Abstract
Objective
To evaluate effects of hearing mode (normal hearing, cochlear implant or hearing aid) on everyday communication among adult unilateral listeners using the Speech, Spatial and Qualities of Hearing scale (SSQ). Individuals with one good, naturally hearing ear were expected to have higher overall ratings than unilateral listeners dependent on a cochlear implant or hearing aid. We anticipated that listening environments reliant on binaural processing for successful communication would be rated most disabling by all unilateral listeners. Regardless of hearing mode, all hearing-impaired participants were expected to have lower ratings than individuals with normal hearing bilaterally. A secondary objective was to compare post-treatment SSQ results of participants who subsequently obtained a cochlear implant for the poorer hearing ear to those of participants with a single normal hearing ear.
Design
Participants were 87 adults recruited as part of ongoing research investigating asymmetric hearing effects. Sixty-six participants were unilateral listeners who had one unaided/non-implanted severe to profound hearing loss ear and were grouped based on hearing mode of the better ear: 30 had one normal hearing ear (i.e., unilateral hearing loss participants); 20 had a unilateral cochlear implant; and 16 had a unilateral hearing aid. Data were also collected from 21 normal-hearing individuals, as well as a subset of participants who subsequently received a cochlear implant in the poorer ear and thus became bilateral listeners. Data analysis was completed at the domain and subscale levels.
Results
A significant mode-of-hearing group effect for the hearing-impaired participants (i.e. with unilateral hearing loss, unilateral cochlear implant or unilateral hearing aid) was identified for two domains (Speech and Qualities) and six subscales (Speech in Quiet, Speech in Noise, Speech in Speech Contexts, Multiple Speech Stream Processing and Switching, Identification of Sound and Objects, and Sound Quality and Naturalness). There was no significant mode-of-hearing group effect for the Spatial domain or the other four subscales (Localization, Distance and Movement, Segregation of Sounds, and Listening Effort). Follow-up analysis indicated the unilateral normal hearing ear group had significantly higher ratings than the unilateral cochlear implant and/or hearing aid groups for the Speech domain and four of the ten subscales; neither the cochlear implant nor hearing aid group had subscale ratings significantly higher than each other or the unilateral hearing loss group. Audibility and sound quality imparted by hearing mode were identified as factors related to subjective listening experience. After cochlear implantation to restore bilateral hearing, SSQ ratings for bilateral cochlear implant and/or cochlear implant plus hearing aid participants were significantly higher than those of the unilateral hearing loss group for Speech in Quiet, Speech in Noise, Localization, Distance and Movement, Listening Effort, and the Spatial domain. Hearing-impaired individuals had significantly poorer ratings in all areas compared to those with bilateral normal hearing.
Conclusions
Adults reliant on a single ear, irrespective of better ear hearing mode, including those with one normal hearing ear, are at a disadvantage in all aspects of everyday listening and communication. Audibility and hearing mode were shown to differentially contribute to listening experience.
Keywords: Unilateral hearing loss, Hearing disability, Cochlear implant, Hearing aid
INTRODUCTION
Often, unilateral hearing loss (UHL) has been left untreated, either because the effects are considered minimal or the individual with UHL has not found satisfaction from available treatment options. This results in many patients who must function on a daily basis with noticeable asymmetry in hearing. As early as 1967, Giolas and Wark reported that adults with UHL had difficulty understanding speech directed towards the impaired ear with competing noise towards the better ear and understanding speech in quiet and noise, regardless of sound location. Twenty adults with UHL reported that “they did not have normal hearing for all practical purposes” (Giolas and Wark 1967). While this initial research shed light on the effects of UHL and brought these concerns into view, four decades later, the same communication difficulties persist for these individuals. The inability to overcome deficits imposed by unilateral hearing loss, in particular when the degree is severe to profound, is a two-fold issue. The first relates to the absence of benefits associated with binaural hearing. Input to both ears imparts advantages for specific communication tasks, including sound localization and speech understanding in noise, areas that have been well established in the literature for both normal-hearing (NH) and hearing-impaired (HI) individuals (Häusler et al. 1983; Hawkins et al. 1987; Bronkhorst and Plomp 1989; Peissig and Kollmeier 1997). The second issue imposed by unilateral hearing loss relates to available treatments. Current device options are unable to restore hearing to the poorer ear to achieve bilateral input or binaural processing. Rather, these devices, such as the CROS (contralateral routing of the signal) hearing aid or BAHA™ (bone-anchored hearing aid), are only able to re-route a signal received at the poorer ear to the better ear. Although these arrangements improve sound awareness and head shadow effects, hearing is not restored to the poorer ear and higher order binaural processing is not possible.
Several studies have tried to better quantify the effects of UHL, as perceived by the individual, using self-assessment questionnaires. For example, Gatehouse and Noble (2004) administered the Speech, Spatial and Qualities of Hearing scale (SSQ) to groups of patients with hearing asymmetry. The SSQ is a 49-item questionnaire that uses a 10-point rating scale (where a 0 rating reflects least ability and 10 reflects greatest ability) to evaluate the effects of hearing loss in terms of disability and function across three domains: Speech Hearing, Spatial Hearing, and Qualities of Hearing (e.g., sound clarity). The SSQ seeks to provide a realistic assessment of daily function by emphasizing the effects of hearing loss in diverse listening environments. In a study of 153 patients prior to hearing aid fitting, responses to the SSQ suggested that greater disability and perceived handicap were linked to two features: auditory sensitivity and asymmetry between ears (Gatehouse and Noble 2004). In a follow-up study, Noble and Gatehouse (2004) defined asymmetric hearing loss as an interaural difference greater than 10 dB based on a pure tone average (PTA) at .5, 1, 2 and 4 kHz. In this analysis almost 33% of the sample had asymmetric hearing loss, and these participants were significantly more disabled for speech recognition, spatial hearing and quality of sound than those with symmetric hearing loss.
More recent studies have examined the effects of greater degrees of hearing asymmetry. For example, Olsen et al (2012) compared SSQ results from individuals with UHL to those with NH to examine whether age, gender, gradual versus sudden onset of hearing loss, or side of hearing loss (ear) affected performance. In the case of UHL, participants had thresholds > 80 dB HL from .25 to 4 kHz in the poorer ear. The contralateral better ear had thresholds < 20 dB HL at .25, .5 and 1 kHz, < 30 dB at 2 kHz, and < 40 dB at 4 kHz resulting in a large hearing difference between ears. For the UHL group, findings suggested no effect of age, gender, side (ear) or onset of hearing loss. At the individual item level, UHL and NH groups did not differ in scores for speech understanding in quiet, talking on the phone or on 10 of 18 sound quality items. However, the groups did differ for understanding speech in difficult situations (e.g. in noise or reverberation), localizing sound, and in amount of effort expended to communicate. The difference in median scores for the significantly different items ranged from 3–6, 3–9 and 2.5–5 scale points for the Speech, Spatial and Qualities domains respectively.
Whereas Olsen and colleagues (2012) studied individuals with UHL of varied durations and etiologies, Douglas and colleagues (2007) quantified perceived hearing disability in adults following acoustic neuroma removal and subsequent onset of UHL (average duration of UHL was five years). Rather than using individual item analyses, Douglas et al (2007) used SSQ subscales, originally described by Gatehouse and Akeroyd (2006), which grouped SSQ items based on processing demands. Compared to a NH control group, the scores of those with UHL were similar for Speech in Quiet (from the Speech domain) and the Identification of Sound and Objects subscale (from the Qualities domain); all other subscale scores were significantly different between groups, particularly understanding speech in any type of noise and localizing unseen sound sources. Results also indicated significantly more effort was expended by the UHL group compared to the NH group.
The SSQ has also been used to evaluate treatment of UHL with sensory devices, such as a CROS hearing aid or BAHA™. Pai et al (2012) examined efficacy of the BAHA™ in treating single-sided deafness (SSD) and used the SSQ to assess device benefit, i.e. pre- and 6 months post-BAHA™ use. SSD participants all had NH or mild loss in the better ear, defined by a PTA of ≤ 25 dB HL at .5, 1, 2 and 3 kHz. Most of the 25 participants had sudden onset of UHL, primarily as a result of a schwannoma resection, and were recruited based on self-reported benefit obtained after a 2-week trial with a softband BAHA™. Results indicated pre-BAHA™ SSQ domain scores were lowest for the Spatial domain, followed by the Speech and Qualities domains. This hierarchical relationship between domains was also observed post-BAHA™ treatment, with the greatest improvement reported for the Speech domain. Dumper et al (2009) used the SSQ to assess BAHA™ benefit for four different patient groups with bilateral conductive hearing loss, unilateral conductive hearing loss, unilateral mixed hearing loss or SSD. Participants with SSD had hearing thresholds in the better ear < 20 dB HL through 2 kHz and a mild to moderate loss at 4 and 8 kHz. Comparisons across groups on the SSQ showed participants with unilateral or bilateral conductive hearing loss rated their abilities as less disabled than those with unilateral mixed or SSD losses. SSD participants rated their abilities lower than the other three groups for Spatial hearing. Hol et al (2010) assessed functional outcome from treatment of UHL after trial periods using a conventional CROS, a completely-in-the-canal CROS (transcranial, CIC) and a BAHA™. All ten participants had NH in the better ear, based on a PTA of < 25 dB HL at .5, 1 and 2 kHz (mean of 12 dB HL). Only the Spatial domain rating was reported, and differences were not significant, indicating contralateral routing of the signal to the better ear did not improve subjective ratings of spatial hearing. The mean unaided score (and standard deviation, SD) was 3.7 (SD 1.5), and the mean aided scores were 5.0 (SD 1.8) for CROS, 4.0 (SD 1.4) for CIC and 4.8 (SD 2.5) for BAHA™. In general, these study results suggest that patients with SSD have the greatest difficulty with spatial hearing and perceive minimal if any improvements in spatial hearing with contralateral routing devices, regardless of the device.
The finding that a contralateral routing of the signal is insufficient to compel a significant change in sound localization or speech understanding, and that subjective ratings of performance or benefit are inconsistent, is not a reflection of poor technology. Rather, the underlying issue is lack of true bilateral input, as evidenced by numerous studies that demonstrated both objective and subjective improvement when bilateral device fitting was possible (e.g., bilateral hearing aid use, Noble and Gatehouse 2006; Gatehouse and Akeroyd 2006; Noble 2010; bilateral cochlear implants, Van Hoesel and Tyler 2003; Van Hoesel 2004; Nobel et al. 2008; Firszt et al. 2008; Noble et al. 2009; Laske et al. 2009; and bimodal fittings, Dunn et al. 2005; Ching et al. 2006). While bilateral device fitting and its benefits are not novel concepts, there are individuals with hearing loss for whom bilateral fitting has not been an option. Examples of this patient population include individuals with asymmetric or unilateral hearing loss, for whom the poorer ear receives no benefit from a hearing aid but does not qualify for a cochlear implant (CI), because the better hearing ear hears ‘too well.’
Minimal information has been published regarding perceived effects of cochlear implantation in patients with a single deaf ear who were traditionally disallowed a CI due to better hearing with a hearing aid in the contralateral ear. Firszt et al (2012a) reported on 10 adults with asymmetric hearing who had a single ear that met traditional CI candidacy guidelines while the contralateral ear with better hearing gained substantial benefit from a hearing aid. As a group, the seven participants with postlingual onset of severe to profound hearing loss (mean length of deafness was 14 years) demonstrated post-implant benefit, including self-reported abilities on the SSQ, primarily on the Speech and Spatial domains. Three pre/perilingual participants in the study reported post-implant benefit in spatial hearing; however, in general there was less post-implant benefit for the other two SSQ domains.
Another patient population for whom there is little published information about the perceived effects of cochlear implantation to restore bilateral input is those with a single deaf ear and a normal or near-normal hearing contralateral ear. Vermeire and Van de Heyning (2009) assessed binaural hearing abilities following cochlear implantation in adults with both SSD and tinnitus and used the SSQ to describe benefit. The SSD participants with a NH ear had mean pre-CI SSQ domain ratings of 3.9 (SD 1.4) for Speech, 3.0 (SD 1.5) for Spatial and 5.8 (SD 1.5) for Qualities. Following cochlear implantation, mean SSQ scores improved significantly for the Speech (mean 6.0, SD 1.4) and Spatial (mean 5.3, SD 1.7) domains but not for the Qualities domain (mean 6.9, SD 1.6). Arndt et al (2010) also assessed outcomes in treating UHL with a CI after other treatment options were tried without success. Again, pre-CI, the Spatial domain was rated the lowest (median 2.29), or most difficult, followed by Speech (median 2.55) and Qualities (median 5.86) domains. And similar to Vermeire and Van de Heyning (2009), post-CI ratings were significantly improved for the Speech and Spatial domains. In a recent pilot study by Firszt et al (2012b), three participants with short-term UHL, who had obtained a CI, were evaluated using a battery of speech perception and sound localization tests and the SSQ. Of the three, two completed pre- and post-CI SSQ assessment. Both participants reported significant post-operative benefit for Spatial hearing, and one reported significant benefit for Speech hearing. In all three of these studies (Vermeire and Van de Heyning 2009; Arndt et al. 2010; Firszt et al. 2012b), subjective improvement in the Speech and Spatial hearing domains was mirrored by performance on objective measures, e.g., speech perception in noise and localization, areas previously shown to have highly variable results with current UHL treatments, i.e., CROS aid or BAHA™.
Collectively, the above studies demonstrate movement by CI clinicians to provide optimal bilateral input and to consider varied degrees and modes of hearing in each ear. This trend was exemplified by Perreault and colleagues (2007) who established fitting guidelines to help determine unilateral or bilateral cochlear implantation for four theoretical patient groups based on usable hearing (e.g., no useable hearing bilaterally, or benefit from only one hearing aid). It was concluded that giving the patient the opportunity for binaural input was of paramount consideration. In order to make confident recommendations, more data are needed on how patients with a single deaf ear function in everyday listening challenges and how mode of hearing in the better ear influences their experiences. As a whole, this information will assist clinicians in determining CI candidacy and will help direct counseling of realistic expectations for patients with a single deaf ear who are considering a CI but have various modes of hearing in the better ear. To date, it is difficult to make direct comparisons between published patient group results in this population due to differences among SSQ studies, including variations in data collection (e.g., interview versus mail method), analysis (e.g., inclusion of domain scores, subscale scores or individual item scores) and reporting (e.g., means versus medians, differences in variance from normal).
The present study provides a direct comparison using the same methodology for three groups of patients, all with a single deaf ear but different modes of hearing in the better, contralateral ear. One group had NH in the better ear and therefore had UHL. The second group used a CI in the better ear, and a third group used a hearing aid (HA) in the better ear. We had two main expectations. First, the UHL group would have overall higher SSQ ratings than either the CI or HA groups, theoretically as a result of mode of stimulation as it relates to auditory sensitivity, frequency resolution and sound quality properties. Second, subscales most reliant on binaural processing for successful communication would be rated the lowest. Additionally, a subset of participants from the CI and HA groups went on to receive a CI in the poorer hearing ear; and post-treatment SSQ results were obtained for these recipients, now in the bilateral CI (CICI) or bimodal (CI in one ear, HA in the contralateral ear; CIHA) condition. This not only allowed pre-to post-treatment comparisons, but also comparison of results between the UHL group and the post-treatment CICI and CIHA groups. The expectation was that post-treatment, the CICI and CIHA groups would have higher ratings than the UHL group for SSQ domains and subscales that posited listening scenarios most sensitive to bilateral hearing. Finally, since previously reported results from NH adults also varied in terms of participant age, data collection, analysis and type of reporting, we compared the results from HI participants to ratings obtained from similarly aged adults with NH bilaterally (NH group). For all analyses, both domain and subscale assessment was included. Analysis at the subscale level has yet to be applied uniformly to each of these HI populations, however this approach provides more details and yields specific areas of deficit, e.g., Speech in Speech Contexts or Segregation of Sounds. Regardless of hearing mode, all HI participants were expected to have lower ratings than NH individuals. By surveying participants in a consistent manner across groups about perceived similarities and differences in communication functioning, and among unilateral listeners (some who subsequently became bilateral listeners), results will assist CI clinicians in treatment recommendations and counseling of expectations in this growing clinical population.
MATERIALS AND METHODS
This study was approved by the Human Research Protection Office at Washington University School of Medicine.
Participants
Participants were 87 adults recruited as part of ongoing research investigating the effects of asymmetric hearing. Twenty-one individuals had NH (unaided thresholds < 30 dB HL) in both ears with a mean bilateral PTA of 10.2 dB HL at .25–6 kHz (range 4.3–22.5 dB HL, SD 4.3). Sixty-six participants had one poorer ear with SPHL and one better hearing ear. The mode of hearing in the better ear fit one of three profiles and resulted in three study groups. The first group had NH in the better ear (UHL group, n=30) with a mean PTA of 13.0 dB HL (range 0.0–26.7 dB HL, SD 6.5). The second group used a CI in one ear (CI group, n=20) and had a mean PTA of 20.2 dB HL (range 12.7–32.7 dB HL, SD 4.8) in the sound field with the device on. The third group used a HA in the better hearing ear (HA group, n=16) and had a mean PTA of 40.0 dB HL (range 28.6–52.4 dB HL, SD 7.2) in the soundfield with the HA on. The HA participants’ unaided hearing levels in the better ear were more varied than the UHL or CI group, although all hearing levels were primarily in the moderate or greater hearing loss range. For example, five participants in this group had some normal or mildly impaired low frequency hearing thresholds but poorer mid to high frequency thresholds. The HA group had a mean unaided PTA of 61.2 dB HL (range 43.6–85.0 dB HL, SD 13.8). All HI participants were unilateral listeners; each had a poorer ear that was not contributing to everyday hearing due to the severity of the hearing loss and lack of amplification or prosthetic device. In order to minimize possible age effects, participants for each group were selected to be of similar ages. Although the HA group was slightly older, the groups were statistically similar for age, p > 0.05. The age range (mean, SD) for the participants was 26.6–73.3 years (50.0, 12.8 years) for the NH group, 25.3–75.9 years (50.5, 13.1 years) for the UHL group, 32.8–75.2 years (53.4, 10.7 years) for the CI group and 26.4–77.4 years (60.3, 17.2 years) for the HA group. Table 1 provides hearing information for the participant groups. The amount of time with “good” hearing is provided both in years and as a percent of life. Years of Good Hearing was calculated as the number of years before onset of any hearing loss plus the number of years of HA use prior to the onset of SPHL for the poorer ear. Percent of Life with Good Hearing is the Years of Good Hearing divided by age. Table 2 shows hearing loss information for the three HI groups.
Table 1.
Group hearing information for NH, UHL, CI and HA participants.
| NH n = 21 |
UHL n = 30 |
CI n = 20 |
HA n = 16 |
||
|---|---|---|---|---|---|
| Years of Good Hearing | mean | 49.1 | 31.5 | 21.3 | 35.4 |
| SD | 12.4 | 22.5 | 13.2 | 23.2 | |
| range | 26.0 – 73.0 | 0.0 – 75.0 | 0.0 – 43.0 | 1.0 – 66.0 | |
|
| |||||
| Percent of Life with Good Hearing | mean | 1.0 | 0.6 | 0.4 | 0.6 |
| SD | 0.0 | 0.4 | 0.3 | 0.3 | |
| range | 1.0 | 0.0 – 1.0 | 0.0 – 0.9 | 0.0 – 0.9 | |
|
| |||||
| Hearing Thresholds Better Hearing Ear (Right ear for NH) | mean | 10.3 | 13.0 | 20.2* | 40.6* |
| SD | 4.7 | 6.5 | 4.8 | 7.1 | |
| range | 3.6 – 22.1 | 0 – 26.7 | 12.7 – 32.7 | 28.6 – 52.4 | |
|
| |||||
| Hearing Thresholds Poorer Hearing Ear (Left ear for NH) | mean | 10.1 | 110.9** | 107.4** | 104.8** |
| SD | 5.0 | 12.2 | 16.5 | 11.6 | |
| range | 2.1 – 22.9 | 72.0 – 120+ | 52.9 – 120+ | 84.3 – 120+ | |
Note: NH = normal hearing; UHL = unilateral hearing loss; CI = cochlear implant; HA = hearing aid; SD = standard deviation; thresholds are averaged for responses .25-6 kHz in decibels hearing level (dB HL);
indicates the threshold could be greater in cases where there was no response at the limits of the audiometer or limits attainable in the sound field
for CI and HA participants, thresholds were obtained with the device in the sound field **for UHL, CI and HA participants, thresholds represent unaided values
Table 2.
Hearing loss information for UHL, CI, and HA participants.
| UHL n = 30 |
CI n = 20 |
HA n = 16 |
||
|---|---|---|---|---|
| Age at Onset of HL Better Hearing Ear (in years) | mean | - | 14.0 | 32.8 |
| SD | - | 12.7 | 24.4 | |
| range | - | 0* – 40 | 0* – 76 | |
|
| ||||
| Age at Onset of HL Poorer Ear (in years) | mean | 31.5 | 13.5 | 29.7 |
| SD | 22.5 | 12.1 | 23.1 | |
| range | 0* – 75 | 0* – 40 | 0* – 66 | |
|
| ||||
| Age at Onset of SPHL Poorer Ear (in years) | mean | 30.8 | 37.7 | 45 |
| SD | 23.3 | 17.9 | 24.1 | |
| range | 0* – 75 | 1 – 61 | 0.6 – 70 | |
|
| ||||
| Length of Deafness Poorer Ear (in years) | mean | 19.1 | 15.0 | 14.7 |
| SD | 21.6 | 14.9 | 12.3 | |
| range | 0.1 – 72.3 | 0.3 – 48.0 | 0.5 – 40.3 | |
Note: UHL = unilateral hearing loss; CI = cochlear implant; HA = hearing aid; HL = hearing loss; SPHL = severe to profound HL; 0* = indicates hearing loss was congenital in onset
All participants from the CI and HA groups received a CI in the poorer ear, becoming bilateral CI recipients (CICI) or bimodal listeners (CIHA; CI on one ear and a HA on the opposite ear). The SSQ was re-administered for these individuals at regular intervals, including three and six months after initial stimulation of the device. The post-treatment (i.e., post-CI) SSQ was administered in the same manner described above, however participants were provided their previous ratings as a reference.
Procedures
Gatehouse and Noble (2004) validated the SSQ in an interview format; however, self-administration using paper and pencil has been used and reported by a number of researchers (Noble et al. 2008; Noble et al. 2009; Laske et al. 2009; Banh et al. 2012). Singh and Pichora-Fuller (2010) also demonstrated comparable reliability for interview and self-administration formats. Therefore, the current participants completed the SSQ in the clinic using a self-administration format and were able to ask questions if needed. HI participants rated each item considering how they listen in everyday life, i.e., with one NH ear, one CI, or one HA. The 10-point scale ratings were recorded to the nearest 0.5 or whole number. SSQ items within each of the three domains were additionally divided into 10 subscales following the same procedures originally described by Gatehouse and Akeroyd (2006). Gatehouse and Akeroyd created these subscales as a means to provide greater detail about communication function in dynamic listening environments. Calculation of both domain and subscale scores also allowed comparison of results among a wider range of studies. Table 3 provides a list of SSQ items included in each subscale. The Speech domain was parsed into four subscales: Speech in Quiet (SiQ), Speech in Noise (SiN), Speech in Speech Contexts (SiSCont) and Multiple Speech Stream Processing and Switching (MultStream). The Spatial domain was split into two subscales: Localization (Loc), and Distance and Movement (DisMov). The Qualities domain was divided into four subscales: Segregation of Sounds (SegSnds), Identification of Sound and Objects (IdSnd), Sound Quality and Naturalness (Qlty) and Listening Effort (Eff). Scores for items within each domain and within each subscale were averaged and yielded three domain and ten subscale ratings for each participant. Following the procedures described by Gatehouse and Akeroyd (2006), six SSQ items (noted in Table 3) were excluded in the process of deriving subscales: one from the Speech domain, two from the Spatial Hearing domain and three from the Qualities domain.
Table 3.
Subscales of the SSQ by individual SSQ item.
| SUBSCALE | SSQ Items | ||
|---|---|---|---|
| SPEECH DOMAIN | SiQ | Speech in Quiet | 2, 3 |
| SiN | Speech in Noise | 1, 4–6 | |
| SiSCont | Speech in Speech Contexts | 7–9, 11 | |
| MultStream | Multiple Speech-stream Processing and Switching | 10, 12, 14 | |
|
| |||
| SPATIAL DOMAIN | Loc | Localization | 1–6 |
| DisMov | Distance and Movement | 7–13, 15, 16 | |
|
| |||
| QUALITIES DOMAIN | SegSnds | Segregation of Sounds | 1–3 |
| IdSnd | Identification of Sound and Objects | 4–7, 13 | |
| Qlty | Sound Quality and Naturalness | 8–12 | |
| Eff | Listening Effort | 14, 18,19 | |
Note: Question 13 was omitted from the Speech domain; questions 14 and 17 were omitted from the Spatial domain; and questions 15, 16 and 17 were omitted from the Qualities domain as suggested by Gatehouse & Akeroyd 2006.
Data Analysis
Data were analyzed for normal distribution and outliers. Data were not normally distributed, and non-parametric rank testing was utilized. Comparisons across multiple groups were conducted with the Kruskal-Wallis H test. Paired comparisons, planned and post hoc, were conducted with the Mann-Whitney U test for independent samples and the Wilcoxon Signed Ranks test for related samples. Significance levels were set as 0.05 and Bonferroni corrections implemented for multiple comparisons.
RESULTS
Comparison of Domain and Subscale Ratings: Normal-Hearing Group versus Hearing-Impaired Groups
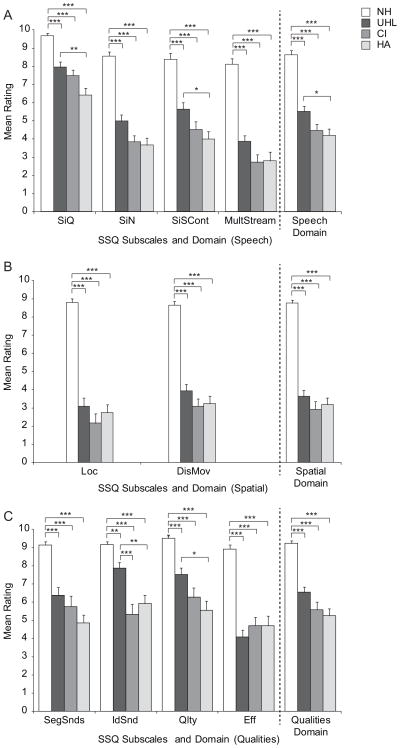
Figure 1 shows the domain (far right of graph) and subscale means organized by SSQ domain (Speech, panel A; Spatial, panel B; Qualities, panel C) for each participant group (white bars for NH, dark gray for UHL, medium gray for CI and light gray for HA). Specific group means, ranges and standard deviations for each domain and subscale are provided in Table 4. The average rankings of NH participants were considerably higher than that of HI participants on all subscales. An initial Kruskal-Wallis H test indicated a significant group difference for all domains, H(3) = 46.3 – 48.4, (all ps < 0.001), and subscales, H(3) = 37.7.8 – 48.3, (all ps < 0.001). Bonferroni-adjusted follow-up comparisons between the NH group and each of the three HI participant groups indicated significantly higher ratings for the NH group on all subscales compared to each of the three HI groups (p < 0.001 for all comparisons except NH versus UHL for the IdSnd subscale for which p < 0.01).
Figure 1.
Group mean ratings are shown for subscales and domains (top panel Speech, middle panel Spatial, lower panel Qualities) for participants with normal hearing (NH), unilateral hearing loss (UHL), a cochlear implant (CI), and a hearing aid (HA). Error bars represent standard error. Brackets and asterisks denote significant comparisons, *** p < 0.001, ** p < 0.01, * p < 0.05.
Table 4.
Domain and Subscale scores for NH, UHL, CI, and HA participants.
| NH | UHL | CI | HA | |||
|---|---|---|---|---|---|---|
| Speech Domain | mean | 8.6 | 5.5 | 4.5 | 4.2 | |
| SD | 1.0 | 1.6 | 1.5 | 1.4 | ||
| range | 6.6 – 10.0 | 1.9 – 8.1 | 1.7 – 7.4 | 2.5 – 6.7 | ||
|
| ||||||
| Spatial Domain | mean | 8.8 | 3.6 | 2.9 | 3.2 | |
| SD | 0.8 | 1.9 | 1.9 | 1.4 | ||
| range | 7.3 – 9.9 | 1.0 – 7.3 | 0.0 – 6.5 | 1.0 – 6.7 | ||
|
| ||||||
| Qualities Domain | mean | 9.2 | 6.5 | 5.6 | 5.3 | |
| SD | 0.7 | 1.6 | 1.9 | 1.6 | ||
| range | 7.6 – 10.0 | 3.6 – 9.2 | 1.6 – 8.6 | 1.9 – 7.9 | ||
|
| ||||||
| Speech in Quiet | SiQ | mean | 9.7 | 7.9 | 7.5 | 6.4 |
| SD | 0.6 | 1.6 | 1.4 | 1.6 | ||
| range | 7.5 – 10.0 | 3.0 – 10.0 | 4.5 – 9.0 | 3.5 – 8.5 | ||
|
| ||||||
| Speech in Noise | SiN | mean | 8.5 | 5.0 | 3.8 | 3.7 |
| SD | 1.0 | 1.9 | 1.4 | 1.6 | ||
| range | 6.8 – 10.0 | 1.5 – 8.0 | 1.5 – 6.4 | 1.6 – 7.3 | ||
|
| ||||||
| Speech in Speech Contexts | SiSCont | mean | 8.4 | 5.6 | 4.5 | 4.0 |
| SD | 1.5 | 2.1 | 1.9 | 1.6 | ||
| range | 5.5 – 10.0 | 1.0 – 9.0 | 1.3 – 8.0 | 2.0 – 7.5 | ||
|
| ||||||
| Multiple Speech Stream Processing and Switching | MultStream | mean | 8.1 | 3.9 | 2.7 | 2.8 |
| SD | 1.4 | 1.7 | 1.9 | 1.9 | ||
| range | 4.7 – 10.0 | 1.0 – 7.3 | 0.0 – 6.7 | 0.5 – 7.0 | ||
|
| ||||||
| Localization | Loc | mean | 8.8 | 3.1 | 2.2 | 2.7 |
| SD | 1.0 | 2.5 | 2.4 | 1.7 | ||
| range | 6.7 – 10.0 | 0.0 – 7.8 | 0.0 – 7.0 | 0.3 – 5.8 | ||
|
| ||||||
| Distance and Movement | DisMov | mean | 8.7 | 3.9 | 3.1 | 3.2 |
| SD | 0.9 | 2.0 | 1.8 | 1.7 | ||
| range | 7.0 – 10.0 | 1.0 –7.3 | 0.0 – 6.2 | 0.8 – 7.3 | ||
|
| ||||||
| Segregation of Sounds | SegSnds | mean | 9.1 | 6.4 | 5.8 | 4.9 |
| SD | 0.9 | 2.2 | 2.6 | 1.7 | ||
| range | 7.3 – 10.0 | 0.7 – 10.0 | 0.3 – 9.3 | 1.7 –7.0 | ||
|
| ||||||
| Identification of Sound and Objects | IdSnd | mean | 9.2 | 7.9 | 5.3 | 5.9 |
| SD | 0.8 | 1.7 | 2.5 | 1.8 | ||
| range | 7.0 – 10.0 | 3.2 – 10.0 | 1.3 – 9.0 | 2.4 – 8.6 | ||
|
| ||||||
| Sound Quality and Naturalness | Qlty | mean | 9.5 | 7.5 | 6.3 | 5.6 |
| SD | 0.6 | 1.9 | 2.3 | 2.1 | ||
| range | 7.8 – 10.0 | 2.9 – 10.0 | 2.0 – 9.6 | 1.6 – 9.6 | ||
|
| ||||||
| Listening Effort | Eff | mean | 8.9 | 4.1 | 4.7 | 4.7 |
| SD | 1.0 | 2.0 | 2.1 | 2.2 | ||
| range | 6.7 – 10.0 | 0.3 – 7.7 | 1.3 – 9.3 | 0.0 – 7.0 | ||
Note: NH = normal hearing; UHL = unilateral hearing loss; CI = cochlear implant; HA = hearing aid
Comparison of Domain and Subscale Ratings: Hearing-Impaired Groups
A second analysis to identify possible group effects was completed for domain and subscale scores of the three HI groups (UHL, CI and HA). Results of Kruskal-Wallis H tests indicated significant group effects for the Speech, H (2) = 8.4, p < 0.05, and Qualities domains, H (2) = 6.3, p < 0.05 which were not present for the Spatial domain, p > 0.05. There were also significant group effects for several subscales: SiQ H(2) = 11.5, p < 0.01; SiN H(2) = 6.8, p < 0.05; SiSCont H(2) = 8.1, p < 0.05; MultStream H(2) = 6.8, p < 0.05; IdSnd H(2) = 18.1, p < 0.001; and Qlty H(2) = 9.5, p < 0.01. There was not a significant group effect for Loc, DisMov, SegSnds and Eff subscales, p > 0.05. When a significant group effect was present, follow-up analysis using a Bonferroni-adjusted Mann-Whitney U test was completed. Significant findings that were identified from these post-hoc comparisons are indicated with brackets in Figure 1. For the Speech domain, the UHL group had significantly higher mean scores than the HA group (UHL vs. HA U = 131.5, z = −2.5, p < 0.05) whereas the CI group was not significantly different from the UHL or HA groups (p > 0.05). The UHL group also had significantly higher mean scores than the HA group on two of four subscales within the Speech domain (SiQ U = 103.0, z = −3.2, p < 0.01; SiSCont U = 122.5, z = −2.7, p < 0.05) and two of four subscales within the Qualities domain (IdSnd U = 89.5, z = −3.4, p < 0.01, Qlty U = 109.5, z = −2.9, p < 0.05). The only significant subscale differences between the UHL and CI groups were higher ratings by the UHL group for IdSnd from the Qualities domain (U = 111.5, z = −3.6, p < 0.001). The CI and HA groups did not differ significantly from each other on any subscale and did not rate themselves significantly higher on any subscale than the UHL group.
Hearing Variables
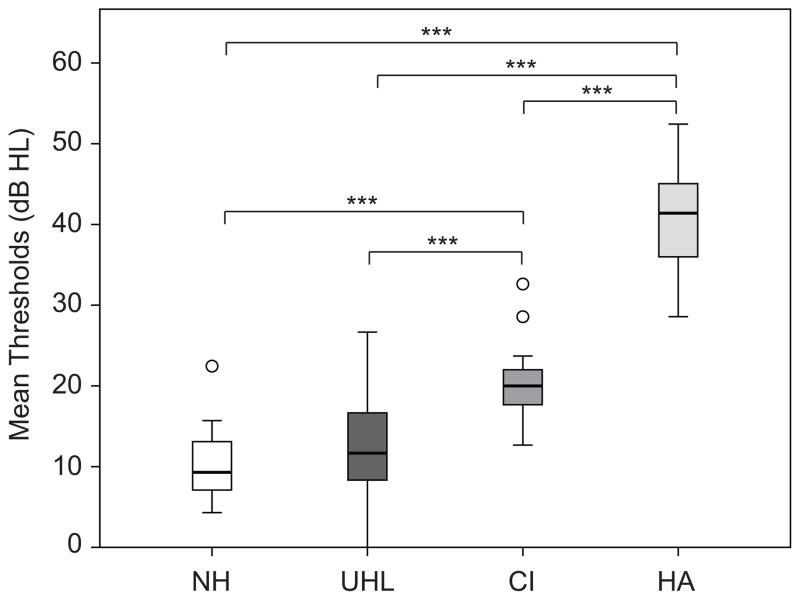
Several variables were compared between the three HI groups and with SSQ ratings: Years of Good Hearing, Percent of Life with Good Hearing, and Better Ear Hearing (NH = average unaided hearing thresholds across ears .25 – 6 kHz; UHL = average unaided hearing thresholds of better ear .25 – 6 kHz; CI and HA = average FM-tone sound field thresholds obtained with the hearing device for the better ear .25 – 6 kHz). There were no significant differences for the amount of time with “good” hearing (in years or as a percent of life) between the three HI groups (ps > 0.05). However there was a significant difference in Better Ear Hearing, H(3) = 42.5, p < 0.001. Figure 2 shows the median and range of Better Ear Hearing for the NH, UHL, CI and HA groups. All HI groups were significantly different from each other in Better Ear Hearing. The NH and UHL groups had significantly better (lower) hearing thresholds than the CI (NH U = 26.0, z = −4.8, p < 0.001; UHL U = 106.0, z = −3.8, p < 0.001) and HA (NH U = 0.0, z = −5.1, p < 0.001; UHL U = 0.0, z = −5.4, p < 0.001) groups, but the better ear hearing of the UHL group was not significantly different from the NH participants hearing (p > 0.05). The HA group had the poorest hearing; the average better ear aided sound field thresholds were significantly higher (poorer) than the CI group’s sound field thresholds (U = 3.0, z = −4.9, p < 0.001).
Figure 2.
Box plots show differences in Better Ear Hearing thresholds for normal hearing (NH), unilateral hearing loss (UHL), cochlear implant (CI) and hearing aid (HA) groups. The box depicts the two mid quartiles transected by the median; tails represent the upper and lower quartiles, and outliers are indicated with open circles. Brackets and asterisks denote significance, *** p < 0.001.
Effect of Treatment on SSQ for Cochlear Implant and Hearing Aid Groups
All participants in the CI and HA groups obtained a CI in the poorer ear, and all completed the SSQ except one participant each in the CI and HA groups. For the CI group, six month post-treatment SSQ ratings were available for 16 participants and three month ratings for an additional three participants (n=19, CICI SSQ ratings). Six-month post-treatment SSQ ratings were available for 12 HA participants and three-month ratings for an additional three participants (n=15, CIHA SSQ ratings). Post-treatment SSQ ratings were significantly higher than pre-treatment ratings on all three domains for the CI group (zs from −3.82 to −3.70, ps < 0.001) and the HA group (zs from −3.41 to −2.98, ps < 0.01). On average, domain ratings increased by 1.58–2.97 points for the CI group and 1.35 – 1.98 for the HA group. Likewise, subscale ratings were significantly higher post-treatment than pre-treatment for both groups and all subscales (zs from −3.82 to −1.76, ps < 0.01) except the Eff subscale for the HA group (p > 0.05). Subscale ratings increased on average by 1.18 – 3.77 points for the CI group and by 1.00 – 2.60 points for the HA group.
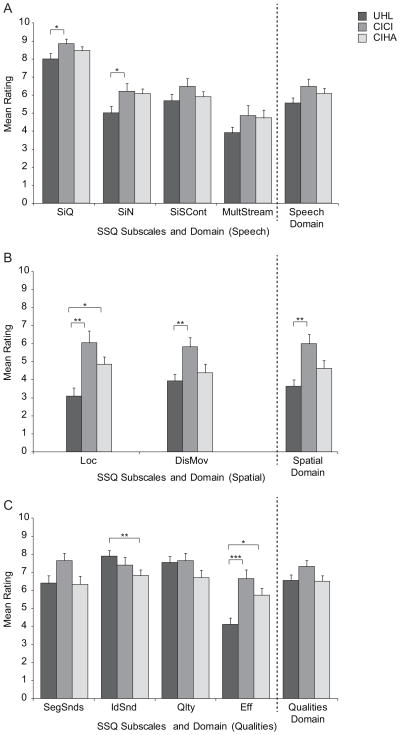
Of interest was the relationship of the UHL group SSQ ratings to the post-treatment ratings of the CI and HA groups who had become bilateral listeners. Figure 3 shows the mean and SD for each subscale and domain rating of the UHL group (dark gray) in relation to post-treatment CICI (medium gray) and CIHA (light gray) group ratings. Specific group means, ranges and standard deviations for each domain and subscale are provided in Table 5. There were two key findings. First, significant differences that were present between the UHL group and the other two HI groups prior to treatment (see Figure 1) were no longer present, with the exception of the IdSnd subscale, for which the UHL group rating was still significantly higher than the CIHA group (U = 108.0, z = −2.72, p < 0.01). And second, the CICI and CIHA group ratings were significantly higher than those of the UHL group for Loc (CICI group U = 132.0, z = −3.14, p < 0.01; CIHA group U = 119.5, z = −2.54, p < 0.05) and Eff (CICI group U = 107.0, z = −3.56, p < 0.001; CIHA group U = 115.0, z = −2.54, p < 0.05). In addition, the CICI group ratings were significantly higher than the UHL group for the SiQ subscale (U = 177.5, z = −2.23, p < 0.05), SiN subscale (U = 185.5, z = −2.05, p < 0.05), DisMov subscale (U = 153.0, z = −2.71, p < 0.01) and the Spatial domain (U = 129.5, z = −3.19, p < 0.01).
Figure 3.
Group mean ratings are shown for subscales and domains (top panel Speech, middle panel Spatial, lower panel Qualities) for participants with unilateral hearing loss (UHL), bilateral cochlear implants (CICI), and bimodal devices (CIHA). Error bars represent standard error. Brackets and asterisks denote significant comparisons, *** p < 0.001, ** p < 0.01, * p < 0.05.
Table 5.
Domain and Subscale scores for UHL, CICI, and CIHA participants.
| UHL | CICI | CIHA | |||
|---|---|---|---|---|---|
| Speech Domain | mean | 5.5 | 6.4 | 6.0 | |
| SD | 1.6 | 1.7 | 1.0 | ||
| range | 1.9 – 8.1 | 3.3 – 9.6 | 4.5 – 7.9 | ||
|
| |||||
| Spatial Domain | mean | 3.6 | 6.0 | 4.6 | |
| SD | 1.9 | 2.2 | 1.7 | ||
| range | 1.0 – 7.3 | 1.5 – 9.5 | 2.4 – 8.4 | ||
|
| |||||
| Qualities Domain | mean | 6.5 | 7.3 | 6.5 | |
| SD | 1.6 | 1.5 | 1.2 | ||
| range | 3.6 – 9.2 | 4.3 – 9.7 | 4.3 – 8.3 | ||
|
| |||||
| Speech in Quiet | SiQ | mean | 7.9 | 8.8 | 8.4 |
| SD | 1.6 | 1.1 | 0.8 | ||
| range | 3.0 – 10.0 | 6.0 – 10.0 | 7.0 – 10.0 | ||
|
| |||||
| Speech in Noise | SiN | mean | 5.0 | 6.2 | 6.0 |
| SD | 1.9 | 1.9 | 1.1 | ||
| range | 1.5 – 8.0 | 3.3 – 9.3 | 4.5 – 7.9 | ||
|
| |||||
| Speech in Speech Contexts | SiSCont | mean | 5.6 | 6.4 | 5.9 |
| SD | 2.1 | 1.9 | 1.1 | ||
| range | 1.0 – 9.0 | 3.3 – 9.8 | 3.8 – 8.0 | ||
|
| |||||
| Multiple Speech Stream Processing and Switching | MultStream | mean | 3.9 | 4.8 | 4.7 |
| SD | 1.7 | 2.4 | 1.7 | ||
| range | 1.0 – 7.3 | 0.7 – 9.7 | 2.0 – 7.2 | ||
|
| |||||
| Localization | Loc | mean | 3.1 | 6.0 | 4.9 |
| SD | 2.5 | 2.9 | 1.6 | ||
| range | 0.0 – 7.8 | 0.5 – 9.5 | 2.5 – 8.2 | ||
|
| |||||
| Distance and Movement | DisMov | mean | 3.9 | 5.8 | 4.4 |
| SD | 2.0 | 2.3 | 1.9 | ||
| range | 1.0 –7.3 | 1.6 – 9.7 | 1.8 – 8.8 | ||
|
| |||||
| Segregation of Sounds | SegSnds | mean | 6.4 | 7.6 | 6.3 |
| SD | 2.2 | 1.8 | 1.8 | ||
| range | 0.7 – 10.0 | 4.5 – 10.0 | 3.3 – 10.0 | ||
|
| |||||
| Identification of Sound and Objects | IdSnd | mean | 7.9 | 7.4 | 6.8 |
| SD | 1.7 | 1.9 | 1.2 | ||
| range | 3.2 – 10.0 | 3.4 – 9.7 | 4.4 – 8.6 | ||
|
| |||||
| Sound Quality and Naturalness | Qlty | mean | 7.5 | 7.6 | 6.7 |
| SD | 1.9 | 1.9 | 1.6 | ||
| range | 2.9 – 10.0 | 4.0 – 9.8 | 3.0 – 9.2 | ||
|
| |||||
| Listening Effort | Eff | mean | 4.1 | 6.6 | 5.7 |
| SD | 2.0 | 2.1 | 1.5 | ||
| range | 0.3 – 7.7 | 3.3 – 10.0 | 3.3 – 8.3 | ||
Note: UHL = unilateral hearing loss; CICI = bilateral cochlear implants; CIHA = cochlear implant and hearing aid
DISCUSSION
In select cases, patients with SSD are being considered for cochlear implantation. As a result, cochlear implant clinicians and researchers are interested in the effects of hearing mode and how individuals with unilateral input function in everyday listening situations. In particular, how does someone with a single NH ear or someone who hears from a single HA function compared to individuals who hear with a unilateral CI? Providing cochlear implants to both ears of patients with bilateral deafness but not implanting the deaf ear of someone with SSD or substantial hearing asymmetry implies that a single NH ear or HA is sufficient for everyday communication whereas a single CI is not. We employed the SSQ and three distinct unilateral listening participant groups to gain an understanding of how hearing mode affects unilateral listeners. Participants all had one ear with poorer hearing that was unable to benefit from a hearing aid and one ear with better hearing ear that was NH or used a CI or a HA. Analysis of SSQ ratings for not only domains but also subscales provided greater detail of how mode of unilateral input differentially affected listening in a variety of scenarios and contexts. It was expected that self-rated ability for SSQ subscales most reliant on binaural hearing would demonstrate the greatest difficulty for all HI participants, particularly items in the Spatial domain. However, what was less clear was how mode of hearing would affect this and other areas of communication. Ratings were also obtained from NH participants of similar ages to the HI study participants so that direct comparisons could be made (independent of study design and methods) between HI groups, and with a NH group of similarly aged participants.
Ratings obtained from the current study identified communication areas that were perceived as challenging for both NH and HI listeners. However, even though the mean NH subscale ratings were below nine for seven of the subscales and as low as 7.9 for MultStream, NH participants ratings for all subscales were significantly higher than those of the other three groups (p < 0.001). This exemplified the lack of similarity between UHL and NH listening experiences even for understanding speech in quiet, despite comparable hearing thresholds, mean PTAs of 8.9 dB HL (NH) and 13.1 dB HL (UHL) at .25–6 kHz. Binaural summation, an effect of the same level signal received by two ears, versus one, results in threshold level signals perceived as approximately 3dB louder and moderate to suprathreshold levels perceived as 6–10 dB louder for NH listeners (Hawkins et al. 1987). This is similar to the 4–6 dB advantage found for signals presented at the most comfortable listening level (Pollack 1952; Christen 1980). A lack of binaural summation for the UHL group likely contributed to differences in SSQ ratings between UHL and NH participants.
Although SSQ scores of NH participants were not a primary focus of the present study, comparison of these NH participant responses to those of other larger studies was of interest. Douglas et al (2007) published SSQ data from 127 NH individuals and found a similar pattern of responses but lower ratings compared to the present data. While mean PTAs were clinically comparable for both NH groups (16.4 dB HL at .5–4 kHz in the Douglas study and 10.2 dB HL at .25–6 kHz in the current study), subtleties imparted by better audibility could have contributed to differing results. Additionally, the age range of participants differed between the two studies. Participants in the Douglas study were similar in mean age to those of the current study (52 vs. 50 yrs) but were older when comparing the age range (50–80 vs. 27–73 yrs). It is possible that the combination of including older participants with slightly poorer thresholds resulted in lower SSQ scores for the participants in Douglas et al (2007) than the current study. More recently, Banh et al (2012) obtained SSQ data from 96 NH individuals, grouped by age to form a young cohort (mean age 19; range 18–22 yrs) and older cohort (mean age 70; range 64–80 yrs). Banh et al (2012) found a significant effect of age at the individual item level; the young cohort had significantly higher ratings for 42 of 46 items. In the present study, NH individuals were specifically recruited who had similar ages as UHL participants. As a result, the NH participants all had good hearing thresholds across all frequencies .25–6 kHz and spanned a larger age range than the other two studies mentioned. In comparison, the Banh study allowed for hearing loss in the older cohort at 4 kHz and above. Interestingly, hearing threshold levels of the NH participants in this study were similar to those of the young cohort from the Banh study (mean thresholds < 10 dB HL for .25 – 8 kHz, estimated from Figure1b); and both groups had nearly identical SSQ ratings. The young cohort had ratings of 8.5 for the Speech domain (8.6 in the current study), 8.6 for the Spatial domain (8.8 in the current study) and 9.4 for the Qualities domain (9.2 in the current study).
Differences between the three HI groups of the current study were present but not prevalent across subscales or domains. Compared to the CI group, the UHL group had significantly higher ratings for only a single Qualities subscale, IdSnd. While significant differences in audibility existed between the two groups, the differences were minimal from a clinical perspective (difference in mean PTAs of 7 dB) and it is unlikely that audibility is the primary factor for the difference in ratings. Rather, the difference is probably related to the nature of the items within the IdSnd subscale, which address sound quality issues associated with pitch, music and prosody. Intrinsic limitations of the CI sound processor make spectral details difficult, if not impossible, to encode and transmit with high fidelity. Compared to the HA group, the UHL group had significantly higher ratings for the Speech domain and two of its four subscales, SiQ and SiSCont, and significantly higher ratings for two Qualities subscales, IdSnd and Qlty. Of the three groups, the HA group had the poorest audibility, significantly worse than both the UHL and CI groups (mean PTAs: HA = 40 dB HL, CI = 20 dB HL, and UHL = 13 dB HL). This substantial gap in hearing threshold levels points to audibility as a key factor in listening experience, particularly understanding speech in quiet and in the presence of one or more competing speech or noise signals. This notion is supported by previous studies that showed increased audibility correlated with improved speech understanding (Humes et al. 1986; Rankovic 1991; Davidson and Skinner 2006), particularly with mild to moderate hearing loss (Ching et al. 1998), high frequency hearing loss (Humes and Roberts 1990) and with CI recipients (Skinner et al. 1997, 1999; Firszt et al. 2004; Holden et al. 2007; Davidson et al. 2009). Significantly lower ratings by the HA group for the IdSnd and Qlty subscales may have resulted from limitations of hearing aids for more severe hearing losses, particularly in the high frequency range. A majority of the individuals in the HA group had unaided thresholds at 3–6 kHz in the severe to profound range and aided thresholds in the moderate to moderately-severe range. Thus it seems that poor audibility, particularly in the high frequencies, may have had a significant impact on these subscale ratings.
The CI and HA groups did not differ for any domain or subscale rating, despite the CI group having significantly better audibility. This suggests factors in addition to audibility contribute to perceived communication abilities. As noted above, IdSnd and Qlty subscales items specifically address musical quality, intonation, pitch and naturalness. Both the CI and HA groups are at a disadvantage, albeit for somewhat different reasons. The CI group has the audibility to access soft input; however, as mentioned, the CI itself lacks the ability to relay the spectral complexities found in musical, prosodic information. The HA group not only has poorer hearing, but participants may receive a suboptimal signal due to potential distortions resulting from mechanical limitations of the hearing aid (e.g., maximum output or high frequency roll-off). However, both groups experience sound through a disrupted auditory system, a consequence of which appears to be insufficient information crucial for speech clarity, musicality and optimal sound quality.
Ratings for the Spatial domain and its subscales, Loc and DisMov were similar for all HI groups and, on average, had the lowest ratings across all subscales. This was not unexpected, because spatial hearing relies predominately on binaural input and processing. And although the HI groups differed in mode of hearing, they all contended with unilateral input, which in these data indicated a greater deficit than could be countered by audibility alone. The finding that the HI groups did not differ in other areas was somewhat unexpected. Apart from spatial hearing, the SegSnds and Eff subscales showed no difference in rating for the UHL, CI and HA groups. SegSnds specifically probes communication scenarios where there are multiple and/or overlapping streams of input, where factors in addition to audibility are needed for reconciliation. Segregating multiple inputs requires a progression of skills from audibility to fidelity of spectral and amplitude information and finally binaural input and higher level processing, for example focus and attention (Rose and Moore 1997; Carlyon et al. 2001; Rose and Moore 2005). Similar ratings for listening effort (Eff) are supported by previous work that demonstrated ease of listening improved when patients were fit bilaterally vs. unilaterally (Feuerstein 1992; Noble and Gatehouse 2006; Noble 2010), suggesting that listening effort is affected by unilateral listening. Critical here is the finding that regardless of the range of hearing and presumably quality of sound for these participants with large interaural asymmetries in hearing, listening effort was affected similarly.
Following cochlear implantation and restoration of bilateral input, the bilateral groups’ ratings were significantly higher compared to pre-treatment ratings for all domains and subscales (with the exception of Eff for the CIHA group). These findings were not unexpected and are in agreement with the current body of literature related to advantages of bilateral device fitting. However, of particular interest was the relationship of the bilateral groups’ ratings to those of the UHL group. Results showed restoration of bilateral input differentially improved CICI and CIHA group ratings suggesting bilateral input is one of several likely components required in dynamic listening contexts.
Recall that only IdSnd was rated as significantly higher by the UHL group than the pre-treatment CI group (Figure 1); all other subscale and domain ratings were not significantly different between these two groups. Post-treatment, this single disparity was eliminated. Additionally, post-treatment CICI group ratings were significantly higher than those of the UHL group for the Spatial domain and five subscales, SiQ, SiN, Loc, DisMov, and Eff. In comparison, post-treatment CIHA group ratings were no longer significantly poorer than the UHL group ratings for the Speech domain and the SiQ, SiSCont and Qlty subscales; however, IdSnd remained significantly lower. Post-treatment CIHA ratings were significantly better than UHL ratings for Loc and Eff. These data suggest: 1) advantages of bilateral input extended to all areas of communication, from understanding speech in quiet to judgments of sound quality to ease of listening; and 2) a CI to restore bilateral input did not degrade SSQ ratings, either in the case of CICI or CIHA for individuals with one SPHL ear.
Shown previously, audibility played a key role in listening experience. Following cochlear implantation, audibility of the CIHA group was more similar to that of the other two groups. The mean PTA (.25 – 6k Hz in dB HL) of the newly implanted ear for the CIHA group was 23.7 (SD 4.2) compared to 18.9. (SD 3.8) for the CICI group and the better ear mean PTA of 13.0 (SD 6.5) for the UHL group. Again, this supports factors in addition to bilateral input and audibility were involved in facilitating successful communication. The subscales for which the UHL, CICI and CIHA groups did not differ (SiSCont, MultStream, SegSnds, and Qlty) all probe complex listening environments that rely on higher level processing skills, i.e., separating multiple streams of input, rapidly switching attention, or discerning subtleties in sound and speech. These challenging environments are further compounded by limitations of the CI sound processor or HA to receive and transmit complex spectral signals inherent to musicality, prosody, or judgments of mood, despite bilateral input and relatively good audibility.
Implications
Study findings that participant groups perceived their daily communication experiences very similarly despite seemingly substantial differences in hearing mode has implications for cochlear implant clinicians and researchers. Previous work has shown poorer ratings for adults with UHL compared to those with NH (Douglas et al. 2007; Vermeire and Van de Heyning 2009; Olsen et al. 2012; Firszt et al. 2012b) and significantly improved ratings for bilateral CIs compared to unilateral CIs (Noble et al. 2008; Laske et al. 2009). However previous studies have not compared ratings directly between the three unilateral listener groups included in the current study. All participants had a single deaf ear that met CI candidacy criteria but due to mode of hearing for the contralateral ear were not all considered CI candidates. Of the three study groups, only the unilateral CI group would routinely be considered for contralateral ear cochlear implantation. The other two groups, one relying on a unilateral HA and the other on unilateral NH, are rarely considered for cochlear implantation of the deaf ear, but rather identified as non-candidates based on the better hearing ear. The current study results suggest that, from a patient’s perspective, this differentiation needs re-evaluation, particularly for individuals reliant on a unilateral HA who reported the greatest communication challenges. After implantation, this group (CIHA) rated themselves comparable to the UHL group with a NH ear on most subscales and higher in terms of spatial hearing and effort.
In addition, some subscales showed similarity in ratings between the UHL and the post-treatment groups, which confirms a need for continued research into the underlying issues related to speech understanding in noise, beyond the scope of audibility or bilateral input. For example, it is possible that difficulties persist due to a mismatch in audibility between ears, technological limitations of a CI or HA in encoding and transmitting spectrally and temporally complex signals with high fidelity, or limitations imposed as a result of a disruption to the normal auditory system.
Acknowledgments
We wish to thank Dorina Kallogjeri, MD, MPH for statistical support and the participants who gave of their time to provide the knowledge gained from this study. We also acknowledge Heidi Frazier, Kristen Lewis and Sarah Zlomke from Midwest Ear Institute, Kansas City, MO for assistance with data collection. This work was supported by R01DC009010 (Firszt) and P30DC04665 from the National Institute on Deafness and Other Communication Disorders.
References
- Arndt S, Aschendorff A, Laszig R, et al. Comparison of pseudobinaural hearing to real binaural hearing rehabilitation after cochlear implantation in patients with unilateral deafness and tinnitus. Otol Neurotol. 2010;32:39–47. doi: 10.1097/MAO.0b013e3181fcf271. [DOI] [PubMed] [Google Scholar]
- Banh J, Singh G, Pichora-Fuller MK. Age affects responses on the speech, spatial, and qualities of hearing scale (SSQ) by adults with minimal audiometric loss. J Am Acad Audiol. 2012;23:81–91. doi: 10.3766/jaaa.23.2.2. [DOI] [PubMed] [Google Scholar]
- Bronkhorst AW, Plomp R. Binaural speech intelligibility in noise for hearing-impaired listeners. J Acoust Soc Am. 1989;86:1374–1383. doi: 10.1121/1.398697. [DOI] [PubMed] [Google Scholar]
- Carlyon RP, Cusack R, Foxton JM, et al. Effects of attention and unilateral neglect on auditory stream segregation. J Exp Psych. 2001;27:115–127. doi: 10.1037//0096-1523.27.1.115. [DOI] [PubMed] [Google Scholar]
- Ching TY, Incerti P, Hill M, van Wanrooj E. An overview of binaural advantages for children and adults who use binaural/bimodal hearing devices. Audiol Neurotol. 2006;11(S1):6–11. doi: 10.1159/000095607. [DOI] [PubMed] [Google Scholar]
- Ching T, Dillon H, Byrne D. Speech recognition of hearing-impaired listeners: predictions from audibility and the limited role of high-frequency amplification. J Acoust Soc Am. 1998;103:1128–1140. doi: 10.1121/1.421224. [DOI] [PubMed] [Google Scholar]
- Christen R. Binaural summation at the most comfortable loudness level. Aus J Audiol. 1980;2:92–98. [Google Scholar]
- Davidson LS, Skinner MW. Audibility and speech perception of children using wide dynamic range compression hearing aids. Am J Audiol. 2006;15:141–153. doi: 10.1044/1059-0889(2006/018). [DOI] [PubMed] [Google Scholar]
- Davidson LS, Skinner MW, Holstad BA, et al. The effect of instantaneous input dynamic range setting on the speech perception of children with the nucleus 24 implant. Ear Hear. 2009;30:340–349. doi: 10.1097/AUD.0b013e31819ec93a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas S, Yeung P, Daudia A, et al. Spatial hearing disability after acoustic neuroma removal. Laryngoscope. 2007;117:1648–1651. doi: 10.1097/MLG.0b013e3180caa162. [DOI] [PubMed] [Google Scholar]
- Dumper J, Hodgetts B, Liu R, et al. Indidcations for bone-anchored hearing aids: a functional outcomes study. J Otolaryngol Head Neck Surg. 2009;38:96–105. [PubMed] [Google Scholar]
- Dunn CC, Tyler RS, Witt SA. Benefit of wearing a hearing aid on the unimplanted ear in adult users of a cochlear implant. J Speech Lang Hear Res. 2005;48:668–680. doi: 10.1044/1092-4388(2005/046). [DOI] [PubMed] [Google Scholar]
- Feuerstein JF. Monaural versus binaural hearing: ease of listening, word recognition, and attentional effort. Ear Hear. 1992;13:80–86. [PubMed] [Google Scholar]
- Firszt JB, Reeder RM, Skinner MW. Restoring hearing symmetry with two cochlear implants or one cochlear implant and a contralateral hearing aid. J Rehab Res Dev. 2008;45:749–768. doi: 10.1682/jrrd.2007.08.0120. [DOI] [PubMed] [Google Scholar]
- Firszt JB, Holden LK, Reeder RM, et al. Cochlear implantation in adults with asymmetric hearing loss. Ear Hear. 2012a;33:521–533. doi: 10.1097/AUD.0b013e31824b9dfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firszt JB, Holden LK, Reeder RM, et al. Auditory abilities after cochlear implantation in adults with unilateral deafness: a pilot study. Otolo Neurotol. 2012b;33:1339–1346. doi: 10.1097/MAO.0b013e318268d52d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firszt JB, Holden LK, Skinner MW, et al. Recognition of speech presented at soft to loud levels by adult cochlear implant recipients of three cochlear implant systems. Ear Hear. 2004;25:375–387. doi: 10.1097/01.aud.0000134552.22205.ee. [DOI] [PubMed] [Google Scholar]
- Gatehouse S, Akeroyd M. Two-eared listening in dynamic situations. Int J Audiol. 2006;45(Suppl):120–124. doi: 10.1080/14992020600783103. [DOI] [PubMed] [Google Scholar]
- Gatehouse S, Noble W. The Speech, Spatial and Qualities of Hearing Scale (SSQ) Int J Audiol. 2004;43:85–99. doi: 10.1080/14992020400050014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giolas TG, Wark DJ. Communication problems associated with unilateral hearing loss. J Speech Hear Disord. 1967;32:336–343. doi: 10.1044/jshd.3204.336. [DOI] [PubMed] [Google Scholar]
- Häusler R, Colburn S, Marr E. Sound localization in subjects with impaired hearing. spatial discrimination and interaural-discrimination tests. Act Oto Laryng. 1983;400(Suppl):1–62. doi: 10.3109/00016488309105590. [DOI] [PubMed] [Google Scholar]
- Hawkins DB, Prosek RA, Walden BE, et al. Binaural loudness summation in the hearing impaired. J Speech Hear Res. 1987;30:37–43. doi: 10.1044/jshr.3001.37. [DOI] [PubMed] [Google Scholar]
- Hol M, Kunst S, Snik A, Cremers C. Pilot study on the effectiveness of the conventional CROS, the transcranial CROS and the BAHA transcranial CROS in adults with unilateral inner ear deafness. Eur Arch Otorhinolaryngol. 2010;267:889–896. doi: 10.1007/s00405-009-1147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden LK, Skinner MW, Fourakis MS, et al. Effect of increased IIDR in the nucleus freedom cochlear implant system. J Am Acad Audiol. 2007;18:777–793. doi: 10.3766/jaaa.18.9.6. [DOI] [PubMed] [Google Scholar]
- Humes LE, Dirks DD, Bell TS, et al. Application of the articulation index and the speech transmission index to the recognition of speech by normal-hearing and hearing-impaired listeners. J Speech Hear Res. 1986;29:447–462. doi: 10.1044/jshr.2904.447. [DOI] [PubMed] [Google Scholar]
- Humes LE, Roberts L. Speech-recognition difficulties of the hearing-impaired elderly: the contributions of audibility. J Speech Hear Res. 1990;33:726–735. doi: 10.1044/jshr.3304.726. [DOI] [PubMed] [Google Scholar]
- Laske RD, Veraguth D, Dillier N, et al. Subjective and objective results after bilateral cochlear implantation in adults. Otol Neurotol. 2009;30:313–318. doi: 10.1097/MAO.0b013e31819bd7e6. [DOI] [PubMed] [Google Scholar]
- Noble W. Assessing binaural hearing: results using the speech, spatial and qualities of hearing scale. J Am Acad Audiol. 2010;21:568–574. doi: 10.3766/jaaa.21.9.2. [DOI] [PubMed] [Google Scholar]
- Noble W, Gatehouse S. Interaural asymmetry of hearing loss, Speech, Spatial and Qualities of Hearing Scale (SSQ) disabilities, and handicap. Int J Audiol. 2004;43:100–114. doi: 10.1080/14992020400050015. [DOI] [PubMed] [Google Scholar]
- Noble W, Gatehouse S. Effects of bilateral versus unilateral hearing aid fitting on abilities measured by the Speech, Spatial, and Qualities of Hearing Scale (SSQ) Int J Audiol. 2006;45:172–181. doi: 10.1080/14992020500376933. [DOI] [PubMed] [Google Scholar]
- Noble W, Tyler R, Dunn C, et al. Unilateral and bilateral cochlear implants and the implant-plus-hearing-aid profile: comparing self-assessed and measured abilities. Int J Audiol. 2008;47:505–514. doi: 10.1080/14992020802070770. [DOI] [PubMed] [Google Scholar]
- Noble W, Tyler RS, Dunn CC, et al. Younger- and older-age adults with unilateral and bilateral cochlear implants: Speech and spatial hearing self-ratings and performance. Otol Neurotol. 2009;30:921–929. doi: 10.1097/MAO.0b013e3181b76b3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen S, Hernvig L, Nielsen L. Self-reported hearing performance among subjects with unilateral sensorineural hearing loss. Audiol Med. 2012;10:83–92. [Google Scholar]
- Pai I, Kelleher C, Nunn T, et al. Outcome of bone-anchored hearing aids for single-sided deafness: a prospective study. Acta Otolaryngol. 2012;132:751–755. doi: 10.3109/00016489.2012.655862. [DOI] [PubMed] [Google Scholar]
- Perreau AE, Tyler RS, Witt S, Dunn C. Selection strategies for binaural and monaural cochlear implantation. Am J Audiol. 2007;16:85–93. doi: 10.1044/1059-0889(2007/011). [DOI] [PubMed] [Google Scholar]
- Peissig J, Kollmeier B. Directivity of binaural noise reduction in spatial multiple noise-source arrangements for normal and impaired listeners. J Acoust Soc Am. 1997;101:1660–1670. doi: 10.1121/1.418150. [DOI] [PubMed] [Google Scholar]
- Pollack I. Comfortable listening levels for pure tones in quiet and noise. J Acoust Soc Am. 1952;24:158–162. [Google Scholar]
- Rancovic C. An application of the articulation index to hearing aid fitting. J Speech Hear Res. 1991;34:391–402. doi: 10.1044/jshr.3402.391. [DOI] [PubMed] [Google Scholar]
- Rose MM, Moore BCJ. Perceptual grouping of tone sequences by normally hearing and hearing-impaired listeners. J Acoust Soc Am. 1997;102:1768–1778. doi: 10.1121/1.420108. [DOI] [PubMed] [Google Scholar]
- Rose MM, Moore BCJ. The relationship between stream segregation and frequency discrimination in normally hearing and hearing-impaired subjects. Hear Res. 2005;204:16–28. doi: 10.1016/j.heares.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Singh G, Pichora-Fuller MK. Older adults’ performance on the speech, spatial, and qualities of hearing scale (SSQ): test-retest reliability and a comparison of interview and self-administration methods. Int J Audiol. 2010;49:733–740. doi: 10.3109/14992027.2010.491097. [DOI] [PubMed] [Google Scholar]
- Skinner MW, Holden LK, Holden TA, et al. Comparison of two methods for selecting minimum stimulation levels used in programming the nucleus 22 cochlear implant. J Speech Lang Hear Res. 1999;42:814–828. doi: 10.1044/jslhr.4204.814. [DOI] [PubMed] [Google Scholar]
- Skinner MW, Holden LK, Holden TA, et al. Speech recognition at simulated soft, conversational, and raised-to-loud vocal efforts by adults with cochlear implants. J Acoust Soc Am. 1997;101:3766–3782. doi: 10.1121/1.418383. [DOI] [PubMed] [Google Scholar]
- Van Hoesel RJM. Exploring the benefits of bilateral cochlear implants. Audiol Neurotol. 2004;9:234–246. doi: 10.1159/000078393. [DOI] [PubMed] [Google Scholar]
- Van Hoesel RJM, Tyler R. Speech perception, localization and lateralization with bilateral cochlear implants. J Acoust Soc Am. 2003;113:1617–1630. doi: 10.1121/1.1539520. [DOI] [PubMed] [Google Scholar]
- Vermeire K, Van de Heyning P. Binaural hearing after cochlear implantation in subjects with unilateral sensorineural deafness and tinnitus. Audiol Neurotol. 2009;14:163–171. doi: 10.1159/000171478. [DOI] [PubMed] [Google Scholar]