Abstract
Genetic factors are implicated in the heritability of drug abuse. However, even with advances in current technology no specific genes have been identified that are critical for the transmission of drug-induced phenotypes to subsequent generations. It is now evident that epigenetic factors contribute to disease heritability and represent a link between genes and the environment. Recently, epigenetic mechanisms have been shown to underlie drug-induced structural, synaptic, and behavioral plasticity by coordinating the expression of gene networks within the brain. Therefore, the epigenome provides a direct mechanism for drugs of abuse to influence the genetic events involved in the development of addiction as well as its heritability to subsequent generations. In this review we discuss the mechanisms underlying intergenerational epigenetic transmission, highlight studies that demonstrate this phenomenon with particular attention to the field of addiction, and identify gaps for future studies.
Introduction
Drug addiction is a chronic, relapsing disorder characterized by compulsive drug taking and seeking despite undesirable consequences (Mendelson and Mello, 1996). Genetic susceptibility together with early life experiences such as environmental factors contributes to the development of addiction. In addition, the transition to compulsive drug seeking has been shown to arise due to altered neuroplasticity and an inability to inhibit drug-associated cues (Luscher and Malenka, 2011). Twin and sibling studies implicate genetic factors in the heritability of drug abuse susceptibility (Cloninger et al., 1981) and it is now clear that substance use disorders are heritable (Bierut et al., 1998; Brook et al., 2002; Cloninger et al., 1981; Merikangas et al., 1998). However, the components that are responsible for the heritability of characteristics that make an individual more susceptible to drug addiction in humans remain largely unknown given that patterns of inheritance cannot be explained by simple genetic mechanisms (Cloninger et al., 1981; Schuckit et al., 1972). The environment also plays a large role in the development of addiction as evidenced by great societal variability in drug use patterns between countries and across time (UNODC, 2012). Therefore, both genetics and the environment contribute to an individual's vulnerability to become addicted following an initial exposure to drugs of abuse.
Recently, it has been demonstrated that epigenetic factors contribute to disease heritability (Danchin et al., 2011; Bohacek et al., 2012) and may provide the missing link between environmental stimuli and genetic heritability. The definition of epigenetics has evolved to include both heritable and stable changes in gene expression within mature, post-mitotic cells without alterations in the DNA sequence (Bird, 2007; Siegmund et al., 2007; Tsankova et al., 2007). Epigenetic mechanisms translate environmental stimuli into stable alterations in chromatin structure that function to activate or repress gene transcription (Jaenisch and Bird, 2003). Recent findings have demonstrated that epigenetic mechanisms contribute to drug-induced structural, synaptic, and behavioral plasticity by orchestrating expression of gene networks in discrete brain nuclei (Renthal and Nestler, 2008; Russo et al., 2010). Thus, epigenetics provides a direct molecular mechanism for drugs of abuse to influence the genetic events involved in the development as well as heritability of addiction.
The goal of this review is to discuss the potential mechanisms whereby environmental exposure to drugs of abuse can cause alterations in the epigenome and thereby be transmitted to future generations. First we will review known mechanisms of transgenerational epigenetic inheritance. Next, we highlight a few recent studies that directly examine potential mechanisms of transgenerational epigenetic inheritance following various environmental stimuli and lastly focus on known mechanisms of transmission in preclinical drug addiction studies.
Epigenetic Mechanisms
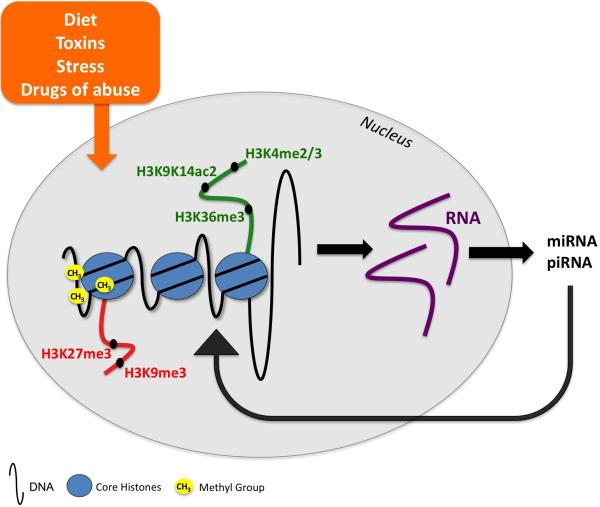
Epigenetics and genetics work together to provide an integrated mechanism of gene expression. Briefly, chromatin consists of DNA wrapped around octamers of histone proteins. The genomic DNA sequence provides the stable nature of an organism. The access of transcription factors and basal transcriptional machinery to DNA sequences including promoter regions is regulated by chromatin structure (Berger, 2007; Li et al., 2007). Chromatin exists in two basic states that are characterized by different levels of condensation. Heterochromatin (condensed chromatin) is associated with inactive gene transcription due to tight packaging of DNA around histone cores, and euchromatin (open chromatin) is associated with active gene transcription due to a more relaxed chromatin structure and accessible DNA sequences (Berger, 2007). The primary epigenetic mechanisms, and the ones discussed in this review, are DNA methylation, histone post-translational modifications, and small noncoding RNAs (See Figure 1). Together, these modifications alter chromatin structure and gene expression and allow cells to respond and adapt in response to their environments. In addition, epigenetic markers allow for the transmission of gene expression from one cell to its daughter cells. These modifications comprise the epigenome, which is defined as the collection of plastic regulatory mechanisms that are responsive to the environment (Waddington, 1942; Holliday and Pugh, 1975; Skinner 2011).
Figure 1. Schematic representation of the epigenetic mechanisms involved in transgenerational epigenetic inheritance.
Environmental stimuli such as diet, toxins, stress, and drugs of abuse lead to epigenetic changes in the germline of the exposed individual that can be transmitted to the subsequent generations. Epigenetic alterations that have thus far been described include, changes in DNA methylation (yellow circles with methyl groups); repressive histone marks (H3K9me3, H3K27me3; red); permissive histone marks (H3K4me2/3, H3K9K14ac2, H3K36me3; green), and noncoding RNAs (miRNA, piRNA). Abbreviations: miRNA, microRNAs; piRNA, Piwi-interacting RNAs.
Germline Transfer
In general, characteristics are inherited by the transfer of DNA through the germline. However, it is now clear that traits can be inherited through the epigenome as well (Anway et al., 2005, 2006; Guerrero-Bosagna et al., 2010; Danchin et al., 2011; Skinner 2011). Until recently, epigenetic modifications were thought to be completely erased in the germline and re-established in each subsequent generation. However, new findings indicate that this erasure is not complete and that epigenetic modifications acquired in one generation can be inherited by the next generation. This phenomenon is known as “transgenerational epigenetic inheritance” (Manolio et al., 2009; Skinner and Guerrero-Bosagna 2009; Skinner 2011; So et al., 2011; Bohacek et al., 2012). Transgenerational epigenetic inheritance requires the transmission of the epigenome via the germline to subsequent generations in the absence of direct environmental exposures. Environmental stimuli influence epigenetic modifications in the germline and can become permanently programmed within the germ cells (Guerrero-Bosagna et al., 2010; Skinner 2011; Bohacek et al., 2012). This mechanism can then allow for the adult onset of disease phenotypes and acquired traits because the somatic cells that are derived from this germline will acquire the modified epigenome (Whitelaw and Whitelaw, 2006; Skinner et al., 2010).
Mechanisms of Germline Transfer
DNA Methylation
DNA methylation is a covalent modification of DNA induced by the addition of a methyl group to cytosines in dinucleotide CpG sequences that results in gene silencing (Figure 1) (Bird, 2002; Tost, 2009 Add References). Environmental factors such as stress (Franklin et al., 2010) and toxins (Guerrero-Bosagna et al., 2010) have been shown to alter DNA methylation at specific genes in germ cells and can be transmitted across multiple generations. More importantly, DNA methylation is involved in genomic imprinting that allows for the selective expression of only one parental allele in the offspring (Paoloni-Giacobino and Chaillet, 2006; Sha, 2008). At imprinted loci, gene silencing is mediated by hypermethylation of DNA regions, which is carried out by DNA methyltransferases that methylate the newly synthesized DNA strand to reproduce the DNA methylation pattern on the daughter cells (Skinner et al., 2011). Therefore, the methylome is maintained through replication during mitosis. Together, these studies demonstrate that DNA methylation is a conceivable mechanism for the transmission and maintenance of epigenetic alterations in response to the environment.
Histone post-translational Modifications
Complex combinations of post-translational modifications of histones alters the affinity of DNA for histone proteins, thereby positively or negatively regulating gene transcription, (Strahl and Allis, 2000). The N-terminal tails of histones contain amino acid residues that are sites for several post-translational modifications such as acetylation, phosphorylation, methylation, and ubiquitination to name a few (Renthal and Nestler, 2008). Specific enzymes function to add or remove associated modifications, demonstrating that these specific histone marks are reversible (Kouzarides, 2007). Until recently, the role of histone modifications in transgenerational inheritance of traits was not clear given that histones are largely replaced by protamines in sperm (Hammoud et al., 2009; Johnson et al., 2011; Bohacek 2012). However, it is now clear that the remaining histones may play a critical role in this process (Steger et al., 2011; Vassoler et al., 2012).
Acetylation of basic lysine residues on histone tails decreases the electrostatic interactions between histones and DNA (Kouzarides, 2007). Therefore, hyperacetylation of promoter regions is associated with increased gene expression, whereas hypoacetylation is correlated with decreased gene expression (Kurdistani et al., 2004). Addition of methyl groups to histones does not change the charge of the amino acid residues and these modifications are relatively stable compared to histone acetylation (Rice and Allis, 2001). Methylation of lysine and arginine residues can occur in mono-(me), di-(me2), or tri-methylated (me3) states with each methylation event having distinct, and sometimes opposite effects on gene transcription (Rice and Allis, 2001).
Histone methylation at gene promoters can either enhance or represse gene transcription depending on the exact amino acid residues that are methylated (Maze and Nestler, 2011). For example, di- and tri-methylation of histone H3 lysine residues 9 (H3K9me2/3) and 27 (H3K27me2/3) decreases gene transcription (Rice and Allis, 2001), while, tri-methylation of histone H3 on lysine residues 4 (H3K4me3) and 36 (H3K36me3) increases gene expression (Rice and Allis, 2001). These histone modifications provide epigenetic mechanisms that can be transmitted from parent to offspring. Indeed, genes involved in spermatogenesis and developmental regulation are associated with methylated histone H3 (H3K4me2 and H3K27me3) (Figure 1) (Hammoud et al., 2009; Brykczynska et al., 2010). In addition, a recent study from our group indicates that histone acetylation is altered in the sperm and seminiferous tubules of the testes in response to chronic exposure to cocaine and that this modification can be transmitted to the offspring (Figure 2) (Vassoler et al., 2012). Together, these findings demonstrate that in addition to DNA methylation, alterations in histone modifications in gonadal tissues contribute to the parental transmission of the epigenome between generations.
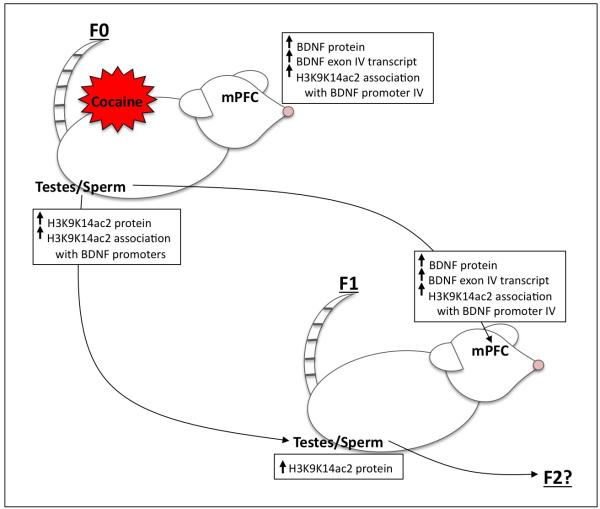
Figure 2. Schematic representation of epigenetic changes in F0 and F1 male rats following paternal cocaine self-administration.
Cocaine self-administration in male rats increases BDNF protein and exon IV transcript levels in the medial prefrontal cortex (mPFC) of rats. These changes are associated with increased H3K9K14ac2 association with BDNF promoter IV. Moreover, there is an increase in total H3K9K14ac2 in the testes as well as associated with BDNF promoters in the sperm of F0 rats. The same molecular alterations are measured in the mPFC of F1 male offspring. In addition, H3K9K14ac2 levels are also increased in the testes of the F1 animals, suggesting that H3K9K14ac2 may be a critical epigenetic mechanism for the transmission of this phenotype. Abbreviations: BDNF, brain-derived neurotrophic factor; H3K9K14ac2, di-acetyl lysine 9, lysine 14, histone H3; mPFC, medial prefrontal cortex.
Non-coding RNAs
Small non-coding RNAs are short RNA sequences that regulate transcriptional and/or translational processes (Ghildiyal and Zamore, 2009; Johnson et al., 2011). Specifically, microRNAs (miRNAs) have emerged as a new class of epigenetic regulators that are capable of altering synaptic plasticity and behavior (Figure 1) (Guarnieri and DiLeone, 2008). miRNAs are a class of non-protein coding RNA transcripts (~19-24 nucleotides) that regulate gene expression at the post-transcriptional level (Ambros, 2004). There are over 800 unique miRNA species in humans (Bentwich et al., 2005; Berezikov et al., 2006), many of which are highly expressed in the brain (Lugli et al., 2008; Sempere et al., 2004). Each miRNA targets on average 200 mRNA transcripts (Friedman et al., 2009; Lewis et al., 2005) with diverse effects on gene expression including mRNA degradation, increased mRNA translation, chromatin remodeling, and DNA methylation (Place et al., 2008; Steitz and Vasudevan, 2009; Vasudevan et al., 2007). Piwi interacting RNAs (piRNA) can also produce stable heritable gene silencing (Figure 1). The mechanism involved in this process is the imperfect base pairing of transcripts that occurs by triggering a secondary small interfering RNA response (Lee et al., 2012; Ashe et al., 2012). In addition, other chromatin modifiers are required in order to maintain piRNA silencing (Ashe et al., 2012). Thus, piRNA link chromatin remodeling with non-coding RNA as mechanisms for the regulation of gene expression and transgenerational epigenetic inheritance.
RNAs were initially thought to be absent in the germline. However, multiple populations of RNAs have been detected in sperm as well as oocytes (Hamatani, 2012; Bohacek et al., 2012). Sperm RNAs are delivered to the oocyte during fertilization and provide a method for the transfer of the epigenome to the offspring (Johnson et al., 2011; Pang et al., 2011; Liu et al., 2012). In addition, non-coding RNAs contribute to chromatin remodeling by binding to promoters and silencing gene expression (Kim et al., 2008; Younger et al., 2011) or inhibiting the replacement of histones by protamines (Johnson et al., 2011). Therefore, the third heritable epigenetic mechanism is through non-coding RNAs.
While each of these mechanisms has been implicated in germline transfer, the precise mechanisms by which these alterations lead to functional changes in the offspring are largely unknown. A recent study by our group has demonstrated that histone modifications within the sperm are altered following cocaine self-administration (Vassoler et al., 2012). However, the mechanisms by which those epigenetic changes alter gene expression in the offspring remain unknown. Currently, there is a clear gap in our understanding of how epigenetic modifications in the germline are translated to altered gene expression in subsequent generations. New and innovative studies are necessary in order to gain a better understanding of the precise mechanisms that are involved in this process.
Behavioral Transfer
Epigenetic transmission can also occur independently of the germline and involve behavioral or social transmission (Jirtle et al., 2007; Youngson and Whitelaw, 2008; Bohacek et al., 2012). During behavioral/social transmission, epigenetic changes occur when environmental factors that bring about the epigenetic modification persist in the environment. For example, in early life, variations in maternal licking and grooming behavior can alter epigenetic marks throughout the genome and determine the level of care administered to the subsequent generation (Champagne and Meaney, 2001; Champagne, 2008; McGowan et al., 2011; Weaver et al., 2004). Thus, daughters receiving greater levels of maternal care will impart increased levels of maternal care on their offspring. In cross-fostering studies, pups from high licking and grooming dams transferred to low licking and grooming mothers not only demonstrate decreased maternal behavior towards their subsequent offspring but also display epigenetic modifications similar to those naturally born to low licking and grooming dams (Weaver, 2007). Therefore, this effect is not permanent and must be instated or reinstated for each new generation.
Examples of Transgenerational Epigenetic Inheritance
While this field is in its infancy, multiple studies have elegantly demonstrated potential or partial mechanisms for transgenerational epigenetic inheritance. For example, following environmental toxins such as vinclozolin (an endocrine disrupter found in agricultural settings), the DNA methylation pattern of the sperm 3 generations removed from the initial exposure (F3 males) is altered at specific promoter regions (Guerrero-Bosagna et al., 2010). Moreover, the promoter of a candidate gene was found to have altered copy number variation (CNV), a permanent change to the genome, suggesting that the epigenome can influence and permanently alter the genetic sequence of the genome (Guerrero-Bosagna et al., 2010). These results are not limited to one environmental toxin as alterations in the DNA methylation pattern of F3 generation sperm was also observed following exposure to pesticides, plastics, dioxin, and jet fuel (Manikkam et al., 2012a; Manikkam et al., 2012b, c, 2013; Tracey et al., 2013). Together, this body of work demonstrates that exposure to environmental toxins during gestation induces a permanent epigenetic change in the germline that transmits adult-onset diseases to future generations in the absence of any subsequent exposure.
Another example involves a model that was recently developed to examine early life stress by unpredictable maternal separation and maternal stress. This manipulation severely affects behavior across multiple generations (Franklin et al., 2011; Franklin et al., 2010; Weiss et al., 2011). The behavioral changes due to maternal separation and stress are accompanied by persistent molecular changes in the stress pathway as well as serotonergic signaling that are transmitted from the F1-F3 generations. The authors examined the sperm and found that DNA methylation was altered at gene promoters that correlated with changes observed in the brains of the stressed males from the subsequent generations (Franklin et al., 2010; Weiss et al., 2011). Another group found that early prenatal stress (during the first week of gestation) caused dysmasculanization in the male offspring that lasted multiple generations, also indicating genomic transfer (Morgan and Bale, 2011). Moreover, an X-linked placental gene, O-linked-N-acetylglucosamine, important for regulating proteins involved with chromatin remodeling, was decreased following prenatal stress (Howerton et al., 2013) and was suggested to play a role in the transmission of this phenotype. Stress during development (prenatal or early life) is not the only circumstance where a stress-related phenotype can be passed on to subsequent generations. Using the chronic social defeat paradigm in adult male rats, it was shown that both male and female offspring display increased anxiety and depressive-like behaviors (Dietz et al., 2011). However, it appears that at least some of these F1 behavioral phenotypes are transmitted behaviorally because only the forced swim test phenotype persists following in vitro fertilization (Dietz et al., 2011).
Parental diet is another factor that has been shown to have transgenerational behavioral and physiological effects. In terms of mechanisms of trait transmission, little is known. However, a few studies have begun to examine possible modes of transmission. Following paternal high fat diet myriad negative effects on offspring development are observed (Binder et al., 2012a; Binder et al., 2012b). Paternal high fat diet also causes subfertility in both male and female offspring and grandoffspring (Fullston et al., 2012). Furthermore, it was shown that diminished reproductive and gamete function are transmitted through the first generation paternal line to both sexes of the second generation and the maternal line to second-generation males (Fullston et al., 2012). This indicates that there are likely changes in the epigenome of the germ cells. Another model found that inadequate paternal folate levels, despite adequate maternal levels, decreases fetal brain DNA methylation levels, insulin-like growth factor 2 expression levels (Kim et al., 2013), placental weight and folate levels, and increases placental folate receptor expression (Kim et al., 2011). These types of methylation changes in the brains of the offspring can possibly arise from alterations to the methylome of the sires, which is then transmitted to the offspring via the epigenome. However, further experimentation is required to confirm these hypotheses.
In terms of drugs of abuse, there are many studies examining the behavioral and physiological consequences of preconception drug exposure (for example: (Abel, 1989; Byrnes et al., 2012; Cicero et al., 1995; He et al., 2006b; Jamerson et al., 2004)). However, those examining the potential mechanisms of underlying heritability remain limited. One of the first studies to show a potential mechanism of transmission demonstrated that the expression of DNA methylatransferase 1 (Dnmt-1) was decreased in the seminiferous tubules of the testes following cocaine self-administration (He et al., 2006a). Dnmt-1 plays a critical role in maintaining methyl groups on imprinted genes in germ cells. Thus, a reduction in Dnmt-1 could be a potential mechanism of transgenerational epigenetic inheritance (He et al., 2006a). It was our goal, together with our colleagues, to identify other potential mechanisms involved in the transmission of transgenerational epigenetic inheritance of cocaine-related traits following prolonged cocaine self-administration. Therefore, we developed a model of paternal cocaine abuse to examine the behavioral and physiological effects in the offspring in order to explore the mechanisms of transmission (Vassoler et al., 2012). Male rats were trained to self-administer cocaine for 60 days (the length of spermatogenesis) and were paired with yoked animals that received saline. Twenty-four hours after the last day of self-administration the animals were bred with naïve females and their offspring were analyzed for behavioral and molecular alterations. Our findings indicate that male, but not female, cocaine-sired offspring, demonstrate delayed acquisition and decreased intake of cocaine during adulthood (Vassoler et al., 2012). Previously, we had shown that in the F0 sires, decreases in brain-derived neurotrophic factor (BDNF) in the medial prefrontal cortex (mPFC) enhanced the rewarding effects of cocaine (Berglind et al., 2007; Sadri-Vakili et al., 2010). Furthermore, we had demonstrated that alterations in BDNF levels in the mPFC were epigenetically regulated (Sadri-Vakili et al., 2010). Specifically, cocaine-induced increases in BDNF were associated with increases in acetylated histone H3 (H3K9K14ac2) in the mPFC. Based on these previous findings, we examined BDNF expression in the mPFC of the offspring to determine whether altered BDNF is an underlying mechanism to blunc cocaine's reinforcing effects. Male but not female cocaine-sired rats demonstrated an increase in both BDNF mRNA and protein in the mPFC (Vassoler et al., 2012) that was caused by increases in histone acetylation (H3K9K14ac2) associated with BDNF promoter IV (Figure 2). This change in histone acetylation in the mPFC was correlated with increased H3K9K14ac2 associated with all BDNF promoters in the sperm of the cocaine sires as measured by chromatin immunoprecipitation (Figure 2) (Vassoler et al., 2012). More importantly, the behavioral phenotype was rescued by the systemic administration of a TrkB receptor (the BDNF receptor) antagonist, ANA-12, suggesting that increases in BDNF in the mPFC are necessary for the decrease in cocaine-seeking in the male progeny. This study is the first to demonstrate that paternal cocaine self-administration causes alterations in the acetylation of histones retained within sperm at a specific gene that is subsequently transmitted to the male offspring. Importantly, this epigenetic alteration resulted in increases in BDNF expression within the mPFC of the offspring. However, for this effect to be considered genuine transgenerational inheritance, a similar phenotype should be measured in the F2 generation because neither the F2 animals nor their germ cells were directly exposed to cocaine. We are currently working to determine the phenotype of our F2 offspring.
When interpreting our findings with respect to addiction, they may represent a means of rapid environmental adaptation serving a protective role for the offspring. Based on our findings, we hypothesize that alterations in histone acetylation together with changes in BDNF levels in the mPFC ensure that any future exposure to cocaine has a blunted rewarding effect, thereby decreasing the vulnerability of the offspring for becoming drug-dependent. While this might represent an evolutionary advantage because it is a rapidly acquired trait that can be passed on across generations, it is in contrast to human epidemiological studies suggesting that children born to drug-dependent individuals are more vulnerable to becoming drug-dependent themselves. However, as discussed earlier, drug availability as well as other environmental factors leads to the development of addiction. In addition, it should also be noted that human genetics studies are correlational, therefore, the genetic basis for family history as a risk factor for developing addiction must be interpreted cautiously (Goldman et al., 2005). However, there is little doubt that addiction is influenced by genetic and environmental factors; even though the exact contribution of each of these factors is complicated and not easily distinguished based on human data. In contrast, our animal experiments focus solely on the influence of cocaine experience in the F0 sires, without the presence of other drugs, and controlled for environmental influences, which is not possible in human studies.
Clearly many unanswered questions still remain and further work is needed in order to elucidate this phenomenon. For example, is BDNF the only gene involved in this process? Because BDNF is involved in neuronal function and numerous behavioral phenotypes (including learning and memory as well as depression), we cannot rule out other, potentially negative, consequences that could result from paternal cocaine exposure. In terms of the mechanisms of transmission, it still remains to be determined if alterations in histone acetylation alone are sufficient for the transmission of the cocaine-resistance phenotype. Future studies employing novel genome- and epigenome-wide methods of analysis will aid in identifying the set of genes and epigenetic mechanisms that are involved in this process.
Conclusions
The evidence presented here demonstrates that rapid environmental adaptation occurs following exposure to a number of stimuli. Epigenetic mechanisms represent the key components by which the environment can influence genetics, and they provide the missing link between genetic heritability and environmental influences on the behavioral and physiological phenotypes of the offspring. Although a number of promising epigenetic mechanisms have been elucidated as potential candidates for mediating transgenerational inheritance, the field is still in its infancy, particularly in regards to the effects of drugs of abuse. As a future direction it is important to look to the known mechanisms involved in the inheritance of traits and/or disorders resulting from exposure to toxins, stress, and diet, given that the mechanisms are likely the same irrespective of the environmental stimuli. Although long-term epigenetic modifications can be maladaptive once the stimulus is no longer present, there is little doubt that transmitting information about the environment to one's offspring is evolutionarily advantageous. In the case of cocaine, paternal transmission of altered BDNF levels via changes in histone acetylation reduced the intake of cocaine in our rodent model.
Highlights.
Environmental exposure to drugs alters the epigenome and is transmitted to future generations.
Epigenome provides a direct mechanism for drugs to alter the genetic events involved in addiction.
Epigenetics presents a mechanism of heritability of addictive phenotypes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel EL. Paternal and maternal alcohol consumption: effects on offspring in two strains of rats. Alcohol Clin Exp Res. 1989;13:533–541. doi: 10.1111/j.1530-0277.1989.tb00373.x. [DOI] [PubMed] [Google Scholar]
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- Bentwich I, Avniel A, Karov Y, Aharonov R, Gilad S, Barad O, Barzilai A, Einat P, Einav U, Meiri E, Sharon E, Spector Y, Bentwich Z. Identification of hundreds of conserved and nonconserved human microRNAs. Nature genetics. 2005;37:766–770. doi: 10.1038/ng1590. [DOI] [PubMed] [Google Scholar]
- Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–9. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anway MD, Leathers C, Skinner MK. Endocrine disruptor vinclozolin induced epigenetic transgenerational adult-onset disease. Endocrinology. 2006;147:5515–23. doi: 10.1210/en.2006-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashe A, Sapetschnig A, Weick EM, Mitchell J, Bagijn MP, Cording AC, Doebley AL, Goldstein LD, Lehrbach NJ, Le Pen J, Pintacuda G, Sakaguchi A, Sarkies P, Ahmeh S, Miska EA. piRNAs can trigger a multigenerational epigenetic memory in the germline of C. elegans. Cell. 2012;150:88–99. doi: 10.1016/j.cell.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentwich I, Avniel A, Karov Y, Aharonov R, Gilad S, Barad O, Barzilai A, Einat P, Einav U, Meiri E, et al. Identification of hundreds of conserved and nonconserved human microRNAs. Nature genetics. 2005;37:766–770. doi: 10.1038/ng1590. [DOI] [PubMed] [Google Scholar]
- Berezikov E, Thuemmler F, van Laake LW, Kondova I, Bontrop R, Cuppen E, Plasterk RH. Diversity of microRNAs in human and chimpanzee brain. Nature genetics. 2006;38:1375–1377. doi: 10.1038/ng1914. [DOI] [PubMed] [Google Scholar]
- Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, See RE, Fuchs RA, Ghee SM, Whitfield TW, Jr., Miller SW, McGinty JF. A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. Eur J Neurosci. 2007;26:757–766. doi: 10.1111/j.1460-9568.2007.05692.x. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Dinwiddie SH, Begleiter H, Crowe RR, Hesselbrock V, Nurnberger JI, Jr., Porjesz B, Schuckit MA, Reich T. Familial transmission of substance dependence: alcohol, marijuana, cocaine, and habitual smoking: a report from the Collaborative Study on the Genetics of Alcoholism. Arch Gen Psychiatry. 1998;55:982–988. doi: 10.1001/archpsyc.55.11.982. [DOI] [PubMed] [Google Scholar]
- Binder NK, Hannan NJ, Gardner DK. Paternal diet-induced obesity retards early mouse embryo development, mitochondrial activity and pregnancy health. PLoS One. 2012a;7:e52304. doi: 10.1371/journal.pone.0052304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder NK, Mitchell M, Gardner DK. Parental diet-induced obesity leads to retarded early mouse embryo development and altered carbohydrate utilisation by the blastocyst. Reprod Fertil Dev. 2012b;24:804–812. doi: 10.1071/RD11256. [DOI] [PubMed] [Google Scholar]
- Bird A. Perceptions of epigenetics. Nature. 2007;447:396–398. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- Brook DW, Brook JS, Richter L, Whiteman M, Arencibia-Mireles O, Masci JR. Marijuana use among the adolescent children of high-risk drug-abusing fathers. Am J Addict. 2002;11:95–110. doi: 10.1080/10550490290087875. [DOI] [PubMed] [Google Scholar]
- Bohacek J, Mansuy IM. Epigenetic inheritance of disease and disease risk. Neuropsychopharmacology. 2012;38:220–236. doi: 10.1038/npp.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brykczynska U, Hisano M, Erkek S, Ramos L, Oakeley EJ, Roloff TC. Repressive and active histone methylation mark distinct promoters in human and mouse spermatozoa. Nat Struct Mol Biol. 2010;17:679–687. doi: 10.1038/nsmb.1821. [DOI] [PubMed] [Google Scholar]
- Byrnes JJ, Johnson NL, Schenk ME, Byrnes EM. Cannabinoid exposure in adolescent female rats induces transgenerational effects on morphine conditioned place preference in male offspring. J Psychopharmacol. 2012;26:1348–1354. doi: 10.1177/0269881112443745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F, Meaney MJ. Like mother, like daughter: evidence for non genomic transmission of parental behavior and stress responsivity. Prog Brain Res. 2001;133:287–302. doi: 10.1016/s0079-6123(01)33022-4. [DOI] [PubMed] [Google Scholar]
- Champagne FA. Epigenetic mechanisms and the transgenerational effects of maternal care. Front Neuroendocrinol. 2008;29:386–397. doi: 10.1016/j.yfrne.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Nock B, O'Connor L, Adams M, Meyer ER. Adverse effects of paternal opiate exposure on offspring development and sensitivity to morphine-induced analgesia. J Pharmacol Exp Ther. 1995;273:386–392. [PubMed] [Google Scholar]
- Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse. Cross-fostering analysis of adopted men. Arch Gen Psychiatry. 1981;38:861–868. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- Danchin E, Charmantier A, Champagne FA, Mesoudi A, Pujol B, Blanchet S, et al. Beyond DNA: Integrating inclusive inheritance into an extended theory of evolution. Nat Rev Genet. 2011;12:475–486. doi: 10.1038/nrg3028. [DOI] [PubMed] [Google Scholar]
- Dietz DM, Laplant Q, Watts EL, Hodes GE, Russo SJ, Feng J, Oosting RS, Vialou V, Nestler EJ. Paternal transmission of stress-induced pathologies. Biol Psychiatry. 2011;70:408–414. doi: 10.1016/j.biopsych.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TB, Linder N, Russig H, Thony B, Mansuy IM. Influence of early stress on social abilities and serotonergic functions across generations in mice. PLoS One. 2011;6:e21842. doi: 10.1371/journal.pone.0021842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TB, Russig H, Weiss IC, Graff J, Linder N, Michalon A, Vizi S, Mansuy IM. Epigenetic transmission of the impact of early stress across generations. Biol Psychiatry. 2010;68:408–415. doi: 10.1016/j.biopsych.2010.05.036. [DOI] [PubMed] [Google Scholar]
- Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome research. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullston T, Palmer NO, Owens JA, Mitchell M, Bakos HW, Lane M. Diet-induced paternal obesity in the absence of diabetes diminishes the reproductive health of two subsequent generations of mice. Hum Reprod. 2012;27:1391–1400. doi: 10.1093/humrep/des030. [DOI] [PubMed] [Google Scholar]
- Ghildiyal M, Zamore PD. Small silencing RNAs: An expanding universe. Nat Rev Genet. 2009;10:94–108. doi: 10.1038/nrg2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- Guarnieri DJ, DiLeone RJ. MicroRNAs: a new class of gene regulators. Annals of medicine. 2008;40:197–208. doi: 10.1080/07853890701771823. [DOI] [PubMed] [Google Scholar]
- Guerrero-Bosagna C, Settles M, Lucker B, Skinner MK. Epigenetic transgenerational actions of vinclozolin on promoter regions of the sperm epigenome. PLoS One. 2010;5:e13100. doi: 10.1371/journal.pone.0013100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamatani T. Human spermatozoal RNAs. Fertil Steril. 2012;97:275–281. doi: 10.1016/j.fertnstert.2011.12.035. [DOI] [PubMed] [Google Scholar]
- Hammoud SS, Nix DA, Zhang H, Purwar J, Carrell DT, Cairns BR. Distinctive chromatin in human sperm packages genes for embryo development. Nature. 2009;460:473–478. doi: 10.1038/nature08162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Lidow IA, Lidow MS. Consequences of paternal cocaine exposure in mice. Neurotoxicol Teratol. 2006a;28:198–209. doi: 10.1016/j.ntt.2005.12.003. [DOI] [PubMed] [Google Scholar]
- He F, Lidow IA, Lidow MS. Inhalational model of cocaine exposure in mice: neuroteratological effects. Neurotoxicol Teratol. 2006b;28:181–197. doi: 10.1016/j.ntt.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Holliday R, Pugh JE. DNA modification mechanisms and gene activity during development. Science. 1975;187:226–32. [PubMed] [Google Scholar]
- Howerton CL, Morgan CP, Fischer DB, Bale TL. O-GlcNAc transferase (OGT) as a placental biomarker of maternal stress and reprogramming of CNS gene transcription in development. Proc Natl Acad Sci U S A. 2013;110:5169–5174. doi: 10.1073/pnas.1300065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nature genetics. 2003;33(Suppl):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- Jamerson PA, Wulser MJ, Kimler BF. Neurobehavioral effects in rat pups whose sires were exposed to alcohol. Brain Res Dev Brain Res. 2004;149:103–111. doi: 10.1016/j.devbrainres.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8:253–62. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NL, Carini L, Schenk ME, Stewart M, Byrnes EM. Adolescent opiate exposure in the female rat induces subtle alterations in maternal care and transgenerational effects on play behavior. Front Psychiatry. 2011;2:29. doi: 10.3389/fpsyt.2011.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Saetrom P, Snove O, Jr., Rossi JJ. MicroRNA-directed transcriptional gene silencing in mammalian cells. Proc Natl Acad Sci. 2008;105:16230–16235. doi: 10.1073/pnas.0808830105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HW, Choi YJ, Kim KN, Tamura T, Chang N. Effect of paternal folate deficiency on placental folate content and folate receptor alpha expression in rats. Nutr Res Pract. 2011;5:112–116. doi: 10.4162/nrp.2011.5.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HW, Kim KN, Choi YJ, Chang N. Effects of paternal folate deficiency on the expression of insulin-like growth factor-2 and global DNA methylation in the fetal brain. Mol Nutr Food Res. 2013;57:671–676. doi: 10.1002/mnfr.201200558. [DOI] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Kurdistani SK, Tavazoie S, Grunstein M. Mapping global histone acetylation patterns to gene expression. Cell. 2004;117:721–733. doi: 10.1016/j.cell.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Lee HC, Gu W, Shirayama M, Youngman E, Conte D, Jr., Mello CC. C. elegans piRNAs mediate the genome-wide surveillance of germline transcripts. Cell. 2012;150:78–87. doi: 10.1016/j.cell.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Li B, Carey M, Workman JL. The role of chromatin during transcription. Cell. 2007;128:707–719. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Liu W-M, Pang RTK, Chiu PCN, Wong BPC, Lao K, Lee K-F, et al. Sperm-borne microRNA-34c is required for the first cleavage division in mouse. Proc Natl Acad Sci. 2012;109:490–494. doi: 10.1073/pnas.1110368109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugli G, Torvik VI, Larson J, Smalheiser NR. Expression of microRNAs and their precursors in synaptic fractions of adult mouse forebrain. Journal of neurochemistry. 2008;106:650–661. doi: 10.1111/j.1471-4159.2008.05413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikkam M, Guerrero-Bosagna C, Tracey R, Haque MM, Skinner MK. Transgenerational actions of environmental compounds on reproductive disease and identification of epigenetic biomarkers of ancestral exposures. PLoS One. 2012a;7:e31901. doi: 10.1371/journal.pone.0031901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Dioxin (TCDD) induces epigenetic transgenerational inheritance of adult onset disease and sperm epimutations. PLoS One. 2012b;7:e46249. doi: 10.1371/journal.pone.0046249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Pesticide and insect repellent mixture (permethrin and DEET) induces epigenetic transgenerational inheritance of disease and sperm epimutations. Reprod Toxicol. 2012c;34:708–719. doi: 10.1016/j.reprotox.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS One. 2013;8:e55387. doi: 10.1371/journal.pone.0055387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maze I, Nestler EJ. The epigenetic landscape of addiction. Annals of the New York Academy of Sciences. 2011;1216:99–113. doi: 10.1111/j.1749-6632.2010.05893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Suderman M, Sasaki A, Huang TC, Hallett M, Meaney MJ, Szyf M. Broad epigenetic signature of maternal care in the brain of adult rats. PLoS One. 2011;6:e14739. doi: 10.1371/journal.pone.0014739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK. Management of cocaine abuse and dependence. The New England journal of medicine. 1996;334:965–972. doi: 10.1056/NEJM199604113341507. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Stolar M, Stevens DE, Goulet J, Preisig MA, Fenton B, Zhang H, O'Malley SS, Rounsaville BJ. Familial transmission of substance use disorders. Arch Gen Psychiatry. 1998;55:973–979. doi: 10.1001/archpsyc.55.11.973. [DOI] [PubMed] [Google Scholar]
- Morgan CP, Bale TL. Early prenatal stress epigenetically programs dysmasculinization in second-generation offspring via the paternal lineage. J Neurosci. 2011;31:11748–11755. doi: 10.1523/JNEUROSCI.1887-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang RTK, Liu W-M, Leung CON, Ye T-M, Kwan PCK, Lee K-F, et al. miR-135A regulates preimplantation embryo development through down-regulation of e3 ubiquitin ligase seven in absentia homolog 1a (SIAH1A) expression. PLoS ONE. 2011;6:e27878. doi: 10.1371/journal.pone.0027878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoloni-Giacobino A, Chaillet J. The role of DMDs in the maintenance of epigenetic states. Cytogenet Genome Res. 2006;113:116–121. doi: 10.1159/000090822. [DOI] [PubMed] [Google Scholar]
- Place RF, Li LC, Pookot D, Noonan EJ, Dahiya R. MicroRNA-373 induces expression of genes with complementary promoter sequences. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1608–1613. doi: 10.1073/pnas.0707594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal W, Nestler EJ. Epigenetic mechanisms in drug addiction. Trends Mol Med. 2008;14:341–350. doi: 10.1016/j.molmed.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JC, Allis CD. Histone methylation versus histone acetylation: new insights into epigenetic regulation. Current opinion in cell biology. 2001;13:263–273. doi: 10.1016/s0955-0674(00)00208-8. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends in neurosciences. 2010;33:267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadri-Vakili G, Kumaresan V, Schmidt HD, Famous KR, Chawla P, Vassoler FM, Overland RP, Xia E, Bass CE, Terwilliger EF, Pierce RC, Cha JH. Cocaine-induced chromatin remodeling increases brain-derived neurotrophic factor transcription in the rat medial prefrontal cortex, which alters the reinforcing efficacy of cocaine. J Neurosci. 2010;30:11735–11744. doi: 10.1523/JNEUROSCI.2328-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Goodwin DA, Winokur G. A study of alcoholism in half siblings. Am J Psychiatry. 1972;128:1132–1136. doi: 10.1176/ajp.128.9.1132. [DOI] [PubMed] [Google Scholar]
- Sempere LF, Freemantle S, Pitha-Rowe I, Moss E, Dmitrovsky E, Ambros V. Expression profiling of mammalian microRNAs uncovers a subset of brain- expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome biology. 2004;5:R13. doi: 10.1186/gb-2004-5-3-r13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha K. A mechanistic view of genomic imprinting. Annu Rev Genomics Hum Genet. 2008;9:197–216. doi: 10.1146/annurev.genom.122007.110031. [DOI] [PubMed] [Google Scholar]
- Siegmund KD, Connor CM, Campan M, Long TI, Weisenberger DJ, Biniszkiewicz D, Jaenisch R, Laird PW, Akbarian S. DNA methylation in the human cerebral cortex is dynamically regulated throughout the life span and involves differentiated neurons. PLoS One. 2007;2:e895. doi: 10.1371/journal.pone.0000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MK. Role of epigenetics in developmental biology and transgenerational inheritance. Birth Defects Res C Embryo Today. 2011;93:51–5. doi: 10.1002/bdrc.20199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MK, Guerrero-Bosagna C. Environmental signals and transgenerational epigenetics. Epigenomics. 2009;1:111–117. doi: 10.2217/epi.09.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MK, Manikkam M, Guerrero-Bosagna C. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol Metab. 2010;21:214–22. doi: 10.1016/j.tem.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So H-C, Gui AHS, Cherny SS, Sham PC. Evaluating the heritability explained by known susceptibility variants: A survey of ten complex diseases. Genetic Epidemiol. 2011;3:310–317. doi: 10.1002/gepi.20579. [DOI] [PubMed] [Google Scholar]
- Steger K, Cavalcanti MC, Schuppe HC. Prognostic markers for competent human spermatozoa: fertilizing capacity and contribution to the embryo. Int J Androl. 2011;34:513–527. doi: 10.1111/j.1365-2605.2010.01129.x. [DOI] [PubMed] [Google Scholar]
- Steitz JA, Vasudevan S. miRNPs: versatile regulators of gene expression in vertebrate cells. Biochemical Society transactions. 2009;37:931–935. doi: 10.1042/BST0370931. [DOI] [PubMed] [Google Scholar]
- Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- Tracey R, Manikkam M, Guerrero-Bosagna C, Skinner MK. Hydrocarbons (jet fuel JP-8) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. Reprod Toxicol. 2013;36:104–116. doi: 10.1016/j.reprotox.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tost J. DNA methylation: An introduction to the biology and the disease- associated changes of a promising biomarker. Methods Mol Biol. 2009;507:3–20. doi: 10.1007/978-1-59745-522-0_1. [DOI] [PubMed] [Google Scholar]
- Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8:355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- UNODC, U. N. O. o. D. a. C. World Drug Report 2012. United Nations Publication Sales No. E. 2012;12:XI.I. [Google Scholar]
- Vassoler FM, White SL, Schmidt HD, Sadri-Vakili G, Pierce RC. Epigenetic inheritance of a cocaine-resistance phenotype. Nat Neurosci. 2013;16:42–47. doi: 10.1038/nn.3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318:1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- Waddington CH. The epigenotype. Endeavour. 1942;1:18–20. [Google Scholar]
- Weaver IC. Epigenetic programming by maternal behavior and pharmacological intervention. Nature versus nurture: let's call the whole thing off. Epigenetics. 2007;2:22–28. doi: 10.4161/epi.2.1.3881. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weiss IC, Franklin TB, Vizi S, Mansuy IM. Inheritable effect of unpredictable maternal separation on behavioral responses in mice. Front Behav Neurosci. 2011;5:3. doi: 10.3389/fnbeh.2011.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngson NA, Whitelaw E. Transgenerational epigenetic effects. Annu Rev Genomics Hum Genet. 2008;9:233–257. doi: 10.1146/annurev.genom.9.081307.164445. [DOI] [PubMed] [Google Scholar]