Abstract
Objectives
There is limited research about cochlear function in adults who are human immunodeficiency virus (HIV) positive (+). The aim of the present study was to collect measures of cochlear function in a large sample of adults with, or at risk for, HIV infection, to evaluate associations between HIV status, HIV treatment, and cochlear function.
Design
Distortion product otoacoustic emissions (DPOAEs) were used to evaluate cochlear function in 506 participants; 329 men, 150 of whom were HIV+, and 177 women, 136 of whom were HIV+. DPOAEs were measured at frequencies 1000, 2000, 3000, 4000, and 6000 Hz. A DPOAE nonresponse (NR) was defined as an absolute DPOAE level less than −15 dB SPL or a difference between the absolute DPOAE level and the background noise level less than 6 dB. The total number of NRs was calculated for each ear. The associations of demographic variables, HIV status, and HIV treatment with number of NRs were evaluated with univariate and multivariate ordinal regression models.
Results
There was a statistically significant increase in the odds of higher numbers of NRs with age, being male, and being non-Black, but not with HIV status. Among HIV+ participants, there were no statistically significant associations of the HIV disease status or treatment variables with higher number of NRs.
Conclusion
The authors found no evidence of impaired cochlear function by HIV disease status or highly active antiretroviral therapy–treated HIV infection in this cross-sectional study.
Otitis externa, acute otitis media, and otitis media with effusion are often seen in HIV-infected (HIV+) individuals (Kohan et al. 1988). Other causes of hearing loss among HIV+ individuals may include use of ototoxic medications (Nadol 1993; Bankaitis and Keith 1995), and pathological changes in the middle ear, mastoids, and inner ear which may be direct effects of the virus itself (Chandrasekhar et al. 1992). HIV+ individuals have been reported to have a significantly higher rate of sensorineural hearing loss, using pure-tone hearing measures, than age- and sex-matched individuals (Ongulo and Oburra 2010; van der Westhuizen et al. 2013).
In research or clinical settings where pure-tone thresholds are not obtained (e.g., newborn hearing screening, cognitively impaired individuals, or subclinical measures of auditory function), cochlear function can be measured. One approach for objectively evaluating cochlear function, specifically the outer hair cells (OHCs), is to measure the properties of otoacoustic emissions (OAEs). One such method is distortion product OAEs (DPOAEs), which are sounds emitted by the OHCs within the organ of Corti when stimulated with two pure-tone frequencies (f1 and f2) (Brownell 1990). The DPOAE response is measured by placing a probe assembly that includes a microphone in the external auditory canal (Kemp 1978). In the absence of any middle ear disease, a decrease in DPOAEs usually reflects diminished OHC function, which may indicate more general cochlear damage that can precede decreases in pure-tone hearing thresholds (Lucertini et al. 2002; Seixas et al. 2005). The focus of the current study was to evaluate cochlear function using DPOAEs among HIV+ compared to HIV- adults.
Researchers have previously examined the effects of HIV infection on OAEs (Soucek and Michaels 1996; Ranjan and Bhat 2008; Khoza-Shangase 2011; van der Westhuizen et al. 2013). Soucek and Michaels (1996) compared transient evoked OAEs (TEOAEs) in a clinical sample of 19 adults with AIDS to those in 7 HIV- adults without any reported otologic abnormalities. The adults with AIDS had more “medium” and “weak” waveforms compared to the controls and significantly lower overall TEOAE response levels. Ranjan and Bhat (2008) reported a case-control study with HIV+ individuals and age-matched HIV- individuals; both groups had normal hearing sensitivity. For HIV+ individuals, 25-50% of ears had reduced DPOAE signal-to-noise ratios when compared to the control participants. In fact, 13.3% of DPOAEs were absent even though the HIV+ participants had normal hearing sensitivity. Recently, van der Westhuizen et al. (2013) reported that over 40% of HIV+ adults tested had abnormal DPOAEs between 1800 and 7200 Hz, although there was not a significant difference in DPOAEs across CDC disease categories. There were, however, no DPOAE data collected among HIV- adults in this study.
There are very limited data on the effects of HIV-related medications on OAEs. The only study that has addressed this topic is that of Khoza-Shangase et al. (2011), who showed that DPOAEs significantly decreased over time in individuals with AIDS who were receiving highly active antiretroviral therapy (HAART). It is not known, however, if low CD4+ T-cell counts or HAART contributed to the decrease in DPOAEs.
The DPOAE data presented in the present paper were collected during an initial phase of a hearing/vestibular screening protocol from a larger study that includes subsequent phases of diagnostic hearing and vestibular measures. The results of that study will be presented separately. In the present report, the aims were: 1) examine the DPOAE characteristics among both HIV-seropositive (HIV+) and HIV-seronegative (HIV-) adults, taking into account age, sex, race, and noise exposure history, and 2) to evaluate if HIV disease status and antiretroviral therapy (ART) affected DPOAEs.
Materials and Methods
The Institutional Review Boards for San Diego State University, Johns Hopkins Bloomberg School of Public Health, Georgetown University, and Whitman-Walker Health approved this study. All participants signed informed consent prior to participating.
Cohort Studies and Participants
The Multicenter AIDS Cohort Study (MACS) is an ongoing, prospective study of the natural and treated history of HIV infection among men who have sex with men in the United States. A total of 6,973 men were recruited: 4,954 in 1984-1985, 668 in 1987-1991, and 1,351 in 2001-2003 at four centers located in Baltimore, MD/Washington, DC, Chicago, IL, Los Angeles, CA, and Pittsburgh, PA. HIV+ and HIV- men were recruited similarly from a combination of sources including gay-focused public media, personal referrals, promotional events, and through medical practices and other research studies that targeted gay men. The median age, race/ethnicity, occupation, education and age of initial of sexual intercourse with other men were comparable between the HIV status groups. Other details about the recruitment and study design have been described previously (Kaslow et al. 1987; Dudley et al. 1995). Participants return every 6 months for a detailed interview, a physical examination, and collection of blood for laboratory testing and storage.
The Women's Interagency HIV Study (WIHS) is a multicenter prospective cohort study that was established in 1994 to study women with or at risk for HIV infection. A total of 3,766 women were enrolled in either 1994-1995 (n=2,623) or 2001-2002 (n=1,143) at 6 centers located in New York City (Bronx and Brooklyn), NY, Chicago, IL, Los Angeles, CA, San Francisco, CA, and Washington, DC. The HIV+ and HIV- women were recruited from primary care and hospital-based clinics, research studies, community centers, women's support groups, HIV testing sites and referrals from enrolled participants and were frequency matched on age, race/ethnicity, education, injection drug use since 1978, and total number of self-reported sexual partners since 1980. Recruitment targets were adjusted to balance the distribution of these characteristics across the sites (Barkan et al. 1998; Bacon et al. 2005). Similar to the MACS, participants return every 6 months for a detailed interview, a physical examination, and collection of blood for laboratory testing and storage.
In both studies, antiretroviral therapy use was assessed at the study visit. The definition of HAART was determined by the DHHS/Kaiser Panel (DHHS/Henry J Kaiser Family Foundation Panel on Clinical Practices for the Treatment of HIV Infection 2008). Participants were classified as using: a) no ART; b) monotherapy; c) combination therapy; or d) HAART. Additionally, variables indicating “ever” use of mono- or combination ART were created using longitudinal data, up to and including the study visit testing date. Clinical AIDS-defining illnesses including a history of pulmonary tuberculosis (TB) were self-reported and conformed to the 1993 CDC definition of AIDS (Centers for Disease Control and Prevention 1992).
In the MACS, HIV RNA was measured using the COBAS Ultrasensitive Amplicor HIV-1 monitor assay (Roche Molecular Systems, Branchburg, NJ), sensitive to 50 copies HIV RNA/mL. In the WIHS, HIV RNA was measured using COBAS AmpliPrep/COBAS TaqMan HIV-1 Test (Roche Molecular Systems, Branchburg, NJ), sensitive to 48 copies HIV RNA/mL. Values were log10 transformed for analysis. CD4+ and CD8+ T-cell counts were measured using standard flow cytometry (Hultin et al. 2007) and a complete blood count was obtained. For HIV+ men and women, CD4+ and CD8+ T-cell counts were measured at each study visit.
Study Design and Procedures
Both HIV+ and HIV- participants from the Baltimore-Washington, DC site of the MACS and the Washington, DC site of the WIHS were enrolled during their routine study visit. Exclusion criteria were: a) currently using hearing aids; b) perforated tympanic membrane; c) a self-reported history of seizures due to oculomotor stimulation; d) use of ototoxic antibiotics, or chemotherapeutic agents within three months of the testing date; e) history of surgery or radiation to the ear or neck; f) recent use of erectile dysfunction drugs and/or alcohol; and g) exposure to significantly high levels of noise (e.g., loud music on iPods or car stereos) within 8 hours of testing. Because DPOAEs were used as a screening measure for hearing loss in this study, individuals with confirmed hearing loss, for example those using hearing aids, were excluded.
A questionnaire assessing the participant's self-reported hearing loss due to: a) perinatal exposure to rubella or cytomegalovirus; b) factors present at birth other than genetic or infectious disease; c) measles or meningitis; d) otitis media; e) ear injury; or f) Meniere's disease or otosclerosis, tinnitus, and noise exposure at work or during leisure activities was interviewer-administered. The questionnaire used was identical to the 2007 National Health Interview Survey (NHIS) Hearing Supplement (Centers for Disease Control and Prevention (CDC) NCHS 2007). Prevalent diabetes (Brown et al. 2005; Tien et al. 2012) and ever use of hormone replacement therapy or thyroid medication were ascertained from the medical history questionnaire and/or laboratory results collected during the semi-annual study visit.
In a quiet room, DPOAEs (2f1-f2) were then measured separately in each ear by placing a foam-tipped probe assembly in the ear canal while the participant was instructed to remain as quiet as possible for the duration of the testing. Two primary frequencies (f1 and f2; f2>f1) with the f2/f1 ratio equal to 1.22, and f2 varying from 1000, 2000, 3000, 4000, and 6000 Hz were generated using a portable platform device (Otodynamics EchoportTM, Audiometrics, San Diego, CA). The levels of these two primary frequencies (L1 and L2) were held constant at 65 and 55 dB SPL, respectively. An examiner began the automated DPOAE protocol by evaluating the ear canal response waveform. Once that waveform stabilized (i.e., the presentation level was maintained), DPOAE data acquisition started. After the frequency sequence began, the examiner could stop the procedure if the participant talked or moved and the signal became erratic; in that case, that frequency sequence was discarded and the procedure was started again from the beginning. Two consecutive frequency sweeps with consistent results (i.e., similar DPOAE levels) were required before testing moved to the opposite ear. If the two tests had inconsistent results, then a third test was performed before moving into the opposite ear. This repeated measurement resolved inconsistent DPOAE results in all cases. Five trained examiners completed all DPOAE testing across the sites.
Non-response (NR) at a frequency was considered to be present if the absolute DPOAE level was less than -15 dB SPL or if the difference between the absolute DPOAE level and the background noise level (i.e., signal-to-noise [S/N] ratio) was less than 6 dB. This approach has been used previously with DPOAE measures to screen for hearing loss in adults (Torre et al. 2003). When a participant had more than one test result at a frequency level with discordant results (i.e., some positive and some NR), the response was considered to be positive for analytic purposes. The total number of NRs was calculated for each ear. For analysis, the number of NRs (0-4) in each ear was used as the outcome variable. All participants with two or more NRs were referred for a full audiometric battery, which included tympanometry to assess whether middle-ear pathology was the reason for the NRs.
Statistical Analyses
Multivariable mixed ordinal regression models were constructed using PROC GLIMMIX (SAS, Version 9.2, Cary, NC). Both ears and all frequencies were included in the analyses. A random subject effect accounted for these repeated measures on each participant. Models were performed for all participants combined and separately for HIV+ participants only. Covariables in the multivariable models included sex, age (in decades), race (Black/non-Black), and history of noise exposure. For models restricted to the HIV+ participants, variables included CD4+ and CD8+ T-cell counts, log10 plasma HIV RNA at the study visit closest to the date of the test, ever having a AIDS-defining condition (Centers for Disease Control and Prevention 1992), ever having used monotherapy or combination antiretroviral therapy, and current HAART.
Results
Five hundred forty-five participants were screened for eligibility for this study; 17 declined to participate (2 women [1 HIV+; 1 HIV-] and 15 men [5 HIV+; 10 HIV-]). Twenty-two participants were excluded for one or more of the following reasons: current hearing aid use (n=11, 3 HIV+; 8 HIV-); history of surgery or radiation to the ear or neck (n=4); recent use of erectile dysfunction drugs and/or alcohol (n=2); history of seizure related to an eye movement disorder (n=2); equipment malfunction (n=2); current perforated ear drum (n=1); and high levels of noise exposure within 8 hours of testing time (n=1); one participant had two exclusions. Thus, 506 participants (mean age = 51.2 years, SD = 10.0 years) completed DPOAE testing: 329 men with 150 (45.6%) being HIV+, and 177 women with 136 (76.8%) being HIV+. CD4+ and CD8+ T-cell counts were obtained on the same day as the DPOAE testing in 98.6% (282 of 286) of the HIV+ participants; two did not have these counts completed and the remaining two had counts obtained in previous visits.
The demographic characteristics of the study participants, stratified by HIV status, are shown in Table 1. HIV+ participants were younger and more likely to be female and of black race compared to the HIV- participants. The duration of current HAART use was comparable between men and women (10.7 vs. 9.9 years). The prevalence of history of noise exposure was similar by HIV status. The proportions of HIV+ participants ever using monotherapy, combination therapy and HAART were 31.8%, 52.4% and 88.1%, respectively. Among HIV+ participants, fewer women than men used HAART currently (70.6% vs. 81.3%). The proportion of undetectable HIV RNA levels was lower among women than men (49.3% vs. 75.3%) but the mean CD4+ and CD8+ T-cell counts were similar. More women than men had had an AIDS-defining condition (40.4% vs. 18.0%, respectively).
Table 1. Characteristics of all participants stratified by HIV status.
| HIV+ | HIV− | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Men | Women | All HIV+ | Men | Women | All HIV− | |
| Total, n | 150 | 136 | 286 | 179 | 41 | 220 |
| Age, mean (SD), yrs | 51.9 (7.8) | 45.8 (8.5) | 49.0 (8.7) | 56.6 (9.0) | 42.4 (10.6) | 54.0 (10.9) |
| Race, n (%) | ||||||
| Non-Black | 73 (48.7) | 28 (20.6) | 101 (35.3) | 141 (78.8) | 11 (26.8) | 152 (69.1) |
| Black | 77 (51.3) | 108 (79.4) | 185 (64.7) | 38 (21.2) | 30 (73.2) | 68 (30.9) |
| Occupational noise exposure, n (%) | ||||||
| Yes | 46 (30.9) | 19 (14.0) | 65 (22.8) | 43 (24.6) | 3 (7.3) | 46 (20.9) |
| No | 103 (69.1) | 117 (86.0) | 220 (77.2) | 132 (75.4) | 38 (92.7) | 170 (78.7) |
| Nonoccupational noise exposure, n (%) | ||||||
| Yes | 81 (54.4) | 71 (53.0) | 152 (53.7) | 108 (61.7) | 27 (65.8) | 135 (62.5) |
| No | 68 (45.6) | 63 (47.0) | 131 (46.3) | 67 (38.3) | 14 (34.2) | 81 (37.5) |
| Ever AIDS, n (%) | 27 (18.0) | 55 (40.4) | 82 (28.7) | |||
| Antiretroviral therapy, n (%) | ||||||
| Ever monotherapy | 39 (26.0) | 52 (38.2) | 91 (31.8) | |||
| Ever combination | 72 (48.0) | 78 (57.4) | 150 (52.4) | |||
| Ever HAART | 139 (92.7) | 113 (83.1) | 252 (88.1) | |||
| Current HAART | 122 (81.3) | 96 (70.6) | 218 (76.2) | |||
| Current HAART use in years, median (IQR) | 10.67 (7.38, 12.17) | 9.92 (7.79, 11.51) | 10.17 (7.51, 12.02) | |||
| Current CD4 cell count cells/µL, mean (SD) | 593.5 (298.2) | 536.9 (316.9) | 566.60 (308.0) | |||
| Current CD8 cell count cells/µL, mean (SD) | 914.3 (421.5) | 795.3 (363.6) | 857.73 (398.8) | |||
| Undetectable HIV RNA, n (%) | 113 (75.3) | 67 (49.3) | 180 (62.9) | |||
| Log (HIV RNA, copies/mL), median (interquartile range) | 3.9 (2.9–4.5) | 3.2 (2.5–4.3) | 3.5 (2.5–4.4) | |||
Current log10 HIV RNA, log level of HIV RNA among HIV+ participants with detectable levels of RNA (n = 37 men and 69 women); Ever AIDS, ever diagnosed with AIDS; HAART, highly active antiretroviral therapy; HIV, human immunodeficiency virus; Undetectable HIV RNA, subjects with undetectable levels of HIV RNA.
The 1000 Hz DPOAE data were excluded because of biologic noise (i.e., respiration and circulation) artifact. As a result, only data from 2000, 3000, 4000, and 6000 Hz were analyzed. The distributions of pooled NRs for both ears among the men were similar by HIV serostatus (Figure 1, top). The frequency of NRs was higher among the HIV+ compared to the HIV-women (Figure 1, bottom). The distribution of DPOAE NRs increased monotonically across the frequencies among both men and women and was similar by HIV status (Figure 2). None of the participants with DPOAE NRs had abnormalities in middle ear function as assessed by peak acoustic admittance and middle ear pressures (data not shown).
Figure 1.
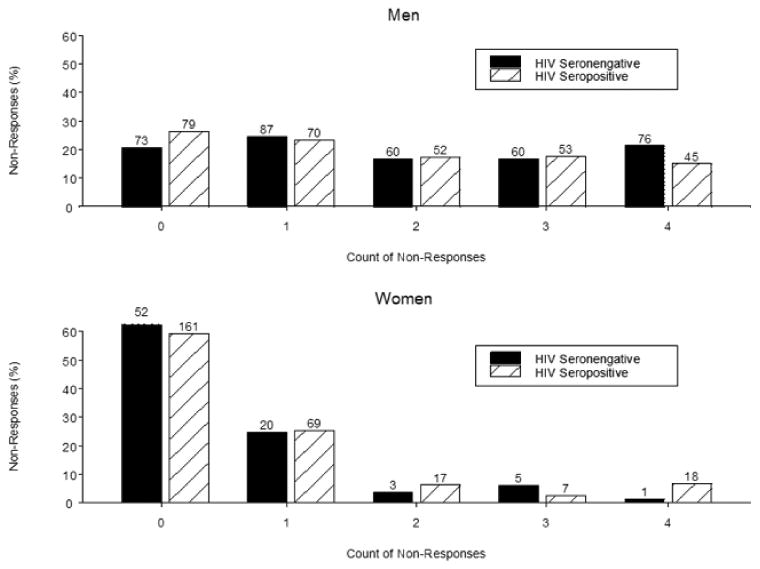
Distribution of pooled DPOAE non-responses across ears by gender and HIV serostatus.
Figure 2.
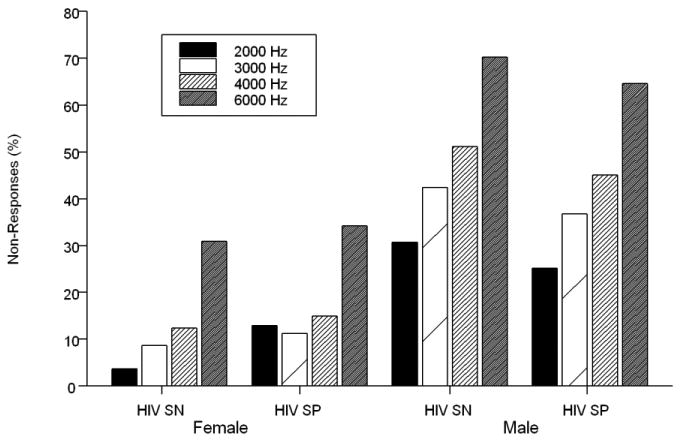
Percentage of DPOAE non-responses for frequencies measured by gender and HIV serostatus (i.e., seronegative [SN] and seropositive [SP]).
Table 2 shows the results of the multivariable ordinal regression analysis for the entire study population. The following variables were significantly associated with a higher number of DPOAE NRs: age, being male and being non-black (Odds Ratio [OR] = 2.83 per 10-year increase, 5.55, and 2.64, respectively). However, occupational and non-occupational noise exposures were not significantly associated with this outcome, nor, after adjusting for these covariates, was HIV status (OR = 1.23; 95% CI [0.7, 2.08]). There were no significant interactions (e.g., sex-by-frequency, sex-by-HIV status, frequency-by-HIV status). In a secondary analysis, individuals were excluded who had known risk factors for hearing loss; specifically, 166 individuals (32 with two or more conditions) who reported one or more of the following conditions were excluded: diabetes (n=82); use of thyroid medication for hypothyroidism (n=29); use of hormone replacement therapy (n=42); perinatal exposure to rubella or cytomegalovirus (n=1); factors present at birth other than genetic or infectious disease (n=1); measles or meningitis (n=1); otitis media (n=12); ear injury such as perforation (n=1); and Meniere's disease or otosclerosis (n=1). With these exclusions, there was a minimal, and nonsignificant, change in the relationship between HIV status and poorer cochlear function (OR = 1.35; 95% CI [0.71, 2.59]).
Table 2. Results of multivariable ordinal regression analyses for all participants for distortion product otoacoustic emissions, with nonresponses defined as either 6 dB S/N or 3 dB S/N.
| 6 dB S/N | 3 dB S/N | |||
|---|---|---|---|---|
|
|
|
|||
| Adjusted Odds Ratio (95% CI) | p | Adjusted Odds Ratio (95% CI) | p | |
| Age, per 10-yr increase | 2.83 (2.11–3.8) | < 0.001 | 3.25 (2.4–4.41) | < 0.001 |
| Male | 5.55 (2.96–10.4) | < 0.001 | 3.81 (2.03–7.15) | < 0.001 |
| Non-Black | 2.64 (1.51–4.63) | < 0.001 | 3.21 (1.82–5.63) | < 0.001 |
| HIV+ | 1.23 (0.7–2.08) | 0.45 | 1.58 (0.93–2.7) | 0.09 |
| Occupational noise exposure | 1.35 (0.75–2.43) | 0.32 | 1.39 (0.78–2.48) | 0.27 |
| Nonoccupational noise exposure | 1.05 (0.64–1.73) | 0.84 | 1.01 (0.61–1.65) | 0.99 |
CI, confidence interval; HIV, human immunodeficiency virus; S/N, signal to noise.
When the analysis was restricted to HIV+ individuals (Table 3), higher age, being male, and being non-black remained significantly associated with higher number of DPOAE NRs. However, current CD4+ and CD8+ T-cell counts, HIV RNA, and all of the HIV treatment variables were not significantly associated with higher number of DPOAE NRs. Additional analyses with nadir CD4+ T-cell counts (replacing current CD4+ T-cell count), peak CD8+ T-cell counts (replacing current CD8+ T-cell count) and ever using HAART (replacing current HAART use) produced similar results (data not shown). Again, the interactions tested above were not statistically significant. Lastly, when a DPOAE NR was defined using a 3 dB S/N ratio rather than 6 dB, the ORs from Tables 2 and 3 changed slightly, but the conclusions did not.
Table 3. Results of multivariable ordinal regression analyses for HIV+ participants only for distortion product otoacoustic emissions, with nonresponses defined as either 6 dB S/N or 3 dB S/N.
| 6 dB S/N | 3 dB S/N | |||
|---|---|---|---|---|
|
|
|
|||
| Adjusted Odds Ratio (95% CI) | p | Adjusted Odds Ratio (95% CI) | p | |
| Age, per 10-yr increase | 2.13 (1.39–3.25) | 0.0005 | 2.14 (1.43–3.22) | 0.0003 |
| Male | 5.80 (2.72–12.35) | < 0.0001 | 4.05 (1.98–8.29) | 0.0001 |
| Non-Black | 2.28 (1.16–4.50) | 0.02 | 2.56 (1.34–4.88) | 0.005 |
| Occupational noise exposure | 0.90 (0.43–1.87) | 0.77 | 0.82 (0.41–1.64) | 0.57 |
| Nonoccupational noise exposure | 0.89 (0.48–1.66) | 0.72 | 0.89 (0.49–1.6) | 0.68 |
| Ever AIDS | 0.79 (0.37–1.67) | 0.53 | 0.92 (0.45–1.88) | 0.82 |
| Ever monotherapy | 1.50 (0.69–3.24) | 0.30 | 1.45 (0.7–3.01) | 0.32 |
| Ever combination therapy | 0.88 (0.44–1.79) | 0.73 | 1.06 (0.54–2.07) | 0.87 |
| Current HAART | 0.84 (0.37–1.93) | 0.68 | 1.09 (0.49–2.43) | 0.83 |
| CD4+ cell count per 100 cells/µL increase | 0.98 (0.87–1.10) | 0.67 | 1.0 (0.89–1.11) | 0.97 |
| CD8+ cell count per 100 cells/µL increase | 0.98 (0.90–1.06) | 0.65 | 0.98 (0.91–1.06) | 0.70 |
| Current log10 HIV RNA per log unit increase | 0.87 (0.61–1.24) | 0.43 | 0.92 (0.65–1.29) | 0.62 |
AIDS, acquired immunodeficiency syndrome; CI, confidence interval; HAART, highly active antiretroviral therapy; HIV, human immunodeficiency virus; S/N, signal to noise.
Discussion
This is the largest study to date to examine cochlear function among individuals with or at risk for HIV infection. We found that poorer cochlear function (i.e., higher number of NRs) was significantly associated with increasing age, being male, and being non-black, but not with HIV status (OR=1.23, 95%CI [0.7-2.08]). Furthermore, among HIV+ participants, antiretroviral therapy, a history of being diagnosed with AIDS, CD4+ or CD8+ T-cell count, and plasma HIV RNA concentration were not significantly associated with poorer cochlear function. However, the association between HIV status and poorer cochlear function was weakly positive, indicating that a relatively modest effect, with an odds ratio as large as two, was not completely ruled out by the data. The purpose of this paper was to evaluate only DPOAEs and HIV, while further phases of data collection for hearing sensitivity are ongoing.
The recording parameters, response criteria, and the conclusions in the present study were similar to those of van der Westhuizen et al. (2013). For both studies, the number of participants was large, DPOAE data were collected for similar frequency ranges, and DPOAEs were defined as absent at a <6 dB S/N ratio. van der Westhuizen et al. (2013) also included analyses defining an abnormal response as DPOAEs between 6-10 dB S/N ratio at 3 of 5 frequencies tested. Most importantly, neither study found a significant effect of HIV infection on DPOAE measures.
The present results do differ from those of two previously published studies. First, Soucek and Michaels (Soucek and Michaels 1996) used visual inspection of the TEOAE waveforms and an overall TEOAE level to define a response. Individuals with AIDS had more “medium” and “weak” waveforms and lower overall TEOAE response levels. This subjective, nominal approach has rarely been used in defining TEOAE responses in other studies. More importantly, all of the individuals with AIDS had mild sensorineural hearing loss, based on pure-tone audiometry between 250 and 8000 Hz. In the second study, DPOAEs were evaluated in a hospital sample of 12 HIV+ and 15 HIV- individuals ranging in age from 20 to 40 years old (Ranjan and Bhat 2008). In HIV+ individuals, the S/N ratios of DPOAEs were reduced or absent in 25%-50% of the frequencies measured, and 50% of the individuals tested had absent DPOAEs, even though all the participants had normal hearing as determined by pure-tone hearing testing. The definition of a DPOAE response in that study, however, was not clearly stated and results for the HIV- were not presented (Ranjan and Bhat 2008).
The presence of middle ear pathology will have an impact on DPOAE NRs. Specifically, an NR can be the result of middle ear pathology rather than decreased OHC function. In the present study, at the time of the DPOAE testing none of the participants reported any middle ear infections or problems. Based on tympanograms obtained from participants with DPOAE NRs, normal middle ear measures (e.g., peak acoustic admittance and middle ear pressure) validated that the NRs were related to OHC function rather than middle ear pathology.
The present study has many strengths. Both of the cohorts studied are well characterized, and standardized testing procedures including extensive training of all examiners were used across the study sites. In fact, all five trained examiners stayed throughout data collection. Most importantly, a clear definition of a DPOAE NR (<6 dB) was used in analyses and in drawing conclusions about loss of cochlear function; further, these conclusions were confirmed when a second definition of a DPOAE NR (<3 dB) was used. Using a S/N ratio as a DPOAE response, for example, is valid only when an acceptable absolute DPOAE level has been obtained. In other words, the absolute DPOAE level has to be higher than the background noise level in order for a non-response to reflect OHC function specifically. It is widely accepted that present or absent DPOAEs (reduced DPOAEs, as in the study by Ranjan and Bhat (2008) are rarely analyzed) are defined only with a cutpoint as small as a 3 dB S/N (Lonsbury-Martin et al. 1990) or as high as 6 or 9 dB S/N, once a valid absolute DPOAE level has been obtained (Torre et al. 2003).
The effect of HAART on cochlear function cannot be separated from that of having HIV infection in this study, since the vast majority of the participants in this study had been immunologically and virologically well-controlled by HAART for many years. Khoza-Shangase (Khoza-Shangase 2011) did report that DPOAEs significantly decreased over time in individuals with AIDS who were receiving HAART at the time of testing. In that study, the participants had more advanced illness (CD4+ T-cell counts below 200 cells/μ1) and were substantially younger (18-50 years) compared with the current study. Pure-tone hearing thresholds in those participants did not change over time (Khoza-Shangase 2011). Therefore, it is unclear whether the decrease in DPOAEs was related to HAART, AIDS, or both. For example, in our study, cochlear function was not impacted adversely by ever having an AIDS diagnosis and no participants in the current study had TB. A limitation of the cross-sectional design of our study was the inability to discern whether DPOAEs decrease over time among immunologically- and virologically-controlled middle-aged adults using HAART.
In the present study, HIV+ and HIV- participants had similar DPOAE NR results. Additionally, long-term HAART treatment was not associated with decreased cochlear function. Since DPOAEs measure only cochlear function and not hearing sensitivity, and since changes in cochlear function probably precede decreases in hearing sensitivity (Marshall et al. 2001), it would not be expected that pure-tone hearing sensitivity would be compromised in HAART-treated HIV-infected people. Further studies are needed to verify this.
Acknowledgments
Supported by the National Institute on Deafness and Other Communication Disorders (NIDCD), National Institutes of Health (NIH) via interagency agreement with NIAID for Cooperative Agreements U01 AI-035042-18 (MACS) and U01 AI-034994-17 (WIHS). Support of the Baltimore-Washington, DC MACS site was provided by the National Institute of Allergy and Infectious Diseases, with additional supplemental funding from the National Cancer Institute (U01-AI-35042, UL1-RR025005 (GCRC). Support of the Metropolitan Washington, DC WIHS site was provided by the National Institute of Allergy and Infectious Diseases (U01-AI-34994) and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (U01-HD-32632).
We thank Ying Li and Haihong Hu, Georgetown University Medical Center, for technical assistance in the preparation of this manuscript.
References
- Bacon MC, von Wyl V, Alden C, et al. The Women's Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12:1013–1019. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankaitis AE, Keith RW. Audiological changes associated with HIV infection. Ear Nose Throat J. 1995;74:353–359. [PubMed] [Google Scholar]
- Barkan S, Melnick S, Preston-Martin S, et al. The Women's Interagency HIV Study. Epidemiology. 1998;9:117–125. [PubMed] [Google Scholar]
- Brown TT, Cole SR, Li X, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165:1179–1184. doi: 10.1001/archinte.165.10.1179. [DOI] [PubMed] [Google Scholar]
- Brownell WE. Outer hair cell electromotility and otoacoustic emissions. Ear Hear. 1990;11:82–92. doi: 10.1097/00003446-199004000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) NCHS. National Health Interview Survey Questionnaire. 2007 Retrieved from ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Survey_Questionnaires/NHIS/2007/English/qadult.pdf.
- Chandrasekhar SS, Siverls V, Sekhar HK. Histopathologic and ultrastructural changes in the temporal bones of HIV-infected human adults. Am J Otol. 1992;13:207–214. [PubMed] [Google Scholar]
- DHHS/Henry J Kaiser Family Foundation Panel on Clinical Practices for the Treatment of HIV Infection. Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents 2008 [Google Scholar]
- Dudley J, Jin S, Hoover D, et al. The Multicenter AIDS Cohort Study: retention after 9 1/2 years. Am J Epidemiol. 1995;142:323–330. doi: 10.1093/oxfordjournals.aje.a117638. [DOI] [PubMed] [Google Scholar]
- Hultin LE, Menendez FA, Hultin PM, et al. Assessing immunophenotyping performance: proficiency-validation for adopting improved flow cytometry methods. Cytometry B Clin Cytom. 2007;72:249–255. doi: 10.1002/cyto.b.20176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaslow RA, Ostrow DG, Detels R, et al. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126:310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- Kemp DT. Stimulated acoustic emissions from within the human auditory system. J Acoust Soc Am. 1978;64:1386–1391. doi: 10.1121/1.382104. [DOI] [PubMed] [Google Scholar]
- Khoza-Shangase K. Highly active antiretroviral therapy: Does it Sound toxic? J Pharm Bioallied Sci. 2011;3:142–153. doi: 10.4103/0975-7406.76494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohan D, Rothstein SG, Cohen NL. Otologic disease in patients with acquired immunodeficiency syndrome. Ann Otol Rhinol Laryngol. 1988;97:636–640. doi: 10.1177/000348948809700611. [DOI] [PubMed] [Google Scholar]
- Lonsbury-Martin BL, Harris FP, Stagner BB, et al. Distortion product emissions in humans. I. Basic properties in normally hearing subjects. Ann Otol Rhinol Laryngol Suppl. 1990;147:3–14. [PubMed] [Google Scholar]
- Lucertini M, Moleti A, Sisto R. On the detection of early cochlear damage by otoacoustic emission analysis. J Acoust Soc Am. 2002;111:972–978. doi: 10.1121/1.1432979. [DOI] [PubMed] [Google Scholar]
- Marshall L, Lapsley Miller JA, Heller LM. Distortion-Product Otoacoustic Emissions as a Screening Tool for Noise-Induced Hearing Loss. Noise Health. 2001;3:43–60. [PubMed] [Google Scholar]
- Nadol JB., Jr Hearing loss. N Engl J Med. 1993;329:1092–1102. doi: 10.1056/NEJM199310073291507. [DOI] [PubMed] [Google Scholar]
- Ongulo B, Oburra H. East Cen Afr J Surg. 2010;15:96–101. [Google Scholar]
- Ranjan R, Bhat JS. DPOAE in HIV infected adults. Online J Health Allied Scs. 2008;7:9. [Google Scholar]
- Seixas NS, Goldman B, Sheppard L, et al. Prospective noise induced changes to hearing among construction industry apprentices. Occup Environ Med. 2005;62:309–317. doi: 10.1136/oem.2004.018143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucek S, Michaels L. The ear in the acquired immunodeficiency syndrome: II. Clinical and audiologic investigation. Am J Otol. 1996;17:35–39. [PubMed] [Google Scholar]
- Tien PC, Schneider MF, Cox C, et al. Association of HIV infection with incident diabetes mellitus: impact of using hemoglobin A1C as a criterion for diabetes. J Acquir Immune Defic Syndr. 2012;61:334–340. doi: 10.1097/QAI.0b013e31826bfc32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre P, III, Cruickshanks K, Nondahl D, et al. Distortion product otoacoustic emission response characteristics in older adults. Ear Hear. 2003;24:20–29. doi: 10.1097/01.AUD.0000051847.66944.2B. [DOI] [PubMed] [Google Scholar]
- van der Westhuizen Y, Swanepoel de W, Heinze B, et al. Auditory and otological manifestations in adults with HIV/AIDS. Int J Audiol. 2013;52:37–43. doi: 10.3109/14992027.2012.721935. [DOI] [PubMed] [Google Scholar]


