Low serotonin (5-hydroxytryptamine, 5-HT) neurotransmission is hypothesized in the pathophysiology of suicide (for review see Bach and Arango, 2012; Mann, 2003; Träskman et al., 1981). Cerebrospinal fluid 5-hydroxyindoleacetic acid (5-HIAA) in depressed suicide attempters is lower compared to controls (Lidberg et al., 2000; Mann et al., 1996; Mann and Malone, 1997; Placidi et al., 2001; Träskman et al., 1981). In four out of five postmortem studies of brainstem (Beskow et al., 1976; Bourne et al., 1968; Lloyd et al., 1974; Pare et al., 1969; Shaw et al., 1967), suicides had less 5-HT or 5-HIAA compared to controls. In contrast, most postmortem studies report no differences in cortical 5-HT or 5-HIAA in suicides compared to controls (Arango and Mann, 1992; Arato et al., 1987; Arranz et al., 1997; Beskow et al., 1976; Cochran et al., 1976; Mahadik et al., 1988).
5-HT in the forebrain is synthesized by brainstem dorsal and median raphe nuclei (DRN and MRN) 5-HT neurons. Tryptophan hydroxylase 2 (TPH2) is the neuron-specific, rate-limiting enzyme for the synthesis of 5-HT (Alenina et al., 2009; Patel et al., 2004; Zhang et al., 2004). We found more 5-HT neurons (Underwood et al., 1999), more TPH2 protein (Boldrini et al., 2005; Underwood et al., 1999) and mRNA (Bach-Mizrachi et al., 2006; Bach-Mizrachi et al., 2008) in DRN and MRN of suicides. We sought to reconcile these three sets of observations in suicides: low 5-HIAA or 5-HT in brainstem, unchanged levels in the cortex and more 5-HT neurons containing more TPH2. We therefore performed a pilot study measuring 5-HT and 5-HIAA by high pressure liquid chromatography (HPLC), sampling the brainstem along the axis of DRN and MRN, and in dorsolateral prefrontal cortex (PFC), in unmedicated controls and depressed suicides, with postmortem intervals of less than 24 hours.
All procedures for brain collection and psychological autopsy were approved by the applicable Institutional Review Boards. This study included 8 nonpsychiatric sudden death controls and 6 suicides (Table 1). Psychiatric diagnosis in the suicides and absence of diagnoses in the controls was determined by the Structured Clinical Interview for DSM IV (SCID-I and II) as part of a psychological autopsy described elsewhere (Kelly and Mann, 1996). All brains were free of gross neuropathology and had negative brain toxicology for psychoactive and neurotoxic drugs. PMI was not different between groups (C: 12.4 ± 3.7 hours; S: 10.5 ± 6.1 hours, p=0.5).
Table 1.
Subject Demographics
| Case | Subject | Age | Sex | PMI | Brain pH | Manner of Death | Brain Toxicology | Axis1 |
|---|---|---|---|---|---|---|---|---|
| 1 | Control | 18 | Male | 16 | 6.8 | MVA-Pedestrian | Clear | NONE |
| 2 | Control | 18 | Male | 11 | 7.07 | Accidental Fall from Height | Clear | NONE |
| 3 | Control | 19 | Male | 13 | 6.84 | CV Disease | Clear | NONE |
| 4 | Control | 32 | Male | 13 | 6.75 | CV Disease | Lidocaine | NONE |
| 5 | Control | 51 | Female | 7 | 6.7 | MVA-Pedestrian | Clear | NONE |
| 6 | Control | 54 | Male | 13 | 6.34 | CV Disease | Clear | NONE |
| 7 | Control | 69 | Male | 8 | 5.8 | Gun Shot Wound | Clear | NONE |
| 8 | Control | 75 | Female | 18 | 6.2 | MVA-Pedestrian | Clear | NONE |
| 9 | Suicide | 26 | Male | 22 | 6.8 | Hanging-Suicide | Clear | Pathological Gambling |
| 10 | Suicide | 33 | Male | 6 | 6.7 | Hanging-Suicide | Clear | Adjustment Disorder |
| 11 | Suicide | 42 | Male | 7 | 6.55 | Hanging-Suicide | Clear | MDD |
| 12 | Suicide | 46 | Male | 6 | 6.6 | Jump from height-Suicide | Clear | Schizophrenia |
| 13 | Suicide | 17 | Male | 12 | 6.4 | Acid Ingestion-Suicide | Clear | MDD |
| 14 | Suicide | 64 | Male | 10 | 6.8 | Acid Ingestion-Suicide | Clear | MDD |
Abbreviations: CV: Cardiovascular Disease, MVA: Motor Vehicle Accident, MDD: Major Depressive Disorder
The brainstem was dissected at autopsy, frozen immediately and stored at -80°C until sectioning in a cryostat. Transverse sections were cut at 60 μm thickness and stored in Eppendorf tubes at -80°C until assayed. pH was measured in cerebellar tissue (Harrison et al., 1995). Sections were collected every millimeter corresponding to 16-20 sections per case. Sets of adjacent sections, stained for Nissl substance, were used to help identify the neuroanatomical levels examined. The goal was to ensure comparable coverage of the raphe along the rostrocaudal axis to account for its anatomical variability. Brodmann Area 9 (BA9) was dissected frozen from coronal slabs of the hemisphere, meninges were removed and samples were dissected as much as possible from gray matter.
5-HT and 5-HIAA were measured in brainstem sections and BA9 using reverse-phase HPLC with electrochemical detection. The samples were homogenized in 0.5 ml ice-cold 0.4M perchloric acid, centrifuged (5 minutes at 14,000g) and a 50 μl aliquot of the supernatant was injected over a Waters HPLC system. The mobile phase contained 0.75 mM sodium phosphate (pH 3.1), 1.4 mM 1-octanesulphonic acid, 10 M ethylenediaminetetraacetic acid (EDTA) and 8% acetonitrile. The flow rate was 1.0 ml/min. A standard curve was generated with external standards. Values were calculated based on peak area and compared to the standard calibration. The inter- and intra-assay coefficients of variation of the assay were <5%. The sensitivity was < 0.5 pmol.
Analysis of Variance (ANOVA, SPSS Statistics Version 17.0 software) was used to determine the statistical significance of differences in total amount of 5-HT or 5-HIAA with fixed variables being group (control or suicide) and covariates being age, PMI, pH or neuroanatomical level after matching based on adjacent Nissl stained sections. Effects of age, PMI or pH were determined using regression analysis.
Higher 5-HT in rostral brainstem was consistent with the greater number of 5-HT synthesizing neurons in the midbrain. 5-HT correlated with 5-HIAA along the rostrocaudal axis (r = 0.837, p = 0.001) of the raphe nuclei measured. Compared with nonpsychiatric control cases, suicides had four times as much total 5-HT (nonpsychiatric controls: 271 ± 58 vs. suicides: 1091 ± 280 picomoles/mg protein, t = -2.87, p = 0.017) and 1.5 times more 5-HIAA (nonpsychiatric controls: 4158 ± 534 vs. suicides: 6404 ± 898 picomoles/mg protein t = -2.15, p = 0.05). The difference in 5-HT between controls and suicides was present throughout the rostrocaudal extent of the brainstem samples (Figure 1; F = 95.9, p < 0.0001). The differences in 5-HT and 5-HIAA amounts in suicides and controls were independent of age and postmortem interval (p > 0.05). The sample size was too small to determine a relationship of MDD or other Axis 1 diagnoses to either 5-HT or 5-HIAA. To index 5-HT turnover, we calculated the ratio of 5-HIAA to 5-HT. The 5-HIAA:5-HT ratio in the brainstem was 15.3 in controls and 5.9 in suicides (co-varying for age and sex, t = 2.76, p = 0.02).
Figure 1.
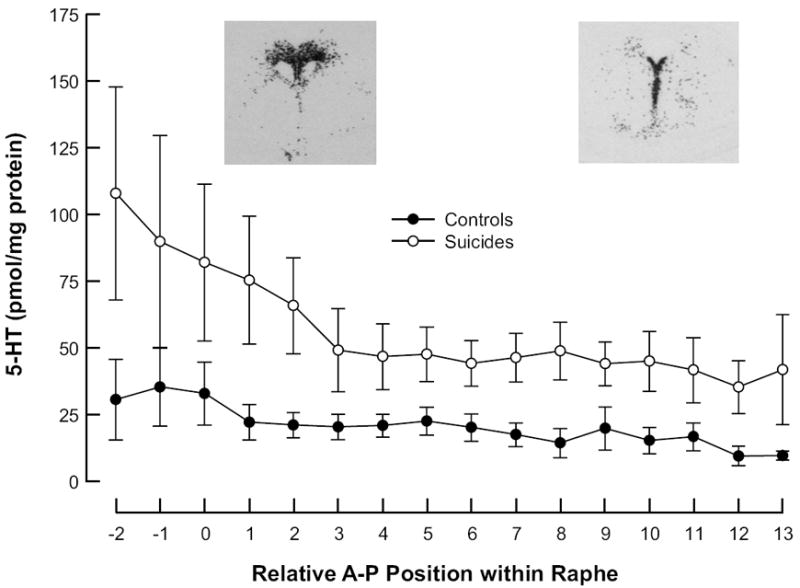
Distribution of 5-HT in the brainstem. Note that suicides (empty circles) have more 5-HT throughout the rostrocaudal extent of the DRN than controls (filled circles). Rostral is to the left, 0mm is the DRN peak area and caudal is to the right; the inserts are corresponding autoradiograms of TPH2 mRNA. These plots include the male subjects only; the 2 control females were excluded from the plot but not the analyses because their values were markedly higher than the males.
5-HT in prefrontal cortex (BA9) of suicides was comparable to nonpsychiatric controls (Controls: 1.6 ± 0.5 vs. Suicides: 2.1 ± 1.9 picomoles/mg protein; F = 0.8, p = 0.376). Mean 5-HIAA in suicides was 42% of controls but was not statistically significant (Controls: 11.6 ± 9.6 vs. Suicides: 5.6 ± 4.4 picomoles/mg protein, F = 2.1, p = 0.174), however, the mean 5-HIAA:5-HT ratio in BA9 was much lower in suicides (6.8 in controls vs. 2.8 in suicides, t = 2.1, p = 0.05). Cortical 5-HIAA levels are comparable with published values (Cheetham et al., 1989; Stanley et al., 1985) suggesting that assay sensitivity and tissue quality are comparable to other studies. Our observed difference in mean cortical 5-HIAA between suicides and controls, although not statistically significant, is comparable to differences observed by others (Beskow et al., 1976; Crow et al., 1984; Owen et al., 1983). In suicides, brainstem 5-HIAA and 5-HT were negatively correlated with cortical 5-HIAA and 5-HT (5-HT: r = - 0.79, p = 0.02, 5-HIAA: r = -0.85, p = 0.007). The same was true for controls (5-HT: r = - 0.85, p = 0.007; 5-HIAA: r = - 0.79 p = 0.02).
We found more brainstem 5-HT and 5-HIAA in suicides compared with nonpsychiatric, sudden death controls. The difference was found throughout the rostrocaudal extent of the brainstem sampled, suggesting that 5-HT synthesis in suicides is greater within all DRN subnuclei and the MRN compared with controls. In both brainstem and prefrontal cortex (PFC), the ratio of 5-HIAA:5-HT was lower in suicides compared with controls suggesting a lower rate of turnover in suicides. However, since brainstem 5-HIAA is still 50% higher in suicides than controls, the results do not indicate lower 5-HT neurotransmission in the brainstem of suicides. In contrast to the brainstem, there was no difference in 5-HT in PFC between suicides and controls suggesting that the extra brainstem 5-HT in suicides is not transported to or released from nerve terminals in the PFC.
Our finding of more brainstem 5-HT and 5-HIAA in suicides is not in agreement with most previous studies. Unlike those studies, we excluded subjects with long postmortem interval (>24h) and either a positive history of psychotropic use or toxicology screen of brain tissue, factors that can lower 5-HT or 5-HIAA levels. We also assayed multiple anatomical levels in the brainstem to encompass the rostral-caudal extent of the DRN and MRN to avoid the risk of systematic, or even random, errors in tissue dissection. The suicide group in our study has a 1.9 hour shorter mean PMI than controls, which was not statistically different and does not likely explain the differences between groups in 5-HT or 5-HIAA. Postmortem studies report that 5-HT decreases initially (Kontur et al., 1994; McIntyre and Stanley, 1984; Palmer et al., 1988) and is largely stable thereafter; 5-HIAA was reported to either increase or remain unchanged. Therefore, the 5-HT and 5-HIAA measures with the 6 to 22 hour range of PMIs in our study are likely stable. Though postmortem degradation would presumably affect both groups equally, we used PMI as a statistical covariate.
Of note, one study found more CSF 5-HT and no difference in CSF 5-HIAA in MDD compared to non-psychiatric controls (Gjerris et al., 1987) raising the possibility that the diagnosis of MDD is associated with elevated 5-HT levels. Our study included only three cases with MDD, and we saw comparable 5-HT and 5-HIAA levels in the non-MDD and MDD cases. Nevertheless, the comparable variability in the measures of 5-HT in the suicides to that of controls, suggests that the levels of 5-HT and 5-HIAA in the suicide group are relatively homogeneous regardless of the different diagnoses. The higher 5-HT in the brainstem of suicides we find is consistent with our observations of more 5-HT neurons, and greater TPH2 mRNA and protein in the brainstem of suicides (Bach-Mizrachi et al., 2006; Bach-Mizrachi et al., 2008; Boldrini et al., 2005; Underwood et al., 1999). There is other evidence of more 5-HIAA in MDD (reviewed in Andrews and Thomson, Jr., 2009). Patients with MDD have higher 5-HIAA in jugular venous blood, argued to reflect higher brain 5-HT neurotransmission and turnover (Barton et al., 2008). While findings of more 5-HT or 5-HIAA in blood and CSF of depressed subjects are not anatomically specific, and certainly not directly comparable to our measures in postmortem brain tissue, they lend support to a possible contribution of MDD to the elevated 5-HT that we detected. More cases are needed to distinguish effects of major depression from effects of suicide.
The main limitation of this pilot study is small sample size. Some of the previous studies in brainstem had comparable sample sizes: (Lloyd et al., 1974, 5 suicides, diagnosis not provided) and, (Cochran et al., 1976, 10 depressed suicides). Other studies (Beskow et al., 1976; Bourne et al., 1968; Pare et al., 1969; Shaw et al., 1967) had more cases, but also had a mixture of diagnoses and were further confounded by either long PMIs or positive toxicology. None of these studies sought to distinguish potential effects of suicide from those of MDD or other diagnoses. In fact, many of the suicides in these studies had diagnoses other than MDD, suggesting that the reported reduction in 5-HT or 5-HIAA in the brainstem in these studies was associated with suicide rather than MDD (Mann et al., 1989). In PFC, we did not find significant differences in 5-HT or 5-HIAA in suicides; this was not likely explained by the small number of cases since a separate analysis of PFC in 35 controls and 37 suicides in our database also did not demonstrate a significant difference. More 5-HT in the brainstem and no change in the cortex of suicides, independent of diagnosis, are suggestive of deficits in serotonergic neurotransmission. A larger number of cases including suicides with MDD and MDD subjects that did not suicide would confirm that the upregulation of 5-HT demonstrated here is a biological correlate of suicide.
Acknowledgments
Supporting Grants Information: This study was supported by the following NIMH grants: MH40210, MH62185 and MH64168.
Reference List
- Alenina N, Kikic D, Todiras M, Mosienko V, Qadri F, Plehm R, Boye P, Vilianovitch L, Sohr R, Tenner K, Hortnagl H, Bader M. Growth retardation and altered autonomic control in mice lacking brain serotonin. Proc Natl Acad Sci U S A. 2009;106:10332–10337. doi: 10.1073/pnas.0810793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews PW, Thomson JA., Jr The bright side of being blue: depression as an adaptation for analyzing complex problems. Psychol Rev. 2009;116:620–654. doi: 10.1037/a0016242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arango V, Mann JJ. Relevance of serotonergic postmortem studies to suicidal behavior. Int Rev Psychiatry. 1992;4:131–140. [Google Scholar]
- Arato M, Tekes K, Tothfalusi L, Magyar K, Palkovits M, Demeter E, Falus A. Serotonergic split brain and suicide. Psychiatry Res. 1987;21:355–356. doi: 10.1016/0165-1781(87)90019-9. [DOI] [PubMed] [Google Scholar]
- Arranz B, Blennow K, Eriksson A, Mansson JE, Marcusson J. Serotonergic, noradrenergic, and dopaminergic measures in suicide brains. Biol Psychiatry. 1997;41:1000–1009. doi: 10.1016/s0006-3223(96)00239-9. [DOI] [PubMed] [Google Scholar]
- Bach H, Arango V. Neuroanatomy of Serotonergic Abnormalities in Suicide. 2012 [PubMed] [Google Scholar]
- Bach-Mizrachi H, Underwood MD, Kassir SA, Bakalian MJ, Sibille E, Tamir H, Mann JJ, Arango V. Neuronal tryptophan hydroxylase mRNA expression in the human dorsal and median raphe nuclei: major depression and suicide. Neuropsychopharmacology. 2006;31:814–824. doi: 10.1038/sj.npp.1300897. [DOI] [PubMed] [Google Scholar]
- Bach-Mizrachi H, Underwood MD, Tin A, Ellis SP, Mann JJ, Arango V. Elevated expression of tryptophan hydroxylase-2 mRNA at the neuronal level in the dorsal and median raphe nuclei of depressed suicides. Mol Psychiatry. 2008;13:507–513. doi: 10.1038/sj.mp.4002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beskow J, Gottfries CG, Roos BE, Winblad B. Determination of monoamine and monoamine metabolites in the human brain: Post mortem studies in a group of suicides and in a control group. Acta Psychiatr Scand. 1976;53:7–20. doi: 10.1111/j.1600-0447.1976.tb00054.x. [DOI] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Mann JJ, Arango V. More tryptophan hydroxylase in the brainstem dorsal raphe nucleus in depressed suicides. Brain Res. 2005;1041:19–28. doi: 10.1016/j.brainres.2005.01.083. [DOI] [PubMed] [Google Scholar]
- Bourne HR, Bunney WE, Jr, Colburn RW, Davis JM, Shaw DM, Coppen AJ. Noradrenaline, 5-hydroxytryptamine, and 5-hydroxyindoleacetic acid in hindbrains of suicidal patients. Lancet. 1968;ii:805–808. doi: 10.1016/s0140-6736(68)92459-8. [DOI] [PubMed] [Google Scholar]
- Cheetham SC, Crompton MR, Czudek C, Horton RW, Katona CLE, Reynolds GP. Serotonin concentrations and turnover in brains of depressed suicides. Brain Res. 1989;502:332–340. doi: 10.1016/0006-8993(89)90629-x. [DOI] [PubMed] [Google Scholar]
- Cochran E, Robins E, Grote S. Regional serotonin levels in brain: a comparison of depressive suicides and alcoholic suicides with controls. Biol Psychiatry. 1976;11:283–294. [PubMed] [Google Scholar]
- Crow TJ, Cross AJ, Cooper SJ, Deakin JFW, Ferrier IN, Johnson JA, Joseph MH, Owen F, Poulter M, Lofthouse R, Corsellis JAN, Chambers DR, Blessed G, Perry EK, Perry RH, Tomlinson BE. Neurotransmitter receptors and monoamine metabolites in the brains of patients with Alzheimer-type dementia and depression, and suicides. Neuropharmacology. 1984;23:1561–1569. doi: 10.1016/0028-3908(84)90100-x. [DOI] [PubMed] [Google Scholar]
- Gjerris A, Srensen AS, Rafaelsen OJ, Werdelin L, Alling C, Linnoila M. 5-HT and 5-HIAA in cerebrospinal fluid in depression. J Affect Disord. 1987;12:13–22. doi: 10.1016/0165-0327(87)90056-5. [DOI] [PubMed] [Google Scholar]
- Kelly TM, Mann JJ. Validity of DSM-III-R diagnosis by psychological autopsy: a comparison with clinician ante-mortem diagnosis. Acta Psychiatr Scand. 1996;94:337–343. doi: 10.1111/j.1600-0447.1996.tb09869.x. [DOI] [PubMed] [Google Scholar]
- Kontur PJ, Al-Tikriti MS, Innis RB, Roth RH. Postmortem stability of monoamines, their metabolites, and receptor binding in rat brain regions. J Neurochem. 1994;62:282–290. doi: 10.1046/j.1471-4159.1994.62010282.x. [DOI] [PubMed] [Google Scholar]
- Lidberg L, Belfrage H, Bertilsson L, Evenden MM, Åsberg M. Suicide attempts and impulse control disorder are related to low cerebrospinal fluid 5-HIAA in mentally disordered violent offenders. Acta Psychiatr Scand. 2000;101:395–402. doi: 10.1034/j.1600-0447.2000.101005395.x. [DOI] [PubMed] [Google Scholar]
- Lloyd KG, Farley IJ, Deck JHN, Hornykiewicz O. Serotonin and 5-hydroxyindoleacetic acid in discrete areas of the brainstem of suicide victims and control patients. Adv Biochem Psychopharmacol. 1974;11:387–397. [PubMed] [Google Scholar]
- Mahadik SP, Laev H, Korenovsky A, Karpiak SE. Haloperidol alters rat CNS cholinergic system: enzymatic and morphological analyses. Biol Psychiatry. 1988;24:199–217. doi: 10.1016/0006-3223(88)90275-2. [DOI] [PubMed] [Google Scholar]
- Mann JJ. Neurobiology of suicidal behaviour. Nature Reviews Neuroscience. 2003;4:819–828. doi: 10.1038/nrn1220. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Arango V, Marzuk PM, Theccanat S, Reis DJ. Evidence for the 5-HT hypothesis of suicide. A review of post-mortem studies. Br J Psychiatry Suppl. 1989;155(suppl 8):7–14. [PubMed] [Google Scholar]
- Mann JJ, Malone KM. Cerebrospinal fluid amines and higher-lethality suicide attempts in depressed inpatients. Biol Psychiatry. 1997;41:162–171. doi: 10.1016/s0006-3223(96)00217-x. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Malone KM, Sweeney JA, Brown RP, Linnoila M, Stanley B, Stanley M. Attempted suicide characteristics and cerebrospinal fluid amine metabolites in depressed inpatients. Neuropsychopharmacology. 1996;15:576–586. doi: 10.1016/S0893-133X(96)00102-9. [DOI] [PubMed] [Google Scholar]
- McIntyre IM, Stanley M. Postmortem and regional changes of serotonin, 5-hydroxyindoleacetic acid, and tryptophan in brain. J Neurochem. 1984;42:1588–1592. doi: 10.1111/j.1471-4159.1984.tb12746.x. [DOI] [PubMed] [Google Scholar]
- Owen F, Cross AJ, Crow TJ, Deakin JFW, Ferrier IN, Lofthouse R, Poulter M. Brain 5-HT2 receptors and suicide. Lancet. 1983;ii:1256. doi: 10.1016/s0140-6736(83)91310-7. [DOI] [PubMed] [Google Scholar]
- Palmer AM, Lowe SL, Francis PT, Bowen DM. Are post-mortem biochemical studies of human brain worthwhile. Biochem Soc Trans. 1988;16:472–475. doi: 10.1042/bst0160472. [DOI] [PubMed] [Google Scholar]
- Pare CMB, Yeung DPH, Price K, Stacey RS. 5-Hydroxytryptamine, noradrenaline, and dopamine in brainstem, hypothalamus, and caudate nucleus of controls and of patients committing suicide by coal-gas poisoning. Lancet. 1969;ii:133–135. doi: 10.1016/s0140-6736(69)92442-8. [DOI] [PubMed] [Google Scholar]
- Patel PD, Pontrello C, Burke S. Robust and tissue-specific expression of TPH2 versus TPH1 in rat raphe and pineal gland. Biol Psychiatry. 2004;55:428–433. doi: 10.1016/j.biopsych.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Placidi GP, Oquendo MA, Malone KM, Huang YY, Ellis SP, Mann JJ. Aggressivity, suicide attempts, and depression: relationship to cerebrospinal fluid monoamine metabolite levels. Biol Psychiatry. 2001;50:783–791. doi: 10.1016/s0006-3223(01)01170-2. [DOI] [PubMed] [Google Scholar]
- Shaw DM, Camps FE, Eccleston EG. 5-Hydroxytryptamine in the hind-brain of depressive suicides. Br J Psychiatry. 1967;113:1407–1411. doi: 10.1192/bjp.113.505.1407. [DOI] [PubMed] [Google Scholar]
- Stanley M, Träskman-Bendz L, Dorovini-Zis K. Correlations between aminergic metabolites simultaneously obtained from human CSF and brain. Life Sci. 1985;37:1279–1286. doi: 10.1016/0024-3205(85)90242-5. [DOI] [PubMed] [Google Scholar]
- Träskman L, Åsberg M, Bertilsson L, Sjöstrand L. Monoamine metabolites in CSF and suicidal behavior. Arch Gen Psychiatry. 1981;38:631–636. doi: 10.1001/archpsyc.1981.01780310031002. [DOI] [PubMed] [Google Scholar]
- Underwood MD, Khaibulina AA, Ellis SP, Moran A, Rice PM, Mann JJ, Arango V. Morphometry of the dorsal raphe nucleus serotonergic neurons in suicide victims. Biol Psychiatry. 1999;46:473–483. doi: 10.1016/s0006-3223(99)00043-8. [DOI] [PubMed] [Google Scholar]
- Zhang X, Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Sci. 2004;305:217. doi: 10.1126/science.1097540. [DOI] [PubMed] [Google Scholar]


