Abstract
Background
Environmental exposures during critical periods of prenatal and early postnatal life affect the development of mammalian body weight regulatory mechanisms, influencing lifelong risk of obesity. The specific biologic processes that mediate the persistence of such effects, however, remain poorly understood.
Objective
The objectives were to determine the developmental timing and physiological basis of the obesity-promoting effect previously reported in offspring of obese agouti viable yellow (Avy/a) mothers.
Design
Newborn offspring of obese agouti viable yellow (Avy/a) and lean (a/a) mothers were cross-fostered shortly after birth to study separately the effects of in utero or suckling-period exposure to Avy/a dams. Body composition, food intake, physical activity, and energy expenditure were measured in offspring shortly after weaning and in adulthood.
Results
Offspring of obese Avy/a dams paradoxically experienced fetal growth restriction, which was followed by adult-onset obesity specifically in females. Our main analyses focused on wild type (a/a) offspring, because a subset of adult Avy/a offspring contracted a kidney disease resembling diabetic nephropathy. Detailed physiological characterization demonstrated that, both shortly after weaning and in adulthood, female wild type mice born to Avy/a mothers are not hyperphagic, but have reduced physical activity and energy expenditure. No such coordinated changes were detected in male offspring. Mediational regression analysis of our longitudinal data supported a causal pathway in which fetal growth restriction persistently reduces physical activity, leading to adult obesity.
Conclusions
Our data are consistent with several recent human epidemiologic studies showing female-specific effects of perinatal nutritional restriction on later obesity, and provide the novel mechanistic insight that this may occur via permanent and sex-specific changes in one’s inherent propensity for physical activity.
Keywords: developmental origins, developmental programming, development, body weight regulation, physical activity, energy expenditure, food intake, metabolic imprinting
INTRODUCTION
The increasing prevalence of obesity, once viewed as a problem of developed countries, is rapidly becoming a worldwide public health crisis1. Environmental exposures during critical ontogenic periods can wield substantial impact on adult metabolic disease risk, highlighting the potential for developmental interventions to curb the obesity pandemic2, 3. In addition to overnutrition4, prenatal undernutrition can also increase later risk of obesity. In particular, epidemiologic data based on birth weight indicate that nutritional restriction during fetal development followed by ample postnatal nutrition (developmental mismatch) promotes obesity5, 6. This hypothesis is supported by recent human quasi-experimental studies7; in independent follow-up studies of three diverse episodes of severe famine (the Dutch famine near the end of World War II)8, the great famine of China (1959–1961) 9, and the Biafran famine during the Nigerian civil war (1967–1970)10, perinatal famine exposure followed by nutritional adequacy increased the risk of adult obesity. Most strikingly, in all three populations this effect was found in women only.
Fetal growth restriction due to placental insufficiency affects a substantial proportion of births, even in developed countries11. Additionally, modernization and improved standards of living underlie a nutrition transition underway in many countries12; millions of individuals who experienced nutritional deficiency early in life are now, several decades later, in an environment of relative food surfeit. Appropriate animal models are urgently needed to improve our understanding of the biology underlying and potential interventions to counter developmental mismatch. Diverse animal models13, 14 have documented that fetal growth restriction followed by postnatal catch-up growth promotes lifelong positive energy balance. Whether this is caused more by increased food intake or reduced energy expenditure, however, remains unclear. Moreover, none of the previous animal models recapitulates the female-specific effect observed in the human studies8–10.
We previously reported transgenerational amplification of obesity in agouti viable yellow (Avy) mice15. The Avy mutation resulted from retrotransposition of an intracisternal A particle upstream of agouti, and causes individually-variable ectopic expression of agouti protein16. Due to its structural similarity to agouti related peptide, ectopic agouti protein antagonizes the melanocortin-4 pathway in the hypothalamus17 causing hyperphagic obesity in Avy/a mice. (The non-agouti (a) allele does not encode functional agouti protein.) Our previous study demonstrated that, compared to Avy/a offspring of a/a dams, isogenic offspring of Avy/a dams become heavier and fatter in adulthood15. It did not, however, resolve whether this obesogenic effect occurs during fetal or early postnatal development, affects a/a as well as Avy/a offspring, or is due to dysregulated food intake, energy expenditure, or physical activity. The current study, designed to answer these questions, found surprisingly that cross-fostering offspring of Avy/a dams to lean a/a dams may provide an apt model of developmental mismatch in humans. Fetal growth restriction followed by postnatal catch-up growth promoted obesity only in females, and this appeared to be mediated not by increased food intake but by persistent blunting of spontaneous physical activity.
MATERIALS AND METHODS
Mouse husbandry, diet, and body composition
Animals were maintained in accordance with all relevant federal guidelines, and the study was approved by the Baylor College of Medicine Animal Care and Use Committee. Avy mice were originally obtained from the colony at the National Center for Toxicological Research18, and have been maintained for hundreds of generations by crossing Avy/a males with their a/a sisters, resulting in an essentially invariant genetic background. All mice were provided ad libitum access to water and diet. During mating, pregnancy, and lactation, Teklad 2919 diet (Harlan Laboratories) was used; otherwise, Teklad 2920X was used. The mouse experiments were conducted in two phases. Phase 1 (Figure 1) was conducted between November, 2009 and July 2010, and Phase 2 (Figures 2, 3, and 4) was conducted between January, 2010 and October, 2011. To establish body weight cutoffs characterizing ‘lean’ and ‘obese’ dams, body composition of 11-wk-old a/a and Avy/a females was measured by dual energy X-ray absorptiometry (Piximus Series Densitometer, GE Medical Systems) as per manufacterer’s instructions.
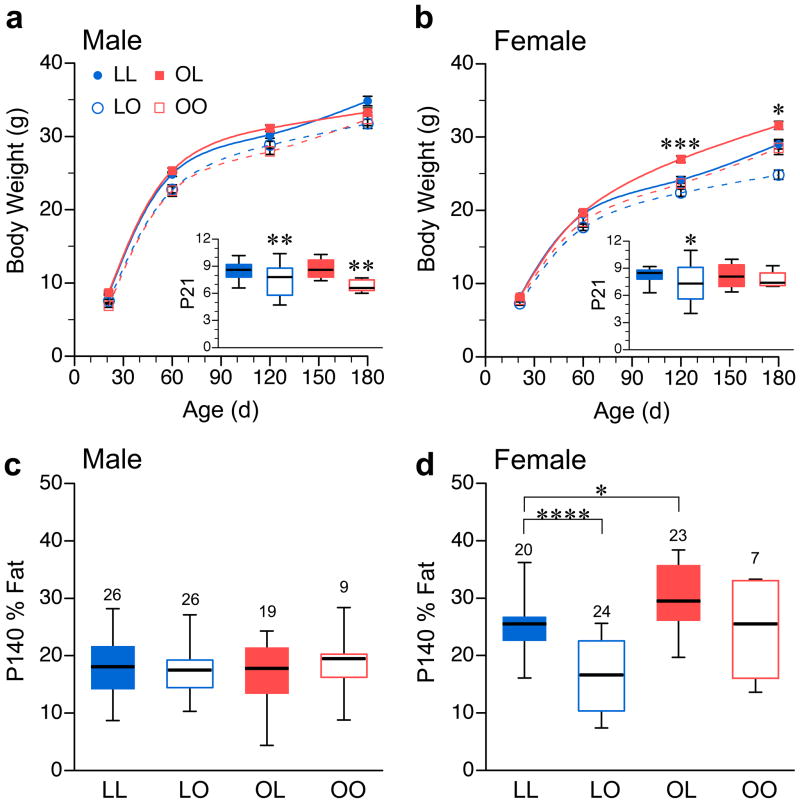
Figure 1.
Fetal growth restriction promotes obesity specifically in female offspring. Body weight vs. age of male (a) and female (b) a/a offspring cross fostered among lean a/a and obese Avy/a dams. Each plot represents mean ± SEM of 20–37 LL offspring from 14–15 litters, 24–50 LO offspring from 28–32 litters, 19–25 OL offspring from 11–12 litters, and 4–9 OO offspring from 3–5 litters. (Detailed sample size tabulation in Table S1a–b.) Asterisks in main plots indicate significance of OL vs. LL differences only. (P values for all group comparisons in Table S1c.) Insets are box plots of body weight at P21, each indicating median, 25th–75th percentiles, and 5th–95th percentiles. (c) and (d) are box plots illustrating group differences in body composition (% fat) of P140 a/a male and female offspring, respectively. Number of mice studied is indicated above each box. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 relative to LL mice of same age and sex.
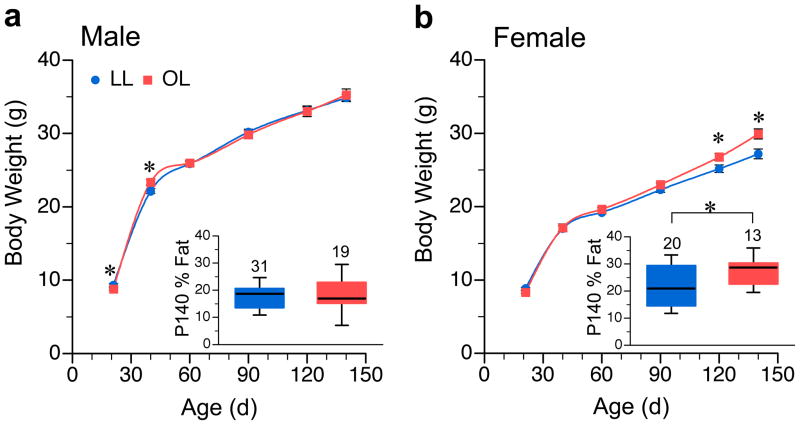
Figure 2.
Independent experiment confirms that fetal growth restriction promotes increased adult body weight and adiposity only in female offspring. Body weight vs. age of a/a male (a) and female (b) LL and OL offspring. Each plot represents mean ± SEM of 21–36 LL offspring from 12–18 litters, and 13–34 OL offspring from 11–17 litters. (Detailed sample size tabulation in Table S2a–b.) Insets are box plots of % body fat at P140, each indicating median, 25th–75th percentiles, and 5th–95th percentiles. Number of mice studied is indicated above each box. *P<0.05 relative to LL mice of same age and sex.
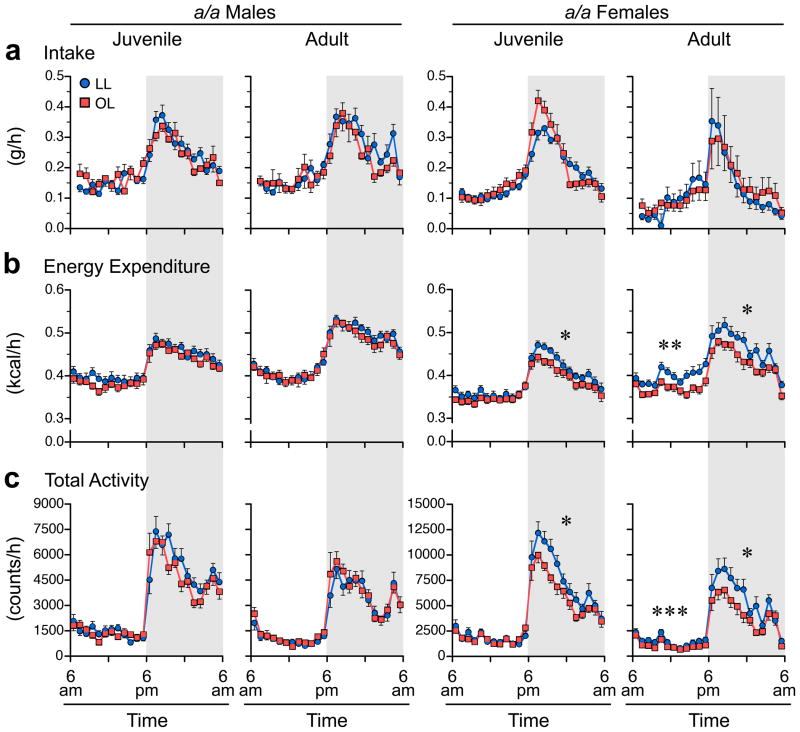
Figure 3.
Fetal growth restriction reduces physical activity and energy expenditure in a/a females but not males. (a) Dietary intake, (b) energy expenditure, and (c) total physical activity of a/a LL and OL mice, by sex and age. (Dietary intake and energy expenditure data have been least-squares normalized for lean mass and fat mass23.) For each mouse, three days of metabolic cage data were collapsed into 24 1-hour averages. Each plot represents mean ± SEM of 12–13 LL offspring from 7 litters, and 12 OL offspring from 8 litters. Statistical analyses were performed separately by light and dark periods (indicated by shading). *P<0.05, ***P<0.001, ****P<0.0001 relative to LL mice of same age and sex.
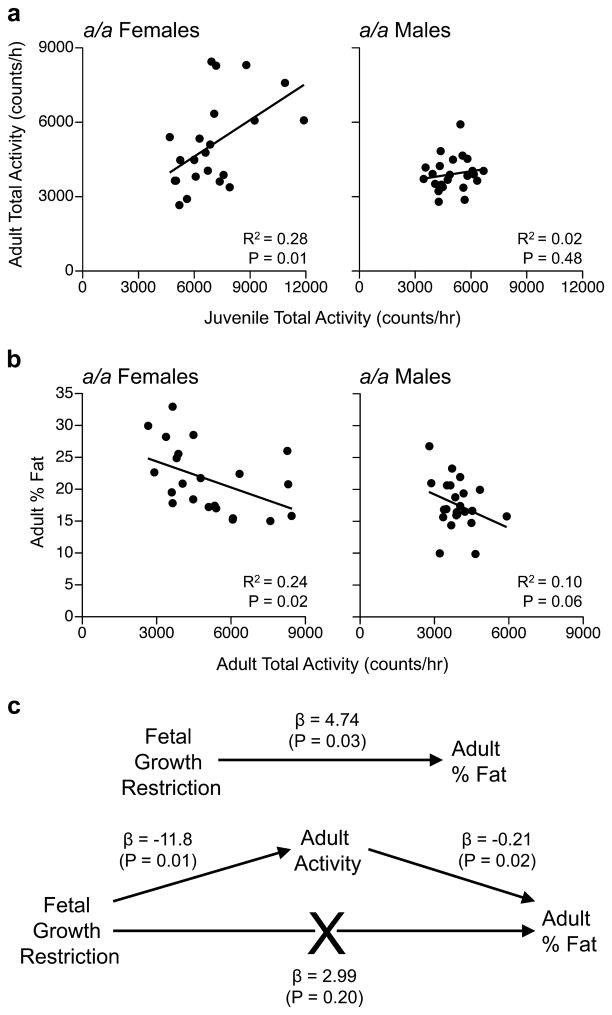
Figure 4.
Physical activity levels ‘track’ into adulthood and predict adult adiposity in females only. (a) Among a/a offspring, dark-period physical activity in juveniles predicts that in adulthood only in females. Each plot includes 11 LL offspring from 7 litters and 11–12 OL offspring from 8 litters. (b) Adult physical activity predicts adult adiposity, again only in females (same offspring as in panel A). (c) Mediational regression analysis focused on a/a female offspring. Fetal growth restriction predicts adult adiposity (top), but this direct pathway is abrogated when adult physical activity is included in the model (bottom), indicating that induced alterations in physical activity mediate the obesogenic effect of fetal growth restriction. (Analysis includes data on female offspring from panels a & b.)
To most closely replicate our previous transgenerational study of obesity in Avy mice15 (in which the Avy allele was passed down the female line) all F0 dams in the current study were born to an Avy/a mother. At age 11 wk, F0 a/a females weighing < 25.0g (lean) and F0 Avy/a females weighing >37.0g (obese) were mated with Avy/a males or a/a males, respectively. As soon as each dam became visibly pregnant, the male was removed from the cage. To maximize acceptance of fostered pups, dams were handled daily during the last several days of pregnancy, by the same technician who would perform the fostering. The F1 offspring were all cross-fostered shortly after birth, usually on postnatal day 1 (P1 – the day after birth) in Phase 1, and usually on P0 in Phase 2. The day of fostering did not differ significantly among the fostering groups. To minimize potential developmental effects associated with variation in litter size, only birth litters of 6–10 pups were included. To minimize potential persistent effects of postnatal litter size19, all litters were culled to a maximum of 8 pups at weaning (i.e. suckling period litter size range was 6–8 pups). Pups were selected for culling on the basis of matching overall litter weight and sex ratio to that of the cross-fostered litter. At weaning (P21), offspring were weighed and classified by sex and genotype (by coat color: a/a mice are black and Avy/a mice range from yellow to brown).
Coat color phenotype of Avy/a mice was further classified by visual estimation of the proportion of brown fur: yellow (<5% brown), slightly mottled (>5% but <50% brown), mottled (about 50% brown), heavily mottled (>50% but <95% brown) and pseudoagouti (>95% brown)20. Epigenetic inheritance occurs when the Avy allele is passed through the female germline21. Because obese Avy/a dams are generally hypomethylated at Avy, their Avy/a offspring tend to have lower methylation (and, hence, yellower coats) than Avy/a offspring born to a/a dams. Consequently, pseudoagouti (completely brown) Avy/a offspring were much more common in the LL and LO groups than in the OL and OO groups. Since pseudoagouti Avy/a mice are protected from the obesity otherwise conferred by the Avy mutation15, 22, we eliminated this potential confound by excluding pseudoagouti F1 offspring from the study, beginning at P21. Offspring were caged by sex in groups of up to 5 mice, and identified individually by ear punching when necessary. Growth of mice was monitored by periodic weighing, and body composition measured at P140 by dual energy X-ray absorptiometry (Piximus Series Densitometer, GE Medical Systems – Phase 1) or by quantitative magnetic resonance (QMR, EchoMRI-100, Echo Medical Systems LLC – Phase 2) (both according to manufacturer’s instructions).
Metabolic cage studies
In Phase 2, a subset of F1 offspring was studied in metabolic cages (Comprehensive Laboratory Animal Monitoring System – CLAMS – Columbus Instruments) at each of P40 and P140. Directly before each study, the flow to each of the 12–16 chambers was verified at 0.5 L/min using a Calibration Analyzer (RT-200, Allied Healthcare Products). The O2 and CO2 analyzers were routinely calibrated using certified calibration gases. Also, infusion of CO2 and N2 mixtures using a Pegas 4000 MF gas blender (Columbus Instruments) was used to evaluate the overall performance of the chambers. Both the RT-200 calibration analyzer and the Pegas 4000 MF gas blender were routinely tested against a Sierra Instruments series 101 Calbench standard (which is traceable to the National Institute of Standards and Technology), as were the concentrations of O2 and CO2 in the reference gas tanks used for daily calibration.
We attempted to perform studies on the same mice at both ages, when possible. We originally attempted to perform the juvenile studies directly after weaning (i.e. at P25), some of the P25 mice were able to wedge themselves into inappropriate areas of the metabolic cages; P40 was therefore chosen for the juvenile studies. In most cases, a maximum of one male and one female of the same genotype were studied from each litter. Adult females were studied without regard to estrus cycle. Starting at P40 (juvenile) or P140 (adult), mice were housed singly in ‘feeder’ cages for three days to allow them to become accustomed to the metabolic cages and eating the powdered diet from the feeder apparatus. No data were collected during these equilibration periods. Directly after the equilibration period, each mouse’s body composition was measured by QMR, as described above, to enable normalization of energy expenditure and food intake data. (QMR can be performed rapidly and without sedation, so there were no carryover effects of sedative exposure on subsequent metabolic measurements.) Directly after the QMR measurement, each mouse was transferred to a metabolic cage in the morning. Beginning at 6:00 am on the following day, 72 hours of data were collected on food intake, energy expenditure (by indirect calorimetry), and physical activity (by beam breaks; coded as x axis activity, ambulatory x-axis activity (i.e. movement along the length of the cage), and z-axis activity (rearing). Total activity data represent the sum of total x axis and total z axis activity. All three components of physical activity (x, x ambulatory, and z) showed similar group, sex, and developmental differences. For simplicity, total activity (sum of x, x ambulatory, and z activity) was analyzed and plotted. All data were collapsed into 24 1-hr averages for each mouse, at each age. Room lights were on from 6:00 am to 6:00 pm; light cycling was corroborated by a room photodetector coupled with the CLAMS instrument.
Statistical approaches
All data were entered independently into two Excel spreadsheets which were compared electronically to eliminate data entry errors. Group differences in body weight and body composition were evaluated by analysis of variance (ANOVA – PROC GLM, SAS Version 9.2). Temporal analysis of food intake, energy expenditure, and physical activity were analyzed by repeated measures ANOVA (SAS PROC Mixed – autoregressive covariance structure) with ‘hour’ as the repeated effect. This approach utilizes the full power of these time-series data while recognizing the non-independence of the 24 multiple measures within each mouse. (The autoregressive covariance structure was chosen because correlations between multiple measurements decline exponentially with the distance (time) between the measurements.) To normalize food intake and energy expenditure data for body weight and body composition, lean mass and fat mass were included in the models as independent variables23. CLAMS data were analyzed separately for the dark (active) and light (resting) periods. Activity counts were skewed right, and were therefore square-root transformed to improve normality prior to analysis. Mediational regression analysis24 was performed by multiple and individual regression (SAS). Statistical significance was based on an α level of 0.05 throughout.
RESULTS
The obesity-promoting effect in offspring of Avy/a mothers occurs before birth
We began by measuring body composition of 11-week-old females to establish pre-pregnancy body-weight cutoffs classifying obese Avy/a (>37 g body weight, >40% fat) and lean a/a dams (<25 g body weight, <22% fat) (Supplemental Figure S1). Obese Avy/a females were crossed with a/a males and lean a/a females were crossed with Avy/a males, producing equal ratios of a/a and Avy/a offspring. Shortly after birth (mostly on postnatal day 1 (P1 - the day after birth)) all litters were cross-fostered, generating four groups: LL – born to and fostered to lean dams, LO – born to lean, fostered to obese, OL – born to obese, fostered to lean, and OO – born to and fostered to obese dams (Table 1). Human offspring of obese mothers tend to have elevated birth weight4. Compared to offspring of lean a/a dams, however, those born to obese Avy/a dams weighed 20% less at P1 (1.21 vs. 1.50 g, P<0.0001) (Supplemental Figure S2a), suggesting fetal growth restriction. At weaning (P21), body weight tended to be lower in pups suckled by Avy/a dams (Figure 1, a and b, insets) particularly in males. (Note: For simplicity, data on a/a offspring only are shown. Similar results were obtained in Avy/a offspring (Supplemental Figure S3)). In adulthood, body weight of LO and OO offspring was generally lower than and never exceeded that of their LL counterparts (Figure 1, a and b, and Supplemental Table S1). These results indicate that obese Avy/a females have impaired lactational performance (consistent with other murine and human studies25) causing persistent stunting in the offspring.
Table 1.
The four groups generated by cross fostering.
| Group | Birth Dam | Foster Dam |
|---|---|---|
| LL | Lean (a/a) | Lean (a/a) |
| LO | Lean (a/a) | Obese (Avy/a) |
| OL | Obese (Avy/a) | Lean (a/a) |
| OO | Obese (Avy/a) | Obese (Avy/a) |
Although lighter at birth, mice born to an obese Avy/a mother and fostered to a lean a/a mother (OL) ‘caught-up’ by weaning (Figure 1, a and b, insets). In adulthood, there was no difference in body weight of OL and LL males (Figure 1a), but OL females became significantly heavier than LL females (Figure 1b). Body composition analysis by dual energy x-ray absorptiometry found no group differences in males (Figure 1c), but showed that adult OL females are fatter than LL (Figure 1d). The increased % fat was due solely to increased fat mass; lean mass was not affected (data not shown). Together, these data indicate that the obesity-promoting effect in offspring of Avy/a dams15 occurs during fetal development, and is specific to females.
Adult weight loss in Avy/a mice is associated with kidney disease
We investigated why the OL vs. LL body weight increment in Avy/a offspring (Supplemental Figure S3b) did not persist from P120 to P180. In a subset of mice in which body weight had been measured at P140, we noted that no a/a mice lost weight from P140-P180, but a significant proportion of Avy/a mice did (P=0.006, Supplemental Figure S4a). Avy/a mice that either lost or gained weight from P140-P180 were necropsied at P180; the former all exhibited kidney disease grossly resembling diabetic nephropathy (Supplemental Figure S4b) but no other overt pathology. Obese Avy/a mice have long been known to develop a phenotype resembling type-2 diabetes17, but we are not aware of previous evidence that they may also provide a useful model of diabetic nephropathy26.
Independent study confirms female-specific effect of fetal growth restriction
To confirm our findings of a sex-specific effect in offspring of Avy/a dams, we repeated the cross-fostering experiment, focusing only on LL and OL mice. To obtain a better measure of birth weight, most of the pups were cross-fostered at P0 rather than P1. As in the first study, offspring of Avy/a dams were significantly smaller at birth (1.18 vs. 1.35 g, P<0.0001, Supplemental Figure S2b) but, when fostered to an a/a dam, generally caught up by P21 (Figure 2). We confirmed fetal growth restriction in offspring of Avy/a dams by measuring fetal weight at 18.5 days of gestation (E18.5). Fetuses of Avy/a dams were 26% smaller than isogenic fetuses of of a/a dams (0.86g vs. 1.17g, P<0.0001, Supplemental Figure S2c) but there was no difference in placental weight (Supplemental Figure S2d). In male a/a offspring, no group differences were observed in either adult body weight (Figure 2a) or adiposity (Figure 2a, inset). As in the previous experiment, adult body weight was elevated in a/a OL females (Figure 2b). (A similar female-specific effect was found in Avy/a offspring (Supplemental Figure S5)). Body composition analysis by quantitative magnetic resonance (QMR) showed, once again, that adult OL a/a females were also fatter than their LL counterparts (Figure 2b, inset); as in the first study, the increased adiposity was purely due to increased fat mass (lean mass was not affected). Hence, this independent study corroborated the findings of the first experiment; offspring of Avy/a dams are growth restricted in utero but, when fostered by a/a dams, exhibit catch-up growth and develop adult-onset obesity specifically in females.
Females exposed to catch-up growth display persistent decreases in physical activity and energy expenditure
To identify alterations in food intake, energy expenditure, or physical activity that precede and therefore may explain OL females’ propensity to overweight, we tested LL and OL offspring in metabolic cages both as juveniles (P40) and as adults (P140). Directly before the metabolic studies we measured body composition by QMR, enabling food intake and energy expenditure data to be least-squares normalized for lean mass and fat mass23. Hence, these data were analyzed as if comparing mice of the same body weight and composition. Food intake of OL mice did not differ from that of isogenic LL mice, in either females or males, as juveniles or as adults (Figure 3a and Supplemental Figure S6a). Similarly, there were no group differences in respiratory exchange ratio (data not shown). We did, however, detect persistent group differences in energy expenditure. Shortly after weaning, energy expenditure of a/a OL females was significantly lower than that of isogenic LL mice, specifically during the dark cycle (Figure 3b); this energy expenditure deficit was associated with reduced physical activity, also during the dark cycle (Figure 3c). Notably, the physical activity and energy expenditure defects of a/a OL females persisted to adulthood, and encompassed both the light and dark cycles (Figure 3, b and c). In Avy/a offspring (Supplemental Figure S6) the picture was less clear. Adult Avy/a females were extremely lethargic, OL even more so than LL (Supplemental Figure S6c). This activity decrement, however, was neither detected in younger mice (Supplemental Figure S6c) nor associated with altered energy expenditure (Supplemental Figure S6b). Adult Avy/a OL males had increased energy expenditure but reduced physical activity during the light on cycle (Supplemental Figure S6, b and c), consistent with previous reports of altered brown adipose tissue development in offspring of obese rodents27. A persistent decrement in dark cycle spontaneous physical activity in Avy/a OL males (Supplemental Figure S6c) was not associated with lower energy expenditure (Supplemental Figure S6b).
Most importantly, contrary to the persistent coordinated changes in energy expenditure and physical activity in a/a OL females, there was not a single significant OL vs. LL difference in a/a males (Figure 3, b and c). The metabolic studies are therefore concordant with our growth and body composition findings (Figures 1 and 2); fetal growth restriction followed by catch up growth promotes positive energy balance only in female offspring. Together, our data suggest this occurs by a persistent and sex-specific blunting of spontaneous physical activity. We tested this model in the subset of a/a mice in which metabolic data were obtained in the same mice both as juveniles and as adults. Remarkably, activity levels tracked from juvenile to adult life (Figure 4a) and adult physical activity was inversely correlated with adiposity (Figure 4b) only in females. A mediational regression analysis24 incorporating these findings (Figure 4c) indicated that the female-specific effect of fetal growth restriction on adult adiposity is indeed mediated at least in part by a persistent down-regulation of spontaneous physical activity.
DISCUSSION
Our results indicate that the Avy mouse may provide an excellent animal model in which to understand how fetal growth restriction followed by nutritional sufficiency – developmental mismatch5 – promotes obesity later in life. This model appears to be unique in recapitulating the female-specific effects of developmental mismatch found in human quasi-experimental studies8–10. Further, our results in this model show that developmental mismatch leads to adult obesity via a persistent female-specific reduction in spontaneous physical activity.
More generally, our results indicate that Avy/a mice may provide a new and apt animal model of fetal growth restriction. Fetal growth restriction causes infants to be born small for gestational age (SGA – defined as birth weight < 10th percentile for gestational age). SGA infants are vulnerable to a host of perinatal complications, and have increased risk for chronic long-term health conditions11. Most established animal models of fetal growth restriction employ either maternal nutritional restriction28 or uterine artery ligation29. In humans, however, fetal growth restriction is generally caused by placental insufficiency30, not maternal nutritional deficiency. Notably, newborn offspring of well-nourished Avy/a females generally weigh below the 10th percentile of isogenic offspring of a/a females (Supplemental Figure S2a–c), consistent with SGA. Since placental size is not compromised (Supplemental Figure S2d), the fetal:placental weight ratio (a proxy for placental efficiency)31 is reduced. This fetal growth restriction could be associated with pre-eclampsia, given that Avy/a mice are obese and diabetic, both of which are risk factors for pre-eclampsia in humans32. Future studies will be required to determine the specific mechanisms mediating fetal growth restriction in offspring of obese Avy/a females.
It has long been postulated that early nutritional insults persistently influence body weight by affecting the development of hypothalamic centers regulating food intake33, 34. Our data show, however, that fetal growth restriction followed by catch-up growth may instead persistently impair central regulation of spontaneous physical activity. Previous animal studies have indicated that prenatal and early postnatal nutrition can cause persistent changes in physical activity. Vickers et al35 reported persistently decreased locomotor activity in the female offspring of rat dams who were undernourished during pregnancy. Activity was monitored for only 15 min in each animal, however, and no data were provided on body weight or adiposity. Bellinger et al36 studied rats born to dams fed a low-protein diet during various stages of pregnancy and found, in one of four groups studied, a female-specific effect of maternal diet on one component of physical activity. But their activity measurements also were conducted for only a very short period of time (30 or 60 min) and, surprisingly, did not differ substantially between the light and dark period. Khan et al37 studied offspring of rat dams fed a lard-rich diet before and during pregnancy and lactation, and detected a female-specific deficit in adult physical activity at one of three ages studied. Again, however, no data on body weight or adiposity were reported. We recently showed that early postnatal overnutrition causes decreased physical activity specifically in females38, but the activity deficit was not detected until adulthood, so it was not possible to draw a clear causal pathway mediated by induced alterations in physical activity. Hence, although previous studies have provided clues that prenatal and postnatal nutrition may cause persistent changes in physical activity specifically in females, our current findings are unique. By longitudinally collecting extensive metabolic and body composition data in a large number of animals, we have shown that fetal growth restriction promotes obesity in females by persistently down-regulating spontaneous physical activity (Figure 4), validating and unifying the previous studies.
Our study does, however, have weaknesses. We have not yet characterized the molecular mechanisms by which fetal growth restriction leads to persistent down-regulation of physical activity. This will be critical to determining if similar processes occur in humans. Compared to our understanding of central mechanisms governing food intake, however, we know relatively little about those controlling voluntary physical activity39, 40. Extensive future studies will be required to elucidate the developmental processes that establish individual propensity for physical activity, and how these are affected by fetal and early postnatal environment. Whereas epigenetic alterations in the central nervous system are almost certainly involved41, these will need to be studied at the level of specific cell types and in the context of potential associated neuroanatomic changes42. Also, although our body composition data indicate that fetal growth restriction did not affect adult muscle mass, we cannot rule out an effect on skeletal muscle innervation. Another limitation of our study is that we do not yet understand the basis of the female-specific effect. One possibility is that androgen-mediated masculinization of the male brain (which occurs during late fetal development43) protects the central nervous system against deleterious effects of fetal growth restriction. Regardless of the molecular mechanism, the males in our study may be viewed as an intrinsic negative control; the absence of any effect in a/a males (which were studied alongside the females) makes those found in a/a females (Figures 1, 2, 3, and 4) even more compelling. Lastly, it is unclear whether the physical activity differences we observed are more akin to volitional exercise or non-exercise activity thermogenesis44 in humans. Future studies of wheel running behavior in OL vs. LL mice should help clarify this, since wheel running may be a reasonable model of human volitional exercise40.
These caveats notwithstanding, the female-specific effect identified here is concordant with multiple recent reports that perinatal famine exposure in humans promotes adult obesity specifically in females8–10, raising the question of whether prenatal and early postnatal undernutrition sex-specifically compromises development of human locomotor function. Indeed, recent human epidemiologic data, including a meta-analysis of Nordic adults45 and a study of stunted Cameroonian children46 indicate that early growth restriction reduces physical activity later in life. From a translational perspective, there is no harm in encouraging parents of children who have experienced fetal growth restriction to make extra effort to promote healthy patterns of physical activity. Otherwise, and particularly in the context of the nutrition transition underway in many parts of the world12, persistent blunting of spontaneous physical activity due to developmental mismatch may in coming decades exacerbate current alarming worldwide trends in physical inactivity47 and, consequently, obesity1.
Supplementary Material
Acknowledgments
Sources of support: This work was supported by grants from NIH/NIDDK (1R01DK081557) and USDA (CRIS 6250-51000-055) to RAW. The body composition and CLAMS studies were performed in the Mouse Metabolic Research Unit at the USDA/ARS Children’s Nutrition Research Center, which is supported by funds from the USDA/ARS.
We thank Adam Gillum (USDA/ARS CNRC) for assistance with the figures, and Firoz Vohra (USDA/ARS CNRC) for assistance with the CLAMS studies. MSB conducted research and wrote the paper; GL and JJK conducted research; RAW designed research, analyzed data, wrote the paper, and had primary responsibility for final content. This work was supported by grants from NIH/NIDDK (1R01DK081557) and USDA (CRIS 6250-51000-055) to RAW. The body composition and CLAMS studies were performed in the Mouse Metabolic Research Unit at the USDA/ARS Children’s Nutrition Research Center, which is supported by funds from the USDA ARS.
Footnotes
Conflict of Interest
No authors declare a conflict of interest.
Supplementary information is available at the journal’s website.
References
- 1.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9. 1 million participants. Lancet. 2011;377(9765):557–67. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atkinson RL, Pietrobelli A, Uauy R, Macdonald IA. Are we attacking the wrong targets in the fight against obesity?: the importance of intervention in women of childbearing age. Int J Obes (Lond) 2012;36(10):1259–60. doi: 10.1038/ijo.2012.149. [DOI] [PubMed] [Google Scholar]
- 3.Gluckman PD, Hanson M, Zimmet P, Forrester T. Losing the war against obesity: the need for a developmental perspective. Science translational medicine. 2011;3(93):93cm19. doi: 10.1126/scitranslmed.3002554. [DOI] [PubMed] [Google Scholar]
- 4.Cnattingius S, Villamor E, Lagerros YT, Wikstrom AK, Granath F. High birth weight and obesity--a vicious circle across generations. Int J Obes (Lond) 2012;36(10):1320–4. doi: 10.1038/ijo.2011.248. [DOI] [PubMed] [Google Scholar]
- 5.Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305(5691):1733–6. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 6.Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. Bmj. 2000;320(7240):967–71. doi: 10.1136/bmj.320.7240.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson WR. Gender-specific effects of early nutritional restriction on adult obesity risk: evidence from quasi-experimental studies. Obesity. 2012;20(12):2464–6. doi: 10.1038/oby.2012.35. [DOI] [PubMed] [Google Scholar]
- 8.Stein AD, Kahn HS, Rundle A, Zybert PA, van der Pal-de Bruin K, Lumey LH. Anthropometric measures in middle age after exposure to famine during gestation: evidence from the Dutch famine. Am J Clin Nutr. 2007;85(3):869–76. doi: 10.1093/ajcn/85.3.869. [DOI] [PubMed] [Google Scholar]
- 9.Yang Z, Zhao W, Zhang X, Mu R, Zhai Y, Kong L, et al. Impact of famine during pregnancy and infancy on health in adulthood. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2008;9 (Suppl 1):95–9. doi: 10.1111/j.1467-789X.2007.00447.x. [DOI] [PubMed] [Google Scholar]
- 10.Hult M, Tornhammar P, Ueda P, Chima C, Bonamy AK, Ozumba B, et al. Hypertension, diabetes and overweight: looming legacies of the Biafran famine. PLoS One. 2010;5(10):e13582. doi: 10.1371/journal.pone.0013582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kinzler WL, Vintzileos AM. Fetal growth restriction: a modern approach. Current opinion in obstetrics & gynecology. 2008;20(2):125–31. doi: 10.1097/GCO.0b013e3282f7320a. [DOI] [PubMed] [Google Scholar]
- 12.Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev. 2012;70(1):3–21. doi: 10.1111/j.1753-4887.2011.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones AP, Friedman MI. Obesity and adipocyte abnormalities in offspring of rats undernourished during pregnancy. Science. 1982;215(4539):1518–1519. doi: 10.1126/science.7063860. [DOI] [PubMed] [Google Scholar]
- 14.Ross MG, Beall MH. Adult sequelae of intrauterine growth restriction. Seminars in perinatology. 2008;32(3):213–8. doi: 10.1053/j.semperi.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waterland RA, Travisano M, Tahiliani KG, Rached MT, Mirza S. Methyl donor supplementation prevents transgenerational amplification of obesity. Int J Obes (Lond) 2008;32(9):1373–9. doi: 10.1038/ijo.2008.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duhl DM, Vrieling H, Miller KA, Wolff GL, Barsh GS. Neomorphic agouti mutations in obese yellow mice. Nat Genet. 1994;8(1):59–65. doi: 10.1038/ng0994-59. [DOI] [PubMed] [Google Scholar]
- 17.Wolff GL, Roberts DW, Mountjoy KG. Physiological consequences of ectopic agouti gene expression: the yellow obese mouse syndrome. Physiol Genomics. 1999;1(3):151–63. doi: 10.1152/physiolgenomics.1999.1.3.151. [DOI] [PubMed] [Google Scholar]
- 18.Wolff GL, Kodell RL, Moore SR, Cooney CA. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J. 1998;12(11):949–957. [PubMed] [Google Scholar]
- 19.Aubert R, Suquet JP, Lemonnier D. Long-term morphological and metabolic effects of early under- and over-nutrition in mice. J Nutr. 1980;110(4):649–661. doi: 10.1093/jn/110.4.649. [DOI] [PubMed] [Google Scholar]
- 20.Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23(15):5293–300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan HD, Sutherland HG, Martin DI, Whitelaw E. Epigenetic inheritance at the agouti locus in the mouse [see comments] Nat Genet. 1999;23(3):314–318. doi: 10.1038/15490. [DOI] [PubMed] [Google Scholar]
- 22.Dolinoy DC, Weidman JR, Waterland RA, Jirtle RL. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect. 2006;114(4):567–72. doi: 10.1289/ehp.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaiyala KJ, Schwartz MW. Toward a more complete (and less controversial) understanding of energy expenditure and its role in obesity pathogenesis. Diabetes. 2011;60(1):17–23. doi: 10.2337/db10-0909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 25.Rasmussen KM. Association of maternal obesity before conception with poor lactation performance. Annu Rev Nutr. 2007;27:103–21. doi: 10.1146/annurev.nutr.27.061406.093738. [DOI] [PubMed] [Google Scholar]
- 26.Brosius FC, 3rd, Alpers CE. New targets for treatment of diabetic nephropathy: what we have learned from animal models. Current opinion in nephrology and hypertension. 2013;22 (1):17–25. doi: 10.1097/MNH.0b013e32835b3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao XQ, Williams SM, Grayson BE, Glavas MM, Cowley MA, Smith MS, et al. Excess weight gain during the early postnatal period is associated with permanent reprogramming of brown adipose tissue adaptive thermogenesis. Endocrinology. 2007;148(9):4150–9. doi: 10.1210/en.2007-0373. [DOI] [PubMed] [Google Scholar]
- 28.Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab. 2000;279(1):E83–7. doi: 10.1152/ajpendo.2000.279.1.E83. [DOI] [PubMed] [Google Scholar]
- 29.Simmons RA, Templeton LJ, Gertz SJ. Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes. 2001;50(10):2279–86. doi: 10.2337/diabetes.50.10.2279. [DOI] [PubMed] [Google Scholar]
- 30.Hendrix N, Berghella V. Non-placental causes of intrauterine growth restriction. Seminars in perinatology. 2008;32(3):161–5. doi: 10.1053/j.semperi.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Wallace JM, Horgan GW, Bhattacharya S. Placental weight and efficiency in relation to maternal body mass index and the risk of pregnancy complications in women delivering singleton babies. Placenta. 2012;33(8):611–8. doi: 10.1016/j.placenta.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Gaillard R, Durmus B, Hofman A, Mackenbach JP, Steegers EA, Jaddoe VW. Risk factors and outcomes of maternal obesity and excessive weight gain during pregnancy. Obesity. 2013;21(5):1046–55. doi: 10.1002/oby.20088. [DOI] [PubMed] [Google Scholar]
- 33.Kennedy GC. The development with age of hypothalamic restraint upon the appetite of the rat. The Journal of endocrinology. 1957;16(1):9–17. doi: 10.1677/joe.0.0160009. [DOI] [PubMed] [Google Scholar]
- 34.Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976;295(7):349–353. doi: 10.1056/NEJM197608122950701. [DOI] [PubMed] [Google Scholar]
- 35.Vickers MH, Breier BH, McCarthy D, Gluckman PD. Sedentary behavior during postnatal life is determined by the prenatal environment and exacerbated by postnatal hypercaloric nutrition. American journal of physiology Regulatory, integrative and comparative physiology. 2003;285(1):R271–3. doi: 10.1152/ajpregu.00051.2003. [DOI] [PubMed] [Google Scholar]
- 36.Bellinger L, Sculley DV, Langley-Evans SC. Exposure to undernutrition in fetal life determines fat distribution, locomotor activity and food intake in ageing rats. Int J Obes (Lond) 2006;30(5):729–38. doi: 10.1038/sj.ijo.0803205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khan IY, Taylor PD, Dekou V, Seed PT, Lakasing L, Graham D, et al. Gender-linked hypertension in offspring of lard-fed pregnant rats. Hypertension. 2003;41(1):168–75. doi: 10.1161/01.hyp.0000047511.97879.fc. [DOI] [PubMed] [Google Scholar]
- 38.Li G, Kohorst JJ, Zhang W, Laritsky E, Kunde-Ramamoorthy G, Baker MS, et al. Early Postnatal Nutrition Determines Adult Physical Activity and Energy Expenditure in Female Mice. Diabetes. 2013 doi: 10.2337/db12-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bauman AE, Reis RS, Sallis JF, Wells JC, Loos RJ, Martin BW, et al. Correlates of physical activity: why are some people physically active and others not? Lancet. 2012;380(9838):258–71. doi: 10.1016/S0140-6736(12)60735-1. [DOI] [PubMed] [Google Scholar]
- 40.Garland T, Jr, Schutz H, Chappell MA, Keeney BK, Meek TH, Copes LE, et al. The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: human and rodent perspectives. The Journal of experimental biology. 2011;214(Pt 2):206–29. doi: 10.1242/jeb.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gluckman PD, Hanson MA, Buklijas T, Low FM, Beedle AS. Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nat Rev Endocrinol. 2009;5(7):401–8. doi: 10.1038/nrendo.2009.102. [DOI] [PubMed] [Google Scholar]
- 42.Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304(5667):108–10. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- 43.Lenz KM, McCarthy MM. Organized for sex - steroid hormones and the developing hypothalamus. Eur J Neurosci. 2010;32(12):2096–104. doi: 10.1111/j.1460-9568.2010.07511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levine JA. Non-exercise activity thermogenesis. The Proceedings of the Nutrition Society. 2003;62(3):667–79. doi: 10.1079/PNS2003281. [DOI] [PubMed] [Google Scholar]
- 45.Andersen LG, Angquist L, Gamborg M, Byberg L, Bengtsson C, Canoy D, et al. Birth weight in relation to leisure time physical activity in adolescence and adulthood: meta-analysis of results from 13 nordic cohorts. PLoS One. 2009;4(12):e8192. doi: 10.1371/journal.pone.0008192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Said-Mohamed R, Bernard JY, Ndzana AC, Pasquet P. Is overweight in stunted preschool children in Cameroon related to reductions in fat oxidation, resting energy expenditure and physical activity? PLoS One. 2012;7(6):e39007. doi: 10.1371/journal.pone.0039007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kohl HW, 3rd, Craig CL, Lambert EV, Inoue S, Alkandari JR, Leetongin G, et al. The pandemic of physical inactivity: global action for public health. Lancet. 2012;380(9838):294–305. doi: 10.1016/S0140-6736(12)60898-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.