of a clinician or examiner may be more sensitive to identifying IADL deficits in MCI[12, 14, 15]. However, these direct assessments add to the time of testing and although they involve direct manipulation of common objects to assess function some of these items may not be a part of activities in which the person routinely engages such as writing a check or mailing a letter. The assessment is not ecologically valid and does not reflect how the person performs in their usual daily environment over time.
An alternative approach to the assessment of daily function is to bring the assessment into the home through remote monitoring using sensors strategically placed in the home environment to continuously track daily function in real time. Thus a home network of motion sensors can provide information about functional activity in particular locations of a residence, while specific instrumentation of devices commonly used such as the telephone, medication caddies or a personal computer provides information about the interaction with these devices. In this paper we present results from a longitudinal cohort study (ISAAC, Intelligent Systems for Assessing Aging Change) comparing older people with MCI to non-cognitively impaired individuals living independently by assessing the specific higher order activity of daily living, computer use. In order to study computer use in the current aging population where computer use is not as prevalent as in the next generations, we initially trained each participant to a level of computer literacy defined as being able to independently send and receive email. We hypothesized that over time those with MCI would spend fewer days and less time on the computer and that their day-to-day variability in usage would increase.
2. Methods
2.1. Study Participants
The research protocol was approved by the Oregon Health and Science University Institutional Review Board. (OHSU IRB #2353). All participants provided written informed consent. Participants were recruited from the Portland, Oregon metropolitan area through advertisement and presentations at local retirement communities as part of the ISAAC longitudinal cohort study. Details of the study protocol for ISAAC have been published elsewhere [17]. Briefly, entry criteria for the study included being a man or woman age 70 or older, living independently (living with a companion or spouse was allowed, but not as caregiver), not demented (Mini-Mental State Examination (MMSE[18]) score > 24; Clinical Dementia Rating[19] scale score ≤ 0.5) and in average health for age. Medical illnesses that would limit physical participation (e.g. wheelchair bound) or likely lead to untimely death (such as certain cancers) were exclusions. A total of 265 participants were enrolled (beginning in 2007). The participants lived in a variety of settings from apartments in organized retirement communities to free standing single-family homes. In this report we present data for 113 participants living alone or who were the only computer user at home.
2.2. Clinical Assessment Procedures
Participants were clinically assessed at baseline and during annual visits in their home using a standardized battery of tests consisting of physical and neurological examinations[17]. In addition to the MMSE, participants were administered the Geriatric Depression Scale,[20] the Functional Activities Questionnaire (FAQ)[21] and medication use was recorded. Health status was further assessed by the modified Cumulative Illness Rating Scale (CIRS)[22]. Diagnosis of MCI was made using the Petersen framework[23] operationalized as absence of dementia, none or minimal functional impairment (dependent on less than three activities on the FAQ)19, normal general cognitive function (MMSE≥24) and objective impairment on one or more of six neuropsychological tests considered to be representative of five cognitive domains (Logical Memory Delayed (memory)[24], Category Fluency: Animals (executive function), Trail Making B (executive function)[25], WAIS Digit Symbol (attention) [26], Boston Naming Test (language)[27], WAIS-R Block Design (visual-perceptive function)[25]. Impairment on neuropsychological testing was defined as a score 1.5 SDs or more below the model-derived predicted mean values stratified by age, education and sex based on previously published normative data[28]. Amnestic MCI was defined as a decrement in the memory domain with or without decrement in other domains. Non-amnestic MCI was defined as a decrement in one or more non-memory domains.
2.3. Home sensor network and unobtrusive data collection
Continuous activity data was collected using an unobtrusive activity assessment system consisting of several types of motion and contact sensors in the home of each participant [17]. Metrics assessed by the sensors include total daily activity, time out of home, and walking speed[29]. These measures although not reported specifically here were used in the context of collecting computer use data to identify general activity such as whether a person was out of the home for extended periods of time and thus not using their computer. Data from all sensors were received by a dedicated research laptop computer placed in the participant's home (not the participant's personal computer), time-stamped and stored in an SQL database. All data were automatically uploaded daily to a central database in the project data center.
2.4. Computer Installation, Setup, and Training
Each participant received a desktop computer (or could choose to use their own). Internet broadband services were provided to facilitate data acquisition and participation in all study activities. At study screening research personnel observed and scored participant proficiency on 20 computer-based tasks such as opening an Internet browser or going to a specific Web site using the Web site address[17]. Based on the results of this proficiency assessment, participants were categorized as novice or intermediate users and were then invited to participate in either a novice or intermediate level computer training program. The Computer and Internet Literacy Program was developed specifically for implementation with older adults. The program consists of six one-hour instructor led sessions designed to achieve computer proficiency, defined as the ability to launch computer programs from the desktop, send and receive email, and navigate the Internet.
Each participant was issued a personal login password to begin each computer session identifying them as a unique user. Participants were asked to use their computers daily. Study participants received and completed a weekly online health questionnaire covering nine areas concerning medication changes, falls, injuries, health changes, emergency room visits, depression, changes to living space, vacations, and visitors. Research assistants contacted participants who failed to complete their online health questionnaire on-time.
An online database and project tracking software, “The Console”, was created to examine the status of daily data transfer and quality[30]. The software also provided a secure interface to access data summaries and plots of activity, alerting staff to equipment malfunction (e.g., dead sensor battery), changes in behavior pattern (e.g., decline in sensor firings), and non-compliance (e.g., failure to complete the weekly health questionnaire). Home monitoring systems were remotely accessed for trouble-shooting or software upgrades. Assistance was available throughout the study by a helpline or email to answer questions and to perform in-person computer fixes.
2.5. Computer Use Algorithm
Computer sessions were calculated using mouse movement data. Each mouse movement of more than five pixels generated a Windows event that was saved and time-stamped. Each day was partitioned into 5 minute periods, and for any period with more than 100 mouse events the computer was considered `in use'. The value of 100 events was chosen empirically to remove a slow drift of mouse position during periods of inactivity. The total time on the computer per day was then estimated as the sum of these 5-minute in-use periods, measured in minutes. Number of days with any computer usage was summed for each month on-study. Mean daily usage (in hours) was the sum of total time on the computer per month divided by total number of days with usage in the month. Coefficient of Variation (COV) of computer use per month was a measure of the variability or consistency in day-to-day usage per month. Monthly COV of usage was calculated as follows: generate total usage per day (including days with no usage), calculate mean and SD for each month. The coefficient of variation equaled the ratio of the monthly standard deviation to its mean multiplied by 100 (a dimensionless number). Mean daily use and COV of usage were log-transformed to achieve normal distributions.
2.6. Data Analysis
Characteristics at baseline including demographics, neuropsychological test scores and general computer experience (novice or more experienced) were compared between cognitively intact and MCI using Student's t-test or Wilcoxon Ranked Sum Test for continuous variables and Pearson Chi-Square test for categorical variables. Computer usage during the baseline month was compared between intact and MCI participants as well. Linear mixed effects models were generated with the three computer activity variables (number of days in use per month, mean daily use (in minutes) and day-to-day variability in use (COV)) as unique outcomes, adjusted for age, gender, education, race, mood (as measured by GDS), and computer proficiency, and including both random intercepts and random time scales (i.e., months from baseline). A sensitivity analysis was performed in which non-white participants were excluded from the analysis. As a post-hoc analysis we repeated the models with MCI sub-grouped as amnestic or non-amnestic MCI subtypes. All analyses were performed using SAS 9.2 (Cary, NC).
3. Results
Baseline characteristics of the 113 computer users are given in Table 1. Participants were older adults (mean age: 85 years), 92 (81%) female, 14 (12%) non-white with a mean of 15 years of education. The volunteers were relatively healthy (mean CIRS: 21; range of possible scores = 14 – 70) and free from dementia (mean MMSE = 29; mean FAQ = .75). About half of participants (46%) were considered novice computer users at entry. Over 230,000 computer sessions from 124,000 participant-days of use across the cohort were recorded during a mean of 36 ± 6 (SD) months of follow-up. Thirty-eight of these participants had MCI (9 amnestic MCI; 29 nonamnestic). MCI participants did not differ from the cognitively intact according to demographics or baseline computer experience. MCI participants scored lower on all six neuropsychological tests as compared to cognitively intact participants. During the study period 15 participants died or withdrew, the drop-out rate was similar among MCI and intact participants.
Table 1.
Baseline characteristics between MCI and cognitively intact participants
| Characteristic | Cognitively Intact (n=75) | MCI (n=38) | P value |
|---|---|---|---|
| Age (yrs) | 84.6 ± 4.3 | 84.3 ± 4.8 | 0.67 |
| Gender (% Women) | 79% | 84% | 0.48 |
| Non-white (%) | 5% | 24% | <0.01 |
| Education (yrs) | 15.4 ± 2.5 | 15.5 ± 2.2 | 0.67 |
| Novice user at entry (%) | 42% | 53% | 0.28 |
| Duration of follow-up (yrs) | 3.0 ± 0.4 | 3.0 ± 0.5 | 0.24 |
| MMSE | 28.9 ± 1.4 | 28.5 ± 1.4 | 0.1 |
| GDS | 0.8 ± 1.1 | 0.9 ± 1.1 | 0.6 |
| FAQ | 0.5 ± 1.3 | 1.1 ± 2.6 | 0.54 |
| CIRS | 21.0 ± 2.7 | 21.3 ± 3.9 | 0.84 |
| Logical Memory Delayed | 12.6 ± 3.6 | 10.2 ± 4.2 | 0.004 |
| Category Fluency: Animals | 19.0 ± 4.9 | 14.5 ± 4.7 | <0.0001 |
| Trail Making Test Part B | 99.4 ± 31.8 | 164.3 ± 69.8 | <0.0001 |
| WAIS Digit Symbol Test | 42.1 ± 8.0 | 31.8 ± 9.9 | <0.0001 |
| Boston Naming Test | 26.8 ± 2.1 | 24.2 ± 3.4 | <0.0001 |
| WAIS-R Block Design | 23.4 ± 6.9 | 18.5 ± 8.0 | 0.005 |
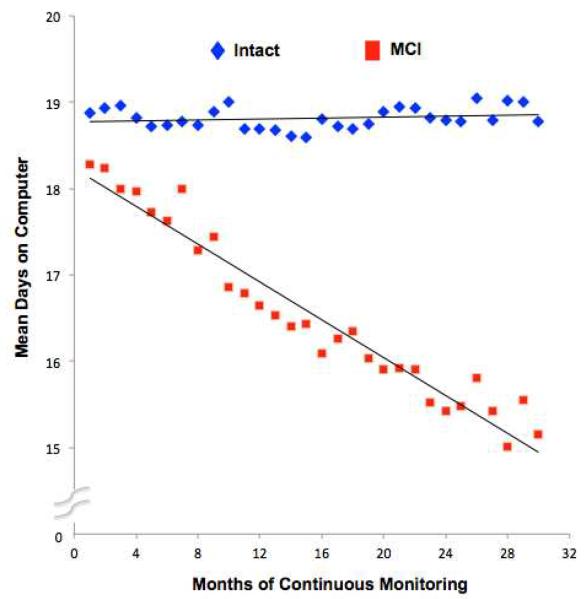
MCI participants did not differ from normal participants during the baseline month according to number of days with use, mean daily use (in hrs) or COV of usage (Table 2). Figure 1 presents the differences in predicted mean days on computer (in hrs) by study month for both MCI and cognitively intact participants. During years 2 and 3 of follow-up MCI participants spent consistently fewer days on the computer than cognitively intact participants. There was no difference in number of days/month with use (p=0.78) at baseline between the MCI and intact groups controlling for age, sex, education, race, mood (as measured by GDS), and computer proficiency. However, there was a decrease in number of days/month with use over time among MCI compared to intact cases (p=0.01) (Model 1, Table 3). There was no difference in mean daily use (p=0.71) at baseline between the MCI and intact groups adjusted for covariates. However, there was a decrease in mean daily use over time among MCI (~1% greater decrease/month, i.e., e−0.008=0.992) compared to intact cases; p=0.01) (Model 2, Table 3). Finally adjusted day-to-day variability in usage did not differ between the MCI and intact groups at baseline (p=0.65). However, there was an increase in variability (COV) of usage over time among MCI compared to intact cases (p=0.002) (Model 3, Table 3). In a sensitivity analysis we excluded non-white participants from the analysis; all results (MCI*time interactions) remained significant.
Table 2.
Computer use during baseline month between MCI and cognitively intact participants
| Activity measure | Cognitively Intact (n=75) | MCI (n=38) | p-value |
|---|---|---|---|
| Baseline days with computer use (#) | 19.8 ± 8.9 | 17.7 ± 8.0 | 0.20 |
| Baseline daily mean use (hrs) | 1.5 ± 1.1 | 1.5 ± 1.7 | 0.06 |
| Baseline coefficient of variation (COV) of usage | 112.2 ± 65.1 | 129.9 ± 66.1 | 0.11 |
Figure 1.
Mean days on computer by study month in MCI and cognitively intact participants.
Table 3.
Results of multivariate linear mixed effects models
| Model 1 Days on Computer/Month | Model 2 Daily Mean Use/Month | Model 3 COV of Use/Month | ||||
|---|---|---|---|---|---|---|
| Covariate | Coefficient | p-value | Coefficient | p-value | Coefficient | p-value |
| Age (yrs) | −0.422 | 0.01* | −0.015 | 0.25 | 0.026 | 0.03* |
| Female | 0.719 | 0.69 | 0.174 | 0.23 | −0.056 | 0.65 |
| Education (yrs) | −0.060 | 0.84 | −0.021 | 0.35 | −0.002 | 0.94 |
| Novice User | −2.858 | 0.06 | −0.549 | <0.0001*** | 0.206 | 0.04* |
| Non-white | −4.762 | 0.06 | 0.005 | 0.98 | 0.373 | 0.03* |
| MCI at baseline | 0.436 | 0.78 | 0.045 | 0.71 | −0.048 | 0.65 |
| Duration (months) | −0.030 | 0.38 | 0.0003 | 0.88 | 0.0003 | 0.90 |
| Novice * Duration | −0.082 | 0.08 | −0.005 | 0.057 | 0.006 | 0.069 |
| MCI * Duration | −0.119 | 0.01* | −0.008 | <0.01** | 0.010 | 0.002** |
p<0.05,
p<0.01,
p<0.0001
In post-hoc analysis MCI participants were sub-classified as amnestic MCI (n=9) and non-amnestic MCI (n=29) and the three mixed effects models were re-generated. In all three instances, both sub-groups showed trends in the original direction with non-amnestic MCI participants maintaining significance (p < 0.05) while amnestic MCI participants did not reach significance. Additionally, since we collect information on when participants take vacations or leave home due to medical issues (i.e. hospitalization) we were able to verify that there were no overall differences in days out of the house between MCI and intact participants (6% of days overall) where presumably no computer use could occur.
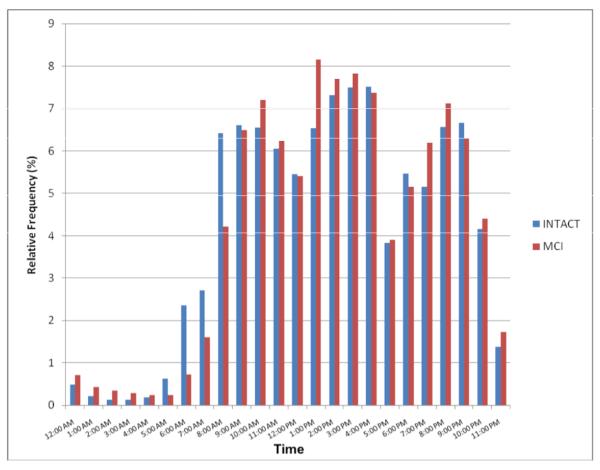
There were significant differences in usage depending on the time of day (p<0.0001), although the relative frequency of when participants logged onto their computers by hour of the day was not different between MCI and cognitively intact participants (Figure 2). On average, the most common time period of day to log on was between 1:00 and 5:00 pm.
Figure 2.
Aggregated computer session start times (relative frequency) between MCI and cognitively intact participants by time of day.
4. Discussion
We have demonstrated for the first time that an IADL – computer use - can be continuously monitored in seniors' homes for years without major intrusions in the participant's usual routine. We have further shown for the first time, that computer use is significantly different over time between independently living older persons with MCI and age-matched, non-cognitively impaired volunteers. This suggests that unobtrusive instrumentation of the home environment is a promising new approach to detecting ecologically valid change in a person's daily function. Computer time use as reported here is a simple measure that may reflect cognitive decline indirectly because users find it more effortful to navigate and use what is often a complex and changing interface. As a result they may increasingly keep their sessions brief and less frequent. There is little data with regard to how people with MCI perceive their computer performance over time. People with MCI have a general lack of awareness of deficit or anosognosia for their cognitive impairment, as well as their functional ability when tested in the clinic[31, 32]. This may extend to their awareness of computer use proficiency. A relative lack of awareness of computer self-efficacy may explain the notable sustained use of their computer, albeit at a lower intensity for many months. At annual ISAAC assessments participants were asked about their attitudes and beliefs regarding computer use (data not shown). There were significant differences in several areas of computer self-efficacy between MCI and cognitively intact persons. MCI volunteers reported less confidence and more anxiety over time while using their computer relative to cognitively intact seniors who gained confidence[33].
Because the cohort of MCI participants was largely of the non-amnestic MCI subtype (a proportion similar to other community cohort studies)[34–37], the computer use results may be characteristic of those with non-amnestic MCI. In post-hoc analyses, we found similar trends in declining computer usage over time among non-amnestic and amnestic MCI alike, but the smaller number of amnestic MCI cases does not allow a definitive conclusion to be made at this time. Further, this cohort of MCI participants is best considered to be composed of very early MCI since they were required to score at a minimum 1.5 SD below the mean of their peers on only one or more of the cognitive tests in the battery. The early MCI character of this group is further supported by the fact that these were people with MCI at entry who lived alone and by definition needed to function independently on their own. Note at entry they were able to learn to use a computer to criterion (able to reliably send and receive email). Nevertheless, during follow-up, we observed a significant decline in cognitive test scores while the functional measures (FAQ) remained relatively stable. Thus, none progressed to dementia. The MCI group's declining scores remained consistently lower compared to the intact group throughout the follow-up period. These studies suggest that continuous assessment of computer use is sensitive to subtle early decline in MCI. It will take longer follow-up to identify the ultimate clinical and neuropathological outcomes of these volunteers.
Other measures of computer use that reflect cognitive function may also be unobtrusively obtained. For example, when a user logs on to the computer typing their password, this forms an opportunity to record inter keystroke intervals. This repeated computer use task thus forms an everyday measure of psychomotor speed akin to finger tapping tasks performed in the clinic[38] which have been shown to change around the time of dementia diagnosis[39]. Other means of taking advantage of the routine use of a computer without formal testing may reside in monitoring game playing behavior patterns for those who enjoy playing computer-based games[40].
The ISAAC cohort is relatively healthy, educated, and largely composed of computer novices at entry. Future research will need to determine the extent to which prior levels of experience or training influences the decline in computer use seen in our MCI cohort. Other factors that may affect computer use may be important to capture in future studies such as lifelong interest in learning and cognitive engagement. Because computer use among the current population over age 65 is relatively low (estimated as 46%,[1]), although growing rapidly, some may argue that this approach is not generalizable. However, as noted in the introduction, the next generation of seniors have begun to turn 65 and this group is a much more active computer using generation[1]. Thus, the majority of future seniors at risk for MCI and dementia may be on-line, accessible through the Internet and with their consent could be assessed without intrusion across a broad range of computer use attributes on a wide-scale for early signals of cognitive decline. This approach is inherently highly scalable and less costly than person-to-person screening. For those who advocate for screening for cognitive impairment (there are clearly pros and cons to the issue), computer use monitoring lowers the cost barrier.
Obviously there are important privacy and security issues that must be kept in mind. As is widely known, currently, anyone routinely using the Internet and any standard search engine such as Google is being monitored for use patterns. Accordingly, there are important considerations as to how and why data is recorded when a computer is used. With this in mind, we and others have studied closely the attitudes and beliefs of older adults and their use of technologies and have found that there is overall wide acceptance of the use of remote unobtrusive health and functional assessments including computer use monitoring as long as it is performed with the intent of providing opportunities for early warning of decline or ongoing health maintenance [41–43]. Thus, for example, in a study of attitudes and beliefs about remote ambient home-based monitoring among older persons, the acceptance by older adults of unobtrusive in-home monitoring was closely tied to the perceived utility of the data generated by such systems[41]. In a study of particular relevance to the current study, a majority of patients with MCI, as well as similarly aged older adults without cognitive impairment were willing to share computer use data specifically with one's doctor or family members [43]. Interestingly, the MCI group was even more accepting of computer use monitoring than those without MCI indicating again that concerns about privacy or security may be less among those with the most perceived potential to gain from ongoing monitoring. In this case we suggest that this reflects the attitude of many older adults who are willing to make concessions to their privacy if such compromises may facilitate their remaining in their home away from institutional care.
For individuals who are not computer users there are many other IADLs that lend themselves to this remote assessment approach. For example, medication-taking behavior can be readily assessed by instrumentation of a common 7-day pill-box so that the time and date of compartment openings is recorded. This approach has shown in a five week study that those with MCI are significantly less able to adhere to a medication taking regimen than those with better cognitive function[4]. Many other commonly used devices such as telephones, televisions and kitchen appliances are amenable to similar instrumentation. Because the set-up process involves a one-time visit to a home, one can imagine that multiple indicators of IADL's can be deployed and interrogated with this approach and theoretically the sensitivity of detecting early change would be increased by combining or fusing multiple domains of activity. Ultimately these data may lead to a convergence of the “formal” episodic assessment of cognitive domains thought to be key to performing meaningful tasks and the direct measurement of these tasks themselves in real time at home or in the community.
Acknowledgements
The study was funded by grants from the National Institutes of Health: P30AG024978, R01AG024059, P30 AG008017, R01 AG033581 and the Intel Corporation. The authors thank all the participants and research staff of the ISAAC Study.
Dr. Kaye receives research support from the Department of Veterans Affairs and the National Institutes of Health (NIH), directs centers that receive research support from the NIH, Johnson & Johnson, Roche, Bristol Myers Squibb; receives compensation for serving on the global advisory board for the INROADS patient registry sponsored by Janssen; receives reimbursement through Medicare or commercial insurance plans for providing clinical assessment and care for patients; is salaried to see patients at the Portland VA Medical Center; and serves on the editorial advisory board of the journals, Alzheimer's & Dementia and Frontiers of Aging Neuroscience.
Dr. Dodge receives research support from the NIH; and serves on the Statistical Review Board for International Psychogeriatrics.
Dr. Hayes receives research support from the NIH. She has a significant financial interest in Intel Corporation, a company that may have a commercial interest in the results of this research and technology. This potential conflict has been reviewed and managed by OHSU.
Dr. Jimison receives research support from the NIH and NSF.
Dr. Pavel has received research support from the NIH and NSF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosures
Ms. Mattek reports no disclosures.
Mr. Campbell reports no disclosures.
Mr. Austin reports no disclosures.
Mr. Hatt reports no disclosures.
Dr. Wild reports no disclosures.
She reports no disclosures.
He reports no disclosures.
References
- 1. [(Accessed January 18, 2012)]; http://pewinternet.org/Reports/2010/Generations-2010.aspx.
- 2. [(Accessed February 14, 2012)]; http://blog.nielsen.com/nielsenwire/online_mobile/generation-app-62-of-mobile-users-25-34-own-smartphones/.
- 3.Petersen RC, Roberts RO, Knopman DS, Boeve BF, Geda YE, Ivnik RJ, Smith GE, Jack CR., Jr. Mild cognitive impairment: ten years later. Archives of Neurology. 2009;66:1447–1455. doi: 10.1001/archneurol.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayes TL, Cobbinah K, Dishongh T, Kaye JA, Kimel J, Labhard M, Leen T, Lundell J, Ozertem U, Pavel M, et al. A study of medication-taking and unobtrusive, intelligent reminding. Telemed J E Health. 2009;15:770–776. doi: 10.1089/tmj.2009.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Artero S, Touchon J, Ritchie K. Disability and mild cognitive impairment: a longitudinal population-based study. International Journal of Geriatric Psychiatry. 2001;16:1092–1097. doi: 10.1002/gps.477. [DOI] [PubMed] [Google Scholar]
- 6.Binegar DL, Hynan LS, Lacritz LH, Weiner MF, Cullum CM. Can a direct IADL measure detect deficits in persons with MCI? Current Alzheimer's Research. 2009;6:48–51. doi: 10.2174/156720509787313880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galasko D, Bennett DA, Sano M, Marson D, Kaye J, Edland SD. ADCS prevention instrument project: Assessment of instrumental activities of daily living for community-dwelling elderly individuals in dementia prevention clinical trials. Alzheimer Disease and Associated Disorders. 2006;20(Suppl 3):S152–169. doi: 10.1097/01.wad.0000213873.25053.2b. [DOI] [PubMed] [Google Scholar]
- 8.Peres K, Helmer C, Amieva H, Orgogozo JM, Rouch I, Dartigues JF, Barberger-Gateau P. Natural history of decline in instrumental activities of daily living performance over the 10 years preceding the clinical diagnosis of dementia: a prospective population-based study. Journal of American Geriatrics Society. 2008;56:37–44. doi: 10.1111/j.1532-5415.2007.01499.x. [DOI] [PubMed] [Google Scholar]
- 9.Wadley VG, Crowe M, Marsiske M, Cook SE, Unverzagt FW, Rosenberg AL, Rexroth D. Changes in everyday function in individuals with psychometrically defined mild cognitive impairment in the Advanced Cognitive Training for Independent and Vital Elderly Study. Journal of American Geriatrics Society. 2007;55:1192–1198. doi: 10.1111/j.1532-5415.2007.01245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farias ST, Mungas D, Reed BR, Harvey D, Cahn-Weiner D, Decarli C. MCI is associated with deficits in everyday functioning. Alzheimer Disease and Associated Disorders. 2006;20:217–223. doi: 10.1097/01.wad.0000213849.51495.d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuokko H, Morris C, Ebert P. Mild cognitive impairment and everyday functioning in older adults. Neurocase. 2005;11:40–47. doi: 10.1080/13554790490896802. [DOI] [PubMed] [Google Scholar]
- 12.Allaire JC, Gamaldo A, Ayotte BJ, Sims R, Whitfield K. Mild cognitive impairment and objective instrumental everyday functioning: the everyday cognition battery memory test. Journal of American Geriatrics Society. 2009;57:120–125. doi: 10.1111/j.1532-5415.2008.02054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bangen KJ, Jak AJ, Schiehser DM, Delano-Wood L, Tuminello E, Han SD, Delis DC, Bondi MW. Complex activities of daily living vary by mild cognitive impairment subtype. Journal of International Neuropsychology Society. 2010;16:630–639. doi: 10.1017/S1355617710000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pereira F, Yassuda M, Oliveira A, Diniz B, Radanovic M, Talib L, Gattaz W. Profiles of functional deficits in mild cognitive impairment and dementia: benefits from objective measurement. Journal of the International Neuropsychological Society. 2010;16:297–305. doi: 10.1017/S1355617709991330. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg TE, Koppel J, Keehlisen L, Christen E, Dreses-Werringloer U, Conejero-Goldberg C, Gordon ML, Davies P. Performance-based measures of everyday function in mild cognitive impairment. American Journal of Psychiatry. 2010;167:845–853. doi: 10.1176/appi.ajp.2010.09050692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim KR, Lee KS, Cheong HK, Eom JS, Oh BH, Hong CH. Characteristic profiles of instrumental activities of daily living in different subtypes of mild cognitive impairment. Dementia and Geriatric Cognitive Disorders. 2009;27:278–285. doi: 10.1159/000204765. [DOI] [PubMed] [Google Scholar]
- 17.Kaye JA, Maxwell SA, Mattek N, Hayes TL, Dodge H, Pavel M, Jimison HB, Wild K, Boise L, Zitzelberger TA. Intelligent Systems For Assessing Aging Changes: home-based, unobtrusive, and continuous assessment of aging. Journa of Gerontology B Psychological Sciences. 2011;66(Suppl 1):i180–190. doi: 10.1093/geronb/gbq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 20.Sheikh J, Yesavge J. Geriatric Depression Scale (GDS. Recent evidence and development of a shorter verson. In: Brink TL, editor. In Clinical Gerontology: A Guide to Assessment and Intervention. The Haworth Press, Inc.; NY: 1986. pp. 165–173. [Google Scholar]
- 21.Pfeffer RI, Kurosaki TT, Harrah CH, Jr., Chance JM, Filos S. Measurement of functional activities in older adults in the community. Journal of Gerontolology. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 22.Parmelee P, Thuras P, Katz I, Lawton M. Validation of the Cumulative Illness Rating Scale in a geriatric residential population. Journal of the American Geriatrics Society. 1995;43:130–137. doi: 10.1111/j.1532-5415.1995.tb06377.x. [DOI] [PubMed] [Google Scholar]
- 23.Peterson R. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 24.Wechsler D. Wechsler Memory Scale - Revised. Psychological Corporation; New York: 1987. [Google Scholar]
- 25.Wechsler D. Manual for the Wechsler Adult Intelligence Scale - revised. Psychological Corporation; New York: 1981. [Google Scholar]
- 26.Jastak S, Wilkinson G. The Wide Range Achievement Test - Revised. Jastak Associates, Inc; Wilmington: 1984. [Google Scholar]
- 27.Kaplan E, Goodglass H, Weintraube S. Boston Naming Test. Lea & Febiger; Philadelphia: 1983. [Google Scholar]
- 28.Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, Cummings J, DeCarli C, Foster NL, Galasko D, et al. The Alzheimer's Disease Centers' Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Disease and Associated Disorders. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hagler S, Austin D, Hayes TL, Kaye J, Pavel M. Unobtrusive and ubiquitous in-home monitoring: a methodology for continuous assessment of gait velocity in elders. IEEE Transactions on Biomedical Engineering. 2010;57:813–820. doi: 10.1109/TBME.2009.2036732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayes T, Kaye J. Gathering the evidence: Supporting large-sale research deployments. Intel Technology Journal. 2009;13:148–167. [Google Scholar]
- 31.Okonkwo OC, Griffith HR, Vance DE, Marson DC, Ball KK, Wadley VG. Awareness of functional difficulties in mild cognitive impairment: a multidomain assessment approach. Journal of American Geriatrics Society. 2009;57:978–984. doi: 10.1111/j.1532-5415.2009.02261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tremont G, Alosco ML. Relationship between cognition and awareness of deficit in mild cognitive impairment. International Journal of Geriatric Psychiatry. 2011;26:299–306. doi: 10.1002/gps.2529. [DOI] [PubMed] [Google Scholar]
- 33.Wild K, Mattek N, Maxwell S, Dodge H, Jimison H, Kaye J. Computer related self-efficacy and anxiety in older adults with and without mild cognitive impairment. Alzheimer's & Dementia. 2012 doi: 10.1016/j.jalz.2011.12.008. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ganguli M, Chang CC, Snitz BE, Saxton JA, Vanderbilt J, Lee CW. Prevalence of mild cognitive impairment by multiple classifications: The Monongahela-Youghiogheny Healthy Aging Team (MYHAT) project. American Journal of Geriatric Psychiatry. 2010;18:674–683. doi: 10.1097/JGP.0b013e3181cdee4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ganguli M, Dodge HH, Shen C, DeKosky ST. Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology. 2004;63:115–121. doi: 10.1212/01.wnl.0000132523.27540.81. [DOI] [PubMed] [Google Scholar]
- 36.Lopez OL, Jagust WJ, DeKosky ST, Becker JT, Fitzpatrick A, Dulberg C, Breitner J, Lyketsos C, Jones B, Kawas C, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Archives of Neurology. 2003;60:1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- 37.Manly JJ, Tang MX, Schupf N, Stern Y, Vonsattel JP, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Annals of Neurology. 2008;63:494–506. doi: 10.1002/ana.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Austin D, Jimison H, Hayes T, Mattek N, Kaye J, Pavel M. Measuring motor speed through typing: a surrogate for the finger tapping test. Behavior Research Methods. 2011;43:903–909. doi: 10.3758/s13428-011-0100-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buracchio T, Dodge HH, Howieson D, Wasserman D, Kaye J. The trajectory of gait speed preceding mild cognitive impairment. Archives of Neurology. 2010;67:980–986. doi: 10.1001/archneurol.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jimison H, Pavel M, McKanna J, Pavel J. Unobtrusive monitoring of computer interactions to detect cognitive status in elders. IEEE Transactions on Information Technology Biomedicine. 2004;8:248–252. doi: 10.1109/titb.2004.835539. [DOI] [PubMed] [Google Scholar]
- 41.Wild K, Boise L, Lundell J, Foucek A. Unobtrusive monitoring of cognitive and physical health: Reactions and perceptions of older adults. Journal of Applied Gerontology. 2008;27:181–200. doi: 10.1177/0733464807311435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steele R, Lo A, Secombe C, Wong Y. Elderly persons' perceptions and acceptance of using wireless sensor networks to assist healthcare. International Journal of Medical Informatics. 2009;78:788–801. doi: 10.1016/j.ijmedinf.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 43.Boise L, Wild K, Mattek N, Ruhl M, Dodge H, Kaye J. Willingness of older adults to share data and privacy concerns after exposure to unobtrusive in-home monitoring. Gerontechnology. 2013 doi: 10.4017/gt.2013.11.3.001.00. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]