Abstract
Drunk driving, a major contributor to alcohol-related mortality, has been linked to a variety of other alcohol-related (e.g., Alcohol Dependence, early age at first drink) and non-alcohol-related externalizing behaviors. In a sample of 517 same-sex twin pairs from the National Longitudinal Study of Adolescent Health, we examined three conceptualizations of the etiology of drunk driving in relation to other externalizing behaviors. A series of behavioral-genetic models found consistent evidence for drunk driving as a manifestation of genetic vulnerabilities toward a spectrum of alcohol-related and non-alcohol-related externalizing behaviors. Most notably, multidimensional scaling analyses produced a genetic “map” with drunk driving located near its center, supporting the strength of drunk driving’s genetic relations with a broad range of externalizing behaviors. In contrast, non-shared environmental associations with drunk driving were weaker and more diffuse. Drunk driving may be a manifestation of genetic vulnerabilities toward a broad externalizing spectrum.
Drunk driving stands out among the consequences of alcohol use because of its prevalence and the immediacy of its health costs for young people. In 2006, 9.5% of high school seniors reported having driven, in just the past two weeks, after consuming 5 or more alcoholic drinks (O’Malley & Johnston, 2007). Given the well-established impairing effects of alcohol on driving performance, it is unsurprising that the impact of drunk driving is severe. In 2011, 31% (9,878) of all U.S. driving fatalities involved a driver with a blood alcohol concentration above the legal limit (.08 g%; NHTSA, 2013). Among those aged 18–24, it is estimated that driving after drinking contributes to nearly half of all traffic deaths and roughly three-quarters of all alcohol-related injury deaths (Hingson, Zha, & Weitzman, 2009).
Drunk driving stands out from other alcohol-related problems theoretically as well. Relative to other consequences of drinking, drunk driving may be more conceptually and empirically similar to a variety of non-alcohol-related delinquent behaviors (Donovan, 1993; Shope & Bingham, 2002; Martin, Sher & Chung, 2011). As such, drunk driving may serve as a particularly valuable outcome to examine in relation to dimensional models of externalizing disorders. Recent research has provided compelling evidence that “externalizing phenomena are well conceived in terms of a broad but coherent group of disorders that vary continuously both within and among syndromes” (Krueger, Markon, Patrick, & Iacono, 2005, p. 546). The current study examines the genetic and environmental etiology of drunk driving and its relations with other externalizing behaviors, with the goal of improving understanding of this clinically significant and dangerous behavior and of the externalizing spectrum more generally. Specifically, we describe three alternative (but not mutually exclusive) conceptualizations of drunk driving: (1) drunk driving as a symptom of disordered alcohol use, (2) drunk driving as a developmental consequence of early drinking initiation, and (3) drunk driving as a manifestation of genetic predispositions towards externalizing behavior generally. To examine the evidence for each of these theoretical conceptualizations, we present genetically informed analyses that clarify how drunk driving relates to the larger taxonomy of externalizing psychopathology.
Logistically, drunk driving requires an individual to have consumed enough alcohol to become intoxicated. As such, drunk driving is commonly conceptualized as a product of disordered alcohol use. In the DSM-IV diagnostic scheme, recurrent drunk driving could be used as an indicator of Alcohol Abuse (i.e., under the “use in situations in which it is physically hazardous” criterion; APA, 1994, p. 199). Empirically, consistent with the conceptualization of drunk driving as a symptom of disordered alcohol use, drunk driving was more common among alcohol-dependent than among non-dependent young adults (Arria, Caldeira, Vincent, Garnier-Dykstra, & O’Grady, 2011). As several researchers have argued, however, drunk driving qualitatively differs from other criteria used to diagnose AUDs, such as acquired tolerance or loss of control over use, in that it does not necessarily reflect “dysfunction in internal mechanisms” specifically relating to alcohol (Martin et al, 2011, p. 685), and the hazardous use criterion has been identified as potentially problematic (Agrawal, Bucholz, & Lynskey, 2010; Hasin, Paykin, Endicott, & Grant, 1999; Keyes & Hasin, 2008). Despite their association across individuals, drunk driving and other indices of AUD might result, in part, from distinct etiological factors.
Additional evidence on the role of alcohol use in drunk driving has come from a developmental perspective, which has emphasized its association with early (i.e., adolescent) drinking experiences. Early initiation of alcohol use has been a consistent correlate of driving after drinking, even when controlling for alcohol use or alcohol dependence (Hingson, Heeren, Levenson, Jamanka, & Voas, 2002; Lynksey, Bucholz, Madden, & Health, 2007; Quinn & Fromme, 2012). This link has led some to raise the possibility that interventions to delay alcohol use initiation might help deter drunk driving later in life (Hingson et al., 2002). Early drinking initiation, however, is itself associated with a nexus of biological vulnerabilities and environmental background risks (Jessor & Jessor, 1975; McGue, Iacono, Legrand, & Elkins, 2001). Thus, it is possible that early drinking is associated with elevated rates of drunk driving not because early alcohol initiation is a direct causal factor but rather because early drinking is a marker of predispositions towards socially problematic behavior (Lynskey et al., 2007; Prescott & Kendler, 1999).
Finally, multivariate analyses of alcohol use, alcohol problems, and delinquent or antisocial behaviors have consistently supported a hierarchical model in which a common, highly heritable disposition toward externalizing behavior accounts for the covariation among these behaviors in adolescents and young adults (Cooper, Wood, Orcutt, & Albino, 2003; Donovan & Jessor, 1985; Krueger et al., 2002; Krueger, Markon, Patrick, Benning, & Kramer, 2007; Young et al., 2009). Drunk driving has not always been tested in models of the externalizing spectrum, but studies that include drunk driving—in addition to other risky driving behaviors—provide support for its categorization as a behavioral expression of the same externalizing tendency (Bingham, Elliot, & Shope, 2007; Caspi et al., 1997; Donovan, 1993; Shope & Bingham, 2002; Vassallo et al., 2008; Zhang, Wieczorek, Welte, Colder, & Nochajski, 2010). These findings raise the possibility that the same genetic influences responsible for other externalizing behaviors might also dispose some youth and young adults to drive while intoxicated, and indeed, several studies suggest that drunk driving is at least partially a product of genetic influences (Anum, 2007; Beaver & Barnes, 2012; Lynskey et al., 2007; Slustke et al., 1999). That is, drunk driving may be best conceptualized as a marker for biological predispositions towards externalizing behavior generally, including deviant behavior not related to substance use.
In the present investigation, we used multivariate, genetically informed data from a diverse sample of twins to clarify drunk driving’s place among the externalizing behaviors. Analyzing multivariate twin data has two key advantages. First, it allowed us to test not only the extent to which associations among variables are due to common underlying genetic influences, but also the extent to which associations are still evident within MZ twin pairs (i.e., after controlling for common genetic influences). The analysis of within-MZ twin pair associations enables a powerful test of causal developmental hypotheses (e.g., Dick, Johnson, Viken, & Rose, 2000; Johnson, Turkheimer, Gottesman, & Bouchard, 2009). Specifically, if early drinking causes drunk driving, then MZ twins who differ in their age at first drink, but who otherwise share virtually 100% of their genetic predispositions and all family-wide environmental background factors, would also differ in their drunk driving in adulthood. Thus, testing whether the association between early drinking initiation and drunk driving persists when comparing MZ twins constitutes a rigorous test of this developmental model of drunk driving. Second, because our analyses used data from a broad array of externalizing behaviors, including antisocial behaviors that do not involve substance use (e.g., truancy, theft), we could estimate the extent to which genetic predispositions towards drunk driving are exclusively shared with disordered alcohol use or overlap more generally with heritable predispositions to a variety of externalizing behaviors. We used multidimensional scaling analyses, in particular, to construct a visual display of similarities between genetic and environmental influences on drink driving and the externalizing spectrum.
Method
Participants
Data were drawn from the National Longitudinal Study of Adolescent Health (Harris, Halpern, Smolen, & Haberstick, 2006). Participants were initially recruited while in grades 7–12 (M age = 16.1 years) using a stratified, school-based sampling design. A sub-sample of participants (N=10,480 females; N=10,264 males) were randomly selected from school rosters to complete a series of In-Home interviews, beginning in 1994–1995. Three follow-up interviews have been completed in 1995–1996 (Wave II; age 11–23 years), 2001–2002 (Wave III; age 18–26 years), and 2007–2009 (Wave IV; age 24–32 years, with a small number of participants aged 33 or 34). The Add Health interviews employed strategies to reduce potential bias due to social desirability, including the use of computer-assisted self-report technology that permitted participants to respond to sensitive questions in private (Harris, 2011).
The current analyses were restricted to same-sex twin pairs in which at least one twin provided non-missing data on drunk driving, alcohol use, or externalizing behavior. Our analyses excluded opposite-sex DZ twins and other non-twin sibling pairs in order to eliminate within-sibling-pair differences in gender, age, and cohort. Individuals who denied any alcohol use through Wave III (i.e., when the early adulthood driving after drinking assessment occurred) were also excluded. This resulted in an analytic sample of 517 twin pairs (139 female-female MZ pairs; 139 male-male MZ pairs; 110 female-female DZ pairs; 129 male-male DZ pairs). Twin pair zygosity was diagnosed through 11 molecular genetic markers and responses to questionnaire items on similarity of physical appearance (Harris et al., 2006). Of the (non-abstaining) included respondents, 526 (55%) were White, 200 (21%) were African-American, 146 (15%) were Hispanic/Latino, 46 (5%) were Asian-American, 23 (2%) were Native American, and 15 (2%) were other ethnicities. At Waves I, III, and IV, included respondents were an average of 16.20 (n = 952, SD = 1.63, range = 12 – 20), 22.00 (n = 768, SD = 1.63, range = 18 – 26), and 29.07 (n = 795, SD = 1.62, range = 25 – 33) years old, respectively.
Brownstein and colleagues (n.d.) have examined the potential for non-response bias at Wave IV of Add Health in detail. Of all those eligible for Wave IV, 80% participated, whereas 8% were not located or contacted, 3% were not able to participate, 9% refused to participate, and less than 1% did not respond for other reasons. Response rates differed across several demographic characteristics, including gender, race/ethnicity, socioeconomic status, and immigration status. However, Brownstein and colleagues (n.d.) found that the effect of non-response on bias in prevalence rates for most health and risk behaviors, including alcohol use and delinquency, was less than 1%. They concluded that “differences in measurements between non-respondents and respondents are most likely due to random variation and so do not reflect appreciable non-response bias” (p. 6). A similar lack of substantive non-response bias has been reported for Waves I–III (Chantala, Kalsbeek, & Andraca, 2005).
Measures
Drunk driving
We constructed a lifetime drunk driving measure from responses at Waves I, II, and III (i.e., through M age = 22). At Wave I, participants who endorsed lifetime alcohol consumption reported whether or not they had “ever driven while drunk.” Alcohol abstainers were coded as having never driven while drunk. At Waves II and III, participants who endorsed past-year alcohol use were asked whether or not they had “driven while drunk” since the Wave I assessment (defined at Wave II as the month of the last interview and at Wave III as June 1995). Abstainers were again coded as having never driven drunk. Because some participants could have driven under the influence of alcohol in the period between Wave I and the Wave II or III assessments but not consumed alcohol in the year immediately preceding Wave II or III, drunk driving for participants who endorsed some prior but not past-year alcohol use at either wave and had not endorsed drunk driving at another wave was coded as missing. This coding scheme resulted in the loss of lifetime drunk driving data for 40 participants (4% of all included individuals). Among the 517 included twin pairs, lifetime drunk driving data were available for 71% (n = 730) of individuals. Of these non-abstaining individuals, 32% (n = 236) reported that they had driven while drunk.
Alcohol Dependence symptoms
Participants who met thresholds for frequency (at least once per week) and quantity (more than two drinks per usual occasion for women, more than three drinks for men) of present or past alcohol consumption were assessed for the lifetime presence of DSM-IV Alcohol Use Disorder (APA, 1994) symptoms at the Wave IV interview (i.e., through M age = 29). Given concerns about the overlap between drunk driving and Alcohol Abuse diagnoses (e.g., Keyes & Hasin, 2008), we limited our analyses to the seven Alcohol Dependence (AD) symptoms (e.g., acquired tolerance, withdrawal symptoms, desire or inability to reduce consumption). Participants who met neither the present nor past consumption thresholds were coded as having zero AD symptoms. On average, participants reported 0.80 symptoms, SD = 1.38.
Age at first drink
We drew upon data from Waves I, II, and IV to create a variable indicating age of onset of alcohol use. At Wave I, participants reported the age, in years, at which they first “had a drink of beer, wine, or liquor when [they] were not with [their] parents or other adults in [their] family.” Then, at Wave II, participants reported the month and year in which they first had a drink when not with parents or other adult family members following Wave I, from which we calculated age at first drink for participants who had not initiated alcohol use by Wave I. Finally, at Wave IV, non-lifetime-abstaining participants were asked to report the age at which they “first had an alcoholic drink.” We used the Wave IV reports for participants without age at first drink data from Wave I or II (i.e., those who did not provide data or who had not yet initiated alcohol use). Participants began drinking at approximately age 15 on average, M = 15.49 years, SD = 3.31.
Alcohol consumption
We used adolescent and adult measures of past-year alcohol use and binge drinking frequency taken from the Wave I (M age = 16) and III (M age = 22) assessments, respectively. At both waves, non-abstaining participants were asked how frequently they had consumed alcohol during the past 12 months. Abstainers were coded as never having consumed alcohol. Participants who endorsed past-year alcohol consumption then reported how frequently they drank “five or more drinks in a row.” Past-year abstainers were again coded as having never binge drank. The alcohol use and binge drinking measures were similar across waves, with the exception that Wave I responses were scored on a 7-point scale where 1 = every day or almost every day and 7 = never, whereas Wave III responses were scored on a 7-point scale where 0 = none and 6 = every day or almost every day. To maintain consistency across waves, we recoded all variables to a scale where 1 = never/none and 7 = every day or almost every day. In adolescence, participants reported using alcohol (M = 2.19, SD = 1.55) and binge drinking (M = 1.69, SD = 1.36) approximately 1 or 2 times in the past year on average. In early adulthood, mean reported alcohol use increased to more than once per month (M = 3.42, SD = 1.66) and binge drinking increased to more than 1 or 2 times in the past year (M = 2.33, SD = 1.56), respectively.
Rule-breaking delinquency
We used adolescent and adult measures of rule-breaking delinquency also taken from the Wave I and III assessments. At Wave I, we used 11 items indicating past-year engagement in a variety of nonviolent delinquent behavior, including lying to parents, stealing something worth more than $50, and selling marijuana or other drugs (Harden & Mendle, 2012). Responses were scored on a 4-point scale where 0 = never and 3 = 5 or more times. The delinquency section of the Wave III interview included eight nonviolent items. Of the eight items, five were identical to those assessed at Wave I (e.g., damaging property, stealing something worth more than $50), and three were new items reflecting adult rule-breaking behavior (e.g., buying, selling, or holding stolen property, deliberately writing a bad check). We summed responses across items to create adolescent (M = 3.35, SD = 4.11) and adult (M = 0.51, SD = 1.18) rule-breaking delinquency scores.
Analytic Approach
Analyses were conducted in four steps. Unless otherwise noted, all models were fit in Mplus version 5 using full-information maximum likelihood (FIML) estimation to account for missing data (Muthén & Muthén, 1998–2007). We controlled for gender by regressing observed variables on twin-pair gender in the Mplus analyses.
Step #1: Univariate twin models
First, for descriptive purposes, we estimated univariate twin models for each of the nine phenotypes (Neale & Maes, 2004). The classical twin model decomposes total variation in an observed phenotype into three latent factors: additive genetic variance (A), shared environmental variance (C, due to environmental factors that make twins similar to one another), and non-shared environmental variance (E, due to environmental factors that make twins different from one another, plus measurement error). An individual twin’s score for phenotype Y can be expressed as follows:
In this equation, μrepresents the mean of phenotype Y, and coefficients a, c, and e represent main effects or paths weights of the A, C, and E factors, respectively, on the phenotype. Terms that can vary across twins within pairs are denoted with the subscript t. We estimated paths a, c, and e, and the ACE factors were standardized to a mean of 0 and a standard deviation of 1 for model identification. The cross-twin correlation between the A factors was fixed to 1.0 in MZ pairs and 0.5 in DZ pairs, consistent with genetic theory.1 By definition, the correlation between C factors was fixed to 1.0—and the correlation between E factors fixed to 0—in all pairs. Squaring a and dividing it by the sum of a2, c2, and e2 generates the familiar heritability coefficient (h2; the proportion of total variation due to additive genetic differences). When the MZ twin correlation exceeded twice the DZ correlation, we also considered models that omitted shared environmental variance and estimated variation due to dominant genetic influences (D). In the ADE model, the D factors are correlated 1.0 in MZ twins and 0.25 in DZ twins.
Step #2: Estimation of genetic and environmental correlation matrices
Next, we entered all nine phenotypes into a single model, decomposed variation in each phenotype into A, C, and E components, and estimated the genetic and environmental correlations among latent ACE components (Mplus script available upon request to the first author). Following Hicks, Krueger, and colleagues (Hicks, Krueger, Iacono, McGue, & Patrick, 2004; Krueger et al., 2002; Krueger et al., 2007), we included adolescent and early adult outcomes in a single model to capture genetic and environmental variances and covariances across a developmentally broad range of phenotypes. The genetic correlation matrix represents the correlations among the A factors of the nine phenotypes. For example, to what extent are the genetic influences on rule-breaking correlated with the genetic influences on drunk driving? Similarly, the non-shared environmental correlation matrix represents the correlations among the E factors of the nine phenotypes. To facilitate convergence, C variances that were not significantly different from zero in the univariate models were fixed to zero in the multivariate model. Because C variance in drunk driving did not significantly differ from zero, we do not present results from the shared environmental correlation matrix here. Finally, we multiplied all correlations with age at first drink by -1 to facilitate interpretation (i.e., so that higher values indicated greater correspondence with genetic or environmental influences on early drinking).
Step #3: Multidimensional scaling analysis
We next conducted multidimensional scaling analyses (MDS) on the genetic and environmental correlation matrices. MDS is a technique for examining the structure of associations among a set of variables and can produce solutions that are “visual in nature and intuitively appealing” (Tucker-Drob & Salthouse, 2009, p. 278). A MDS solution represents each variable as a point in Euclidean space, and the magnitude of the association between each pair-wise combination of variables is represented as the distance between them (Guttman, 1954; Snow, Kyollen, & Marshalek, 1984; Turkheimer, Ford, & Oltmanns, 2008). Thus, variables that are more highly related are represented as closer together on the “map” produced by an MDS solution. In “non-metric” MDS, which is the type of analysis used in the current paper, the distances between points on the map correspond with the observed “dissimilarities” between items monotonically but not linearly. That is, the distances between points on the map correspond only with the rank ordering of the pairwise correlations (with more positively correlated pairs closer together on the map).
Thus, like factor analysis, MDS involves (1) estimating the similarities among the variables, (2) determining a reduced number of dimensions (k) that can account for observed similarities, (3) mapping the variables to the k-dimensional space, and (4) characterizing the resulting map (Turkheimer et al., 2008). With regards to this final step, factor analytic results are most often characterized by rotating to simple structure, i.e., selecting a coordinate system that maximizes the factor loading of each item onto one dimension and minimizes loadings on all other dimensions. (Rotation to simple structure is simply a convenience, and other rotation procedures are available; Jennrich & Bentler, 2011; Sass & Schmitt, 2010). As illustrated by Maraun’s (1997) example of the color wheel, however, rotated coordinate systems may not always capture interesting features of the “configural structure” of multivariate data, particularly data that do not conform to simple structure (Turkheimer et al., 2008, p. 1614). Put more generally, our understanding of how clinically relevant behaviors relate to one another can be enriched by examining their interrelations using MDS, which results in a descriptive visual representation of their multivariate structure.
One pattern that can be observed in MDS solutions of psychological variables is a radex, a circular disk in which points can be characterized both by their distance from their center of circle and by the angle along the circle at which the points lie (Guttman, 1954). The center of the radex contains variables that have the strongest average associations with all of the other variables. The radex is a two-dimensional solution, but higher-order solutions are sometimes necessary to capture the pattern of dissimilarity among the variables. We transformed the phenotypic, genetic, and non-shared environmental correlation matrices to “dissimilarity” matrices [dissimilarity = (1−r)/2, producing values that range between 0 and 1]. The dissimilarity matrices were submitted to MDS using the “sammon” function in the software program R (R Core Development Team, 2009; Venables and Ripley, 2002). Lack of fit (“stress”) in an MDS solution can be evaluated by comparing the original “dissimilarity” matrix with the distances from the MDS map. Stress values less than 0.05 indicate that an MDS solution provides a good representation of the data (Borg & Groenen, 2005). MDS solutions with stress < .05 were selected.
Step #4: Bivariate Cholesky decompositions
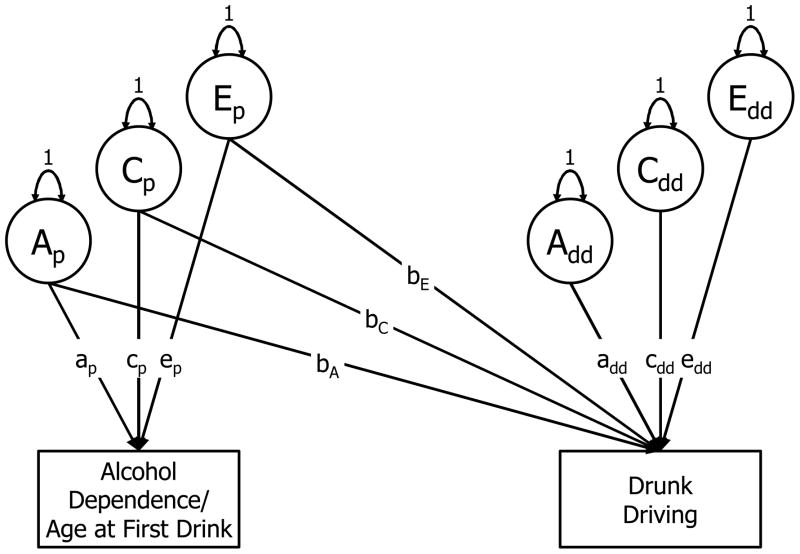
Finally, we used bivariate Cholesky decompositions to “zoom in” on portions of the genetic and environmental maps estimated in Step #3, in order to (a) examine more closely drunk driving’s associations with AD symptoms and age at first drink, and (b) evaluate the statistical significance of these associations. As shown in Figure 1, this approach apportions variation in each of the observed phenotypes into ACE components and additionally decomposes the association between the phenotypes into genetic and environmental influences. It can be expressed using the following two simultaneous equations for drunk driving (Ydd,t) and a putative precursor phenotype (Yp,t; i.e., AD symptoms or age at first drink):
Figure 1.
Path diagram of bivariate Cholesky decomposition of association between alcohol dependence (or age at first drink) and drunk driving. For illustrative purposes, model is shown for one twin only.
In these equations, terms that can vary across twins within pairs are denoted with the subscript t. The μ terms represent means of continuous variables and thresholds of categorical variables (Prescott, 2004). All latent ACE factors are again standardized to have a mean of 0 and a standard deviation of 1, and the paths from the ACE factors to the phenotypes are estimated. Whereas the precursor phenotype (Yp,t) is regressed on its ACE factors only, drunk driving (Ydd,t) is regressed on the ACE factors for both drunk driving and the precursor phenotype. As a result, factors Ap,t, Cp,t, and Ep,t represent genetic, shared environmental, and non-shared environmental variance in the precursor phenotype and its overlap with variance in drunk driving, whereas factors Add,t, Cdd,t, and Edd,t represent variance unique to drunk driving.
Notably, the cross-trait coefficients (bA, bC, and bE) reflect the degree to which genetic and environmental influences explain covariation between the two outcomes. In particular, the regression on E is equivalent to a test of the within-twin-pair association: Do MZ twins who differ in a predictor phenotype also differ in their drunk driving? This path can provides a strong test of developmental hypotheses free of confounding by genetic and shared environmental factors that are common to twins raised in the same home. For example, a significant E cross-path for age at first drink would be consistent with causal hypotheses implicating early alcohol use as a precipitant of drunk driving.
Results
Step #1: Genetic and Environmental Influences on Drunk Driving and Other Externalizing Behaviors
Twin pair correlations and univariate twin parameter estimates are summarized in Table 1. About half of the variance in drunk driving (52%) was due to additive genes; 32% was due to non-shared environmental influences, and the remainder (16%) was due to shared environmental influences, which could be fixed to zero without significant loss of model fit, Satorra-Bentler scaled Δ χ2 (1) = 0.29, p = .59. The overall fit of an AE model was good, χ2 (4) = 5.24, p = .26, CFI = .99, RMSEA = .04. For the remaining externalizing behaviors, heritability point estimates ranged from 0% (for adolescent rule-breaking) to 48% (for adolescent binge drinking) and significantly differed from 0% for all behaviors but adolescent rule-breaking.2 In contrast, the contribution of the shared environment ranged from 0% (for AD symptoms and adult binge drinking and rule-breaking) to 40% (for adolescent rule-breaking) and significantly differed from 0% for adolescent alcohol use and rule-breaking only. Heritability estimates are expected to diverge somewhat from the values obtained in previous studies of the Add Health data because participants who denied ever drinking alcohol by young adulthood (Wave III) were excluded.
Table 1.
Twin-Pair Correlations and Univariate Twin Model Parameters
| Variable | Twin Correlations
|
Twin Model Parametersa
|
|||
|---|---|---|---|---|---|
| rMZ | rDZ | h2 | c2 | e2 | |
| 1. Drunk Driving | .68 | .42 | .52 | .16b | .32 |
| 2. Alcohol Dependence Symptoms | .36 | .11 | .36 | .00b | .64 |
| 3. (Early) Age at First Drink | .57 | .32 | .43 | .12b | .45 |
| Adolescent | |||||
| 4. Alcohol Use | .55 | .35 | .33 | .20 | .47 |
| 5. Binge Drinking | .52 | .28 | .48 | .04b | .48 |
| 6. Rule-Breaking Delinquency | .40 | .40 | .00b | .40 | .60 |
| Adult | |||||
| 7. Alcohol Use | .37 | .26 | .27 | .11b | .62 |
| 8. Binge Drinking | .46 | .20 | .46 | .00b | .54 |
| 9. Rule-Breaking Delinquency | .30 | .10 | .26 | .00b | .74 |
Note. Twin-pair correlations and univariate model parameters are controlled for pair gender.
Proportions of variance attributable to additive genetics (h2), the shared environment (c2), and the non-shared environment (e2).
Twin model parameter did not significantly differ from zero, p < .05.
Steps #2 and #3: Multidimensional Scaling Maps of the Externalizing Spectrum
Table 2 presents the phenotypic correlation matrix (estimated in R using one twin per pair), and Table 3 presents the full genetic and non-shared environmental correlation matrices generated by the multivariate ACE model, χ2 (265) = 445.25, p < .001, CFI = .94, RMSEA = .05.
Table 2.
Phenotypic Correlation Matrix
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| 1. Drunk Driving | -- | |||||||
| 2. Alcohol Dependence Symptoms | .32* | -- | ||||||
| 3. (Early) Age at First Drink | .17* | .19* | -- | |||||
| Adolescent | ||||||||
| 4. Alcohol Use | .34* | .19* | .50* | -- | ||||
| 5. Binge Drinking | .38* | .16* | .37* | .77* | -- | |||
| 6. Rule-Breaking Delinquency | .25* | .23* | .35* | .33* | .30* | -- | ||
| Adult | ||||||||
| 7. Alcohol Use | .32* | .28* | .08 | .15* | .08 | .06 | -- | |
| 8. Binge Drinking | .32* | .30* | .03 | .15* | .12* | .13* | .72* | -- |
| 9. Rule-Breaking Delinquency | .15* | .07 | .03 | .02 | −.04 | .15* | .06 | .10* |
Note. Correlations estimated in one twin per twin-pair.
p < .05.
Table 3.
Estimated Genetic and Non-Shared Environmental Correlation Matrices
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| 1. Drunk Driving | -- | .22 | −.15 | .01 | .10 | .11 | .34 | .40 | .28 |
| 2. Alcohol Dependence Symptoms | .53 | -- | .15 | .03 | .07 | .27 | .04 | .13 | .11 |
| 3. (Early) Age at First Drink | .43 | .26 | -- | −.03 | .02 | .32 | −.03 | −.01 | .18 |
| Adolescent | |||||||||
| 4. Alcohol Use | .53 | .21 | .74 | -- | .58 | .19 | .05 | .13 | .07 |
| 5. Binge Drinking | .45 | .12 | .53 | .90 | -- | .28 | .11 | .14 | −.07 |
| 6. Rule-Breaking Delinquency | .53 | −.04 | .48 | .36 | .41 | -- | .07 | .09 | .06 |
| Adult | |||||||||
| 7. Alcohol Use | .56 | .82 | .24 | .20 | .02 | .13 | -- | .56 | .05 |
| 8. Binge Drinking | .44 | .78 | .15 | .12 | .06 | .22 | .93 | -- | .07 |
| 9. Rule-Breaking Delinquency | .15 | .16 | .11 | −.01 | .01 | .60 | .15 | .24 | -- |
Note. Genetic correlations are below the diagonal. Non-shared environmental correlations are above the diagonal. Values were obtained from Mplus technical output (TECH4), which does not provide information regarding statistical significance of correlations.
For each of the phenotypic, genetic, and non-shared environmental matrices, we estimated a series of MDS solutions ranging from one to five dimensions. Investigation of the stress of each solution indicated that two-dimensional solutions provided good fit to the phenotypic (stress = 0.04) and genetic (stress = 0.01) dissimilarity matrices. For the non-shared environmental matrix, the stress of the two-dimensional solution was borderline (stress = 0.07), whereas the three-dimensional solution satisfied the .05 cut-off (stress = 0.01). We therefore present both the two- and three-dimensional non-shared environmental solutions.
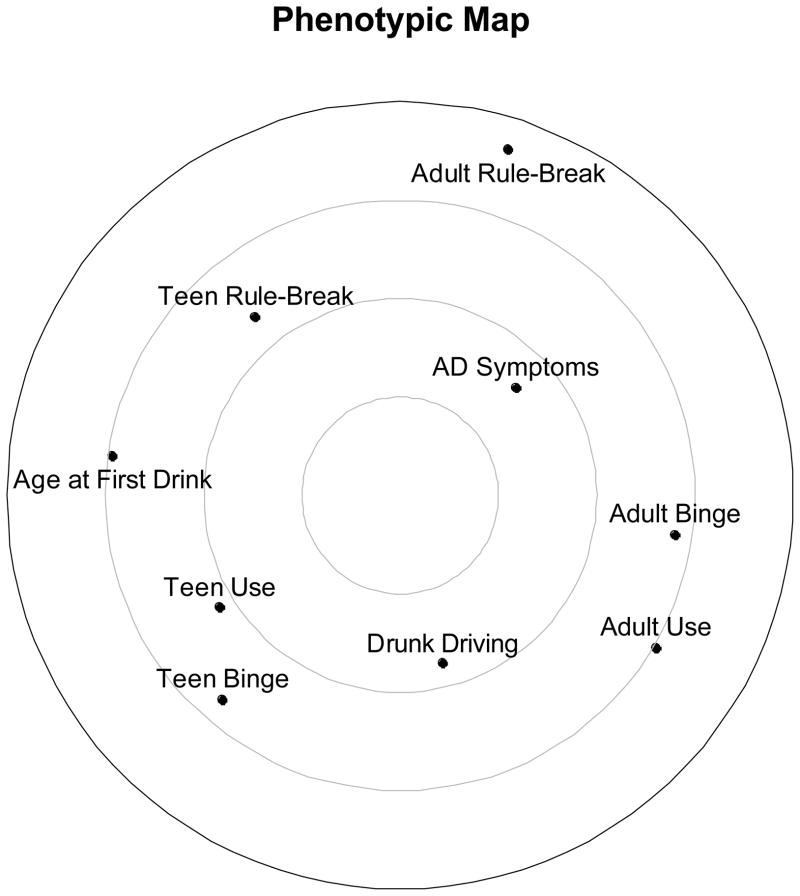
For a preliminary examination, we plotted the phenotypic MDS solution—the phenotypic “map”—in Figure 2. The distance between two points on the radex corresponds, inversely, to the strength of their association, with clusters (e.g., adolescent alcohol use and binge drinking) representing closely associated phenotypes. The phenotypic MDS solution indicated a relatively diffuse pattern of associations with drunk driving. The map lacked a “core” phenotype or phenotypes, which would have indicated behaviors that were closely linked with the range of other externalizing behaviors. Among the behaviors included in this analysis, drunk driving was located most proximally to adolescent and adult alcohol use and binge drinking and was more distal to rule-breaking behaviors. This pattern reflected the finding that phenotypic associations with drunk driving were strongest among indices of alcohol consumption.
Figure 2.
Two-dimensional map of phenotypic associations among drunk driving and other externalizing behaviors.
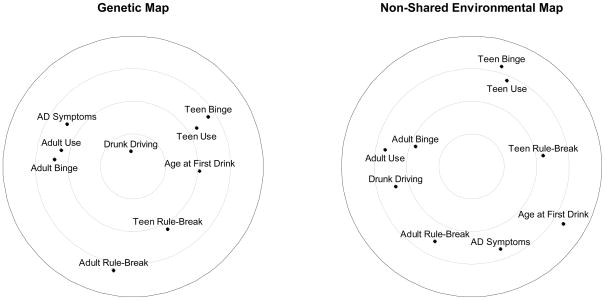
Genetic MDS solution
The MDS solution for the genetic dissimilarity matrix—the genetic “map”—is shown on the left side of Figure 3. Clusters (e.g., adult alcohol use, binge drinking, and AD symptoms) indicate phenotypes that closely share genetic influences. Drunk driving was located at approximately the center of the radex, indicating that it had the strongest average genetic association with all other externalizing behaviors, average rg = .45. Further, whereas genetic variance in adult alcohol-related behaviors, adolescent alcohol-related behaviors, and rule-breaking delinquency formed three fairly distinct clusters at various positions around the radex, drunk driving was approximately equidistant from the three clusters. This positioning suggests that genetic influences on drunk driving are consistently shared with multiple other alcohol-related phenotypes across adolescence and early adulthood. Further, although adolescent rule-breaking delinquency appeared relatively distinct from most alcohol-related outcomes, it remained more proximal to drunk driving. Thus, drunk driving may reflect genetic risk not only for alcohol use and its consequences but also for non-substance-related adolescent delinquent behavior. In contrast, genetic influences on drunk driving were more distinct from genetic influences on adult rule-breaking.
Figure 3.
Two-dimensional maps of genetic and non-shared environmental associations among drunk driving and other externalizing behaviors.
Non-shared environmental MDS solutions
The two-dimensional MDS solution for the non-shared environmental dissimilarity matrix—the non-shared environment “map”—is shown on the right side of Figure 3. Relative to the genetic map, the two-dimensional solution suggested a more diffuse pattern of associations among non-shared environmental influences on externalizing behaviors. Non-shared environmental variance in drunk driving was more closely shared with other adult behaviors, particularly alcohol use and binge drinking, but was quite distinct from adolescent externalizing behaviors and was positioned away from the center of the radex.
The stress results suggested that a three-dimensional solution might provide a stronger fit to the non-shared environmental matrix. Two horizontally rotated images of the three-dimensional non-shared environmental solution are displayed in Figure 4, and a rotating image of the solution is available as supplemental material online. The diffuse pattern of non-shared environmental associations illustrated by the two-dimensional solution is further apparent in the three-dimensional map. Notably, the adolescent and adult behaviors were again mapped onto opposite poles, but the non-shared environmental variances in adult behaviors were further distinguished across the second and third dimensions, with drunk driving appearing more distal to the other adult variables.
Figure 4.
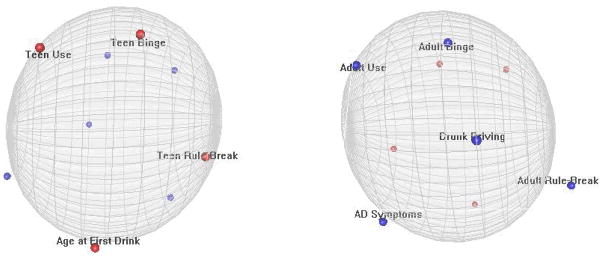
Two horizontally rotated images of the three-dimensional map of non-shared environmental associations among drunk driving and other externalizing behaviors.
In sum, the genetic MDS map supported the central role of common, genetically influenced externalizing predispositions in the links between drunk driving and other alcohol-related and delinquent behaviors. In contrast, there was no “core” apparent in the non-shared environmental map, indicating little commonality in the unique environmental experiences that affect each phenotype.
Step #4a: Association between Alcohol Dependence Symptoms and Drunk Driving
The MDS results provided support for the conceptualization of drunk driving as one of many manifestations of genetic predispositions toward broadly defined externalizing behavior. Another perspective is that drunk driving is a symptom of one particular substance-related behavior, disordered alcohol use. All previous analyses suggested that the relation between AD symptoms and drunk driving is driven by common underlying genetic factors. Notably, the genetic correlation (r = .53) was more than double the non-shared environmental correlation (r = .22), and the non-shared environmental MDS solutions put AD symptoms and drunk driving on opposite sides of the “map.” To provide a final confirmatory test of this conclusion, we fit a bivariate Cholesky decomposition of the association between adult AD symptoms and drunk driving (N = 270 MZ pairs and 231 DZ pairs because of missingness on AD symptoms and drunk driving).
We began by estimating a full decomposition as described in Figure 1, which fit the data well, χ2 (13) = 16.43, p = .23, CFI = .98, RMSEA = .03. However, given the lack of significant shared environmental variation in AD symptoms and drunk driving, all C paths could be constrained to zero (i.e., resulting in an AE model) without significant decrement in model fit, Satorra-Bentler scaled Δχ2 (3) = 0.43, p = .93. Parameter estimates from the AE model are summarized in the left-hand column of Table 4.
Table 4.
Unstandardized Parameter Estimates from Bivariate Cholesky Decompositions
| Parameter | Model
|
|
|---|---|---|
| Alcohol Dependence | Age at First Drink | |
| Alcohol Dependence/Age at First Drink | ||
| Genetic path (ap) | 0.77* (.08) | 2.58* (.13) |
| Shared environmental path (cp) | [0.00] | [0.00] |
| Non-shared environmental path (ep) | 1.15* (.05) | 2.03* (.13) |
| Regression Coefficients | ||
| Genetic path (bA) | 0.46* (.11) | −0.35* (.10) |
| Shared environmental path (bC) | [0.00] | [0.00] |
| Non-shared environmental path (bE) | 0.11 (.06) | 0.08 (.09) |
| Residual Variance in Drunk Driving | ||
| Genetic path (add) | 0.69* (.11) | 0.75* (.11) |
| Shared environmental path (cdd) | [0.00] | [0.00] |
| Non-shared environmental path (edd) | 0.55* (.09) | 0.55* (.09) |
|
| ||
| Model Fit Indices | ||
| χ2 (df) | 16.64 (16) | 17.57 (16) |
| CFI | 1.00 | 0.99 |
| RMSEA | .01 | .02 |
Note. Values are unstandardized parameter (standard error) unless otherwise noted. Bracketed values were constrained to equal zero without significant decrement in model fit, ps > .93. A Bonferroni correction for the total number of statistical tests across both final models (α= .05/14 = .0036) did not alter the statistical significance of the parameters and fit indices reported here.
p < .05.
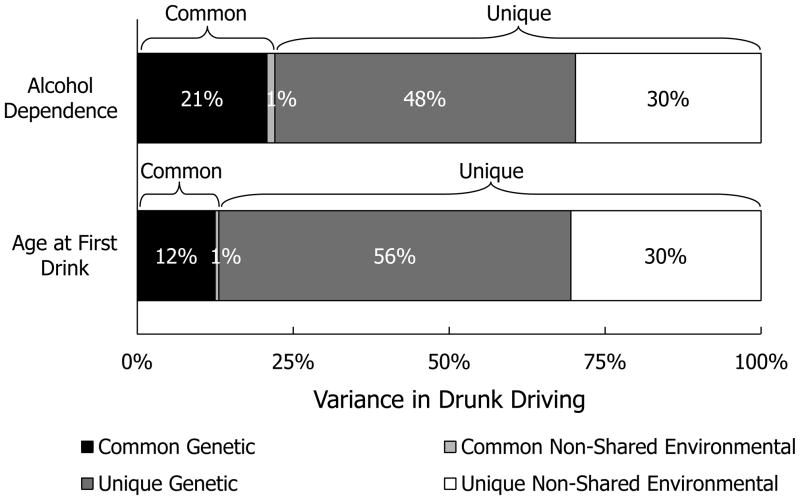
Overall, 22% of the variation in drunk driving was shared with variation in AD symptoms. The cross-paths from the A and E components of AD symptoms to drunk driving (parameters bA and bE) are of particular interest. Notably, only the A regression path was significantly different from zero. Individuals with more AD symptoms were also more likely to drive drunk, but this association was mostly attributable to the two phenotypes’ sharing common underlying genetic influences. In contrast, the E regression was not significantly different from zero: MZ twins who reported more alcohol dependence symptoms than their co-twins were not more likely than their co-twins to drive drunk. Despite the genetically driven overlap between AD symptoms and drunk driving, however, most (70%) of the genetic influences on drunk driving were unique of AD symptoms. See Figure 5 for a plot of the genetic and non-shared environmental variance in drunk driving unique of and shared with AD symptoms. Thus, whereas this analytic step again provided support for the role of common genetic influences in explaining the association between AD symptoms and drunk driving, it also suggested that drunk driving may not be purely a product of disordered alcohol use.
Figure 5.
Genetic and non-shared environmental variance in drunk driving common to—and unique of—alcohol dependence symptoms and age at first drink.
Step #4b: Association between Age at First Drink and Drunk Driving
Our previous analyses also failed to provide support for the hypothesis that early drinking initiation has an environmentally mediated link with drunk driving: The non-shared environmental correlation was small and in the direction opposite from what would be predicted (r =−.15), and the non-shared environmental MDS solutions put age at first drinking and drunk driving on opposite sides of the map. In our final analytic step, we decomposed the association between age at first drink and drunk driving to provide a direct, confirmatory test of this conclusion (N = 266 MZ pairs and 229 DZ pairs).
We again began with a full Cholesky decomposition, χ2 (13) = 17.61, p = .17, CFI = .98, RMSEA = .04, but ultimately selected an AE model, in which all (non-significant) C paths were constrained to zero without loss of fit, Satorra-Bentler scaled Δχ2 (3) = 0.36, p = .95. These results are summarized in the right-hand column of Table 4 and in Figure 5. Again only the A regression cross-path was significantly different from zero, indicating that common underlying genetic influences drove the association between (earlier) age at first drink and drunk driving. Net of this genetically driven similarity, in contrast, the E regression was not significantly different from zero. That is, contrary to the predictions of a causal developmental hypothesis linking early alcohol exposure to drunk driving, MZ twins who took their first drinks at different ages did not significantly differ in their likelihood of drunk driving. Age at first drink explained 13% of the variation in drunk driving; of this shared variance, 96% was attributable to common genetic influences. Consistent with the AD symptoms Cholesky decomposition, however, a substantial majority (82%) of the genetic influences on drunk driving were unique of genetic influences on age at first drink.
Sensitivity Analyses
Several components of our analyses may have been sensitive to differing modeling approaches, specifications or restrictions. Consequently, we conducted a series of analyses to test the robustness of our findings across alternative approaches.
Exploratory factor analyses
As an alternative to MDS, we conducted an exploratory factor analysis (EFA) of the genetic and non-shared environmental correlation matrices using maximum likelihood estimation in Mplus (N = 517, or the number of twin-pairs). Because we anticipated a complex factor structure, with drunk driving related to multiple factors, we used CF-Facparsim rotation, which minimizes factor complexity but not variable complexity (Schmitt, 2011). One variable (adult alcohol use) was omitted to facilitate convergence. EFA of the genetic correlation matrix indicated a three-factor solution: (1) adult alcohol outcomes (largest loadings for adult AD symptoms and adult binge drinking); (2) adolescent alcohol outcomes (largest loadings for age at first drink, adolescent binge drinking, and adolescent alcohol use), and (3) non-alcohol-related forms of deviance (largest loadings for adolescent rule-breaking and adult rule-breaking). Notably, drunk driving significantly loaded on all three factors, with cross-loadings ranging from .23 to .47). Fit of the three-factor solution for the genetic correlation matrix was adequate, χ2 (7) = 43.22, p < .001, CFI = .99, TLI = .97, SRMR = .03.
Analysis of the non-shared environmental matrix indicated a four-factor solution. Rather than showing cross-loadings with all factors, as seen with the genetic EFA, non-shared environmental influences on drunk driving had a significant loading on only one factor, shared with adult binge drinking. Fit of the four-factor solution for the non-shared environmental matrix was good, χ2 (2) = 2.10, p = .35, CFI = 1.00, TLI = 1.00, SRMR = .01). Complete details regarding EFA results are available from the first author upon request.
Age range restriction
The Wave I (“adolescent”) sample included participants with an average age of 16.20 years but with an age range of 12 – 20 years. In order to assess the contribution of age heterogeneity at the initial assessment to our results, we estimated the phenotypic correlations among the key study variables for a sub-sample restricted to participants who were ages 14–18 at Wave I. We again selected one twin per pair for phenotypic analyses. The pattern of correlations was highly similar to that obtained for the full sample. The correlations between adolescent behaviors and the remaining study variables in the restricted sample differed from the correlations obtained in the full sample by −.04 to .02 (mean difference = −.01) for adolescent alcohol use frequency, by −.04 to .02 for adolescent binge drinking (mean difference =−.01), and −.02 to .03 for adolescent rule-breaking (mean difference = .004).
Similarly, we re-estimated the bivariate Cholesky models with a restricted sample of participants who were age 21 or older at Wave III. The patterns of results were unchanged. For the model of drunk driving and AD symptoms (N = 226 MZ pairs and 182 DZ pairs), the genetic cross-path was significant (unstandardized bA = 0.52, SE = .13, p < .001), whereas the non-shared environmental cross-path was not (bE = 0.12, SE = .07, p = .07). Similarly, for the model of drunk driving and age at first drink (N = 223 MZ pairs and 180 DZ pairs), the genetic cross-path was significant (bA = −0.33, SE = .12, p < .01), whereas the non-shared environmental cross-path was not (bE = −.001, SE = .13, p = .99).
Early ages at first drink
Of the participants who provided data on age at first drink, less than 4% (n = 28) reported that they first drank alcohol in early childhood (before age 10 years). As a post-hoc sensitivity analysis, we estimate the bivariate Cholesky model of age at first drink and drunk driving using only participants who reported an age at first drink of 10 years old or greater (N = 252 MZ pairs and 218 DZ pairs). The pattern of results was unchanged: The association between age at first drink and drunk driving could be entirely attributed to common genetic influences (unstandardized bA = −0.41; SE = .10, p < .001), whereas the non-shared environmental cross-path was not significantly different from zero (bE = .09, SE = .11, p = .43).
Discussion
Drunk driving is a major contributor to the public health costs of substance use among young people in the U.S. Although historical trends indicate that alcohol-related traffic fatalities are becoming incrementally less prevalent (NHTSA, 2013), it is clear that more effective means of prevention are still needed. Toward that end, several etiological conceptualizations have been proposed, each with their own implications for intervention. Drunk driving has varyingly been proposed as 1) a symptom or indicator of AUD; 2) a developmental consequence of early drinking initiation; and 3) one of a range of manifestations of genetic predispositions to externalizing behavior broadly defined.
Of these conceptualizations, which are not necessarily mutually exclusive, the results of this study most clearly support the third. They suggest that drunk driving be recognized not simply as a consequence of alcohol use but also as a component of a genetically influenced externalizing spectrum of behavior. Previous research has found significant associations between drunk driving and a range of alcohol-related and non-alcohol-related delinquent behavior (e.g., Donovan, 1993; Shope & Bingham, 2002; Zhang et al., 2010). The current study replicates and extends these findings. Genetic influences on drunk driving were moderately-to-strongly correlated with genetic influences on adolescent and adult alcohol-related behaviors. Although genetic influences accounted for little overall variation in adolescent non-substance-use-related delinquency, these influences were also strongly associated with genetic influences on drunk driving. In contrast, the greater genetic variation in adult rule-breaking was more modestly associated with genetic variation in drunk driving. Our MDS maps located genetic variance in drunk driving centrally on the radex and proximally to genetic variance in the other externalizing behaviors. In contrast, non-shared environmental variance in drunk driving was moderately associated with other adult alcohol consumption indices but only weakly associated with other externalizing behaviors. On both two- and three-dimensional non-shared environmental MDS maps, drunk driving was located proximally only to the other adult alcohol consumption indices. In sum, drunk driving may be one expression among many of genetically influenced predispositions toward externalizing behavior.
As indicated by its central position on the genetic radex, drunk driving may be a strong indicator of the common genetic influences linking externalizing behaviors. As such, it may be valuable to include drunk driving in measurement models of the externalizing behavior spectrum when examining potential endophenotypes (e.g., response inhibition; Young et al., 2009). In addition, drunk driving may be a useful phenotype to include in genome-wide association scans for genetic markers of the adolescent externalizing behavior spectrum. Moreover, it is notable that drunk driving was located less centrally on the phenotypic MDS map relative to its position on the genetic radex. This difference underscores how quantitative genetic methods, which provided evidence of drunk driving’s particular value as a marker of genetic predispositions for externalizing behavior, can inform molecular genetic studies. For example, one recent field study of bar patrons found that homozygous carriers of the short variant of the serotonin transporter gene promoter region polymorphism (5-HTTLPR) who had been drinking were more willing to drive than were individuals homozygous for the long variant who had been drinking (Thombs et al., 2011). Whether this polymorphism or others serve as common risk factors for drunk driving and other externalizing behaviors will be an important topic for future research.
In supporting the externalizing conceptualization of drunk driving, do the current results argue against other proposed conceptualizations? One such approach frames drunk driving as a consequence or symptom of disordered alcohol use. The DSM-IV criterion of “recurrent [alcohol] use in situations in which it is physically hazardous (e.g., driving an automobile or operating a machine when impaired by [alcohol] use)” was the most commonly reported indicator of Alcohol Abuse (APA, 1994, p. 199; Harford, Grant, Yi, & Chen, 2005; Hasin & Paykin, 1999). Because a diagnosis of Alcohol Abuse required endorsement of only one such criterion, studies have estimated that 42–69% of Alcohol Abuse cases met diagnostic criteria on the basis of drunk driving alone (Hasin et al., 1999; Keyes & Hasin, 2008), at least in U.S. studies that explicitly linked automobile use to the hazardous use criterion (Mewton, Slade, Memedovic, & Teesson, 2012). Moreover, the recently released DSM-5 has retained a criterion of “recurrent alcohol use in situations in which it is physically hazardous” for AUD, and, although the criterion does not explicitly refer to drunk driving, DSM-5 also includes the statement that individuals with AUDs “may use alcohol in physically hazardous circumstances (e.g., driving an automobile, swimming, operating machinery while intoxicated)” (APA, 2013, pp. 491, 492). Concerns have been raised, however, that hazardous use—and drunk driving in particular—may qualitatively differ from other AUD criteria (Martin, Chung, & Langenbucher, 2008; Martin et al., 2011).
Results presented here complement prior research on distinctions between drunk driving and disordered alcohol use. Despite a significant genetic overlap, 70% of the genetic variance in drunk driving was unique of AD symptoms. That is, influences on AD symptoms could not entirely explain the genetic etiology of drunk driving. Considered along with the shared genetic variation between drunk driving and adolescent rule-breaking and, to a lesser but non-zero extent, adult rule-breaking, incomplete genetic overlap between drunk driving and AD symptoms could support the contention that drunk driving is a poor criterion for AUDs. Rather, drunk driving may fit into a broader dimensional conceptualization of externalizing disorders, as recommended by Martin and colleagues (2008). On the other hand, however, the imperfect genetic correlation found here may also have resulted from a lack of developmental specificity and other measurement error. We cannot determine, for example, whether the genetic association would have been stronger if the periods assessed by our lifetime indices of drunk driving and AD symptoms overlapped completely. This uncertainty highlights the need both for replication of these findings using precise measurements and, more broadly, for development specificity regarding hypotheses and measurements in future behavioral genetic research.
The well-documented association between earlier age of drinking initiation and eventual driving after drinking has raised the possibility of a developmental hypothesis by which early drinking or its attendant experiences may ultimately lead to drunk driving (Hingson et al. 2002; Lynskey et al., 2007). An advantage of the current twin-comparison design is that it can provide a strong test of this casual relation. If early drinking initiation or other non-shared environmental experiences that coincide with early initiation lead to eventual drunk driving, then MZ twins who begin drinking earlier than their co-twins should also be more likely to drive drunk. Within-twin-pair differences in age at first drink were not, however, associated with differences in drunk driving, which is inconsistent with a causal role of early drinking initiation in the etiology of drunk driving. Common genetic predispositions may explain the co-occurrence within individuals of drunk driving and early age at first drink, along with the other adolescent externalizing behaviors assessed here. Efforts to delay drinking initiation among adolescents may serve to additionally delay initiation of drunk driving, but these results suggest they may not reduce its long-term prevalence.
Several methodological limitations are worth consideration in interpreting the results of this investigation. First, this study relied exclusively on self-report of intoxicated driving and other outcomes. Although Add Health employed data-collection strategies to maximize validity, self-report of potentially illicit behavior may nevertheless have been susceptible to social desirability bias, which could artificially deflate prevalence rates and, if the same bias affects multiple outcomes, inflate phenotypic associations. Second, we found little evidence that AD or early alcohol use initiation led causally to drunk driving, but the current research design cannot provide information regarding the potential influence of acute alcohol intoxication on decisions to drive after drinking. Under certain impelling conditions, alcohol intoxication can increase risk-taking, and decisions to drive after drinking may be susceptible to similar effects (Giancola, Josephs, Parrott, & Duke, 2010). Third, our analyses used a “lifetime” measure of drunk driving, which collapsed drunk driving across adolescence and early adulthood. The pattern of genetic and environmental associations between drunk driving and other externalizing behaviors may differ when considering only drunk driving that occurred in adulthood. As noted above, there may be a stronger genetic association between adult drunk driving and adult AD symptoms. Moreover, drunk driving was assessed at Waves II and III only for participants who endorsed any past-year alcohol use. It is therefore possible that missingness on the lifetime drunk driving score used here was not entirely random. The current findings should be tested for replication in a sample with more comprehensive measurement of drunk driving.
Finally, it is important to note that each of our analytic steps necessarily involved simplifying assumptions. In particular, our two-stage modeling approach, in which we generated genetic and non-shared-environmental correlation matrices and then used those correlations as inputs for MDS, resulted in loss of information regarding uncertainty in the estimation of the correlations. Additionally, our models assumed linear relationships among observed and latent variables, without any gene × environment interactions, and they did not assess moderation of genetic or environmental influences across gender or ethnicity. From a theoretical perspective, however, examining how genetic influences on drunk driving vary across time, context, and demographic groups represents an important area for future research. For example, the heritability of rule-breaking delinquency increases through adolescence (Burt & Neiderhiser, 2009), and we found that genetic associations between drunk driving and rule-breaking decreased in magnitude from adolescence to early adulthood. Future research is needed to determine whether these differences reflect changing patterns of genetic and environmental influences on drunk driving across development. In addition, although some research has found that greater environmental adversity increases the impact of genetic influences on adolescent externalizing behavior (Hicks, South, DiRago, Iacono, & McGue, 2009), drunk driving may be unique among such behaviors in that greater socioeconomic status could actually increase access to its necessary conditions (e.g., availability of automotive transportation; Keyes & Hasin, 2008). Thus, whereas the current investigation suggests that drunk driving may be one component of the genetically influenced externalizing spectrum, it additionally demonstrates the value of a developmental and behavioral genetic approach in illuminating further complexities in its etiology.
Supplementary Material
Rotating image of the three-dimensional multidimensional scaling map of non-shared environmental associations among drunk driving and other externalizing behaviors. Light Blue = Drunk Driving, Black = Alcohol Dependence Symptoms, Pink = Early Age at First Drink, Yellow = Adolescent Alcohol Use, Orange = Adolescent Binge Drinking, Red = Adolescent Rule-Breaking Delinquency, Blue = Adult Alcohol Use, Green = Adult Binge Drinking, Gray = Adult Rule-Breaking Delinquency.
Acknowledgments
Patrick D. Quinn’s contribution was supported by National Institute on Alcohol Abuse and Alcoholism grant F31-AA020725 and the Waggoner Center for Alcohol and Addiction Research. Dr. K. Paige Harden is a Faculty Research Associate of the Population Research Center at The University of Texas at Austin, which is supported by National Institute of Child Health and Human Development grant 5-R24-HD042849. We thank Elliot Tucker-Drob, Mijke Rhemtulla, and Daniel Briley for helpful comments on previous drafts of this manuscript and Eric Turkheimer and Erik Pettersson for guidance regarding multidimensional scaling analyses.
This research uses data from Add Health, a program project directed by Kathleen Mullan Harris and designed by J. Richard Udry, Peter S. Bearman, and Kathleen Mullan Harris at the University of North Carolina at Chapel Hill and funded by grant P01-HD31921 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with cooperative funding from 23 other federal agencies and foundations. Special acknowledgment is due to Ronald R. Rindfuss and Barbara Entwisle for assistance in the original design. Information on how to obtain the Add Health data files is available on the Add Health website (http://www.cpc.unc.edu/addhealth). No direct support was received from grant P01-HD31921 for this analysis.
Footnotes
Several assumptions upon which behavioral genetic models such as those tested here are built are likely overly simplistic (Charney, 2012). In particular, monozygotic twins may not have or express identical genomes. However, we note that the product of any within-monozygotic-twin-pair genetic differences will be an underestimation of heritability and common genetic variance across phenotypes. Moreover, even if genes differ across twins, monozygotic co-twin comparisons can be understood as quite highly matched genetic and environmental controls, the rigor of which will still exceed that of many standard epidemiological designs.
The MZ twin-pair correlation was more than twice the DZ correlation for adult binge drinking and rule-breaking and AD symptoms, none of which showed significant evidence of shared environmental variance. In these cases, we tested for dominant genetic variances (D) by comparing AE models to ADE models. The AE and ADE models fit equivalently for all three behaviors: adult binge drinking, Δ χ2 (1) = 0.21, p = .65, adult rule-breaking, Δχ2 (1) = 0.62, p = .43, and AD symptoms, Δχ2 (1) = 2.28, p = .13. In the adult binge drinking ADE model, the D parameter did not significantly differ from zero (p = .36), whereas the A parameter did, p = .04. However, in the AD symptoms ADE model, the D parameter (p < .001)—but not the A parameter (p > .99)—was significant, and neither the D (p = .12) nor A (p = .80) parameters were significant in the adult rule-breaking ADE model. In all three cases, DE and ADE models also fit equivalently, Δχ2s (1) < 1.00, ps > .32. Given that the AE and DE models are not nested and therefore could not be directly compared, we selected the AE models in all three cases to maintain consistency with drunk driving and other modeled phenotypes. This approach is also consistent with prior studies not finding support for dominance effects on AD symptoms (Agrawal et al., 2009; Sartor et al., 2010).
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/abn
Contributor Information
Patrick D. Quinn, Department of Psychology, The University of Texas at Austin
K. Paige Harden, Department of Psychology and Population Research Center, The University of Texas at Austin
References
- Agrawal A, Bucholz KK, Lynskey MT. DSM-IV alcohol abuse due to hazardous use: A less severe form of abuse? Journal of Studies on Alcohol and Drugs. 2010;71:857–863. doi: 10.15288/jsad.2010.71.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Sartor CE, Lynskey MT, Grant JD, Pergadia ML, Grucza R, et al. Evidence for an interaction between age at first drink and genetic influences on DSM-IV alcohol dependence symptoms. Alcoholism: Clinical and Experimental Research. 2009;33:2047–2056. doi: 10.1111/j.1530-0277.2009.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. DSM-IV. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- Anum EA. Unpublished dissertation. Virginia Commonwealth University; 2007. Heritability and adverse motor vehicle outcomes. [Google Scholar]
- Arria AM, Caldeira KM, Vincent KB, Garnier-Dykstra LM, O’Grady KE. Substance-related traffic-risk behaviors among college students. Drug and Alcohol Dependence. 2011;118:306–312. doi: 10.1016/j.drugalcdep.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver KM, Barnes JC. Genetic and nonshared environmental factors affect the likelihood of being charged with driving under the influence (DUI) and driving while intoxicated (DWI) Addictive Behaviors. 2012;37:1377–1381. doi: 10.1016/j.addbeh.2012.06.012. [DOI] [PubMed] [Google Scholar]
- Bingham CR, Elliott MR, Shope JT. Social and behavioral characteristics of young adult drink/drivers adjusted for level of alcohol use. Alcoholism: Clinical and Experimental Research. 2007;31:655–664. doi: 10.1111/j.1530-0277.2007.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg I, Groenen PJF. Modern multidimensional scaling: Theory and applications. 2. New York, NY: Springer-Verlag; 2005. [Google Scholar]
- Brownstein N, Kalsbeek WD, Tabor J, Entzel P, Daza E, Harris KM. Non-Response in Wave IV of the National Longitudinal Study of Adolescent Health. n.d Retrieved May 21, 2013, from http://www.cpc.unc.edu/projects/addhealth/data/guides/W4_nonresponse.pdf.
- Burt SA, Neiderhiser JM. Aggressive versus nonaggressive antisocial behavior: Distinctive etiological moderation by age. Developmental Psychology. 2009;45:1164–1176. doi: 10.1037/a0016130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Begg D, Dickson N, Harrington H, Langley J, Moffitt TE, Silva PA. Personality differences predict health-risk behaviors in young adulthood: Evidence from a longitudinal study. Journal of Personality and Social Psychology. 1997;73:1052–1063. doi: 10.1037//0022-3514.73.5.1052. [DOI] [PubMed] [Google Scholar]
- Chantala K, Kalsbeek WD, Andraca E. Non-Response in Wave III of the Add Health Study. 2005 Retrieved May 24, 2013, from http://www.cpc.unc.edu/projects/addhealth/data/guides/W3nonres.pdf.
- Charney E. Behavior genetics and postgenomics. Behavioral and Brain Sciences. 2012;35:331–358. doi: 10.1017/S0140525X11002226. [DOI] [PubMed] [Google Scholar]
- Cooper ML, Wood PK, Orcutt HK, Albino A. Personality and the predisposition to engage in risky or problem behaviors during adolescence. Journal of Personality and Social Psychology. 2003;84:390–410. doi: 10.1037//0022-3514.84.2.390. [DOI] [PubMed] [Google Scholar]
- Dick DM, Johnson JK, Viken RJ, Rose RJ. Testing between-family associations in within-family comparisons. Psychological Science. 2000;11:409–413. doi: 10.1111/1467-9280.00279. [DOI] [PubMed] [Google Scholar]
- Donovan JE. Young adult drinking-driving: Behavioral and psychosocial correlates. Journal of Studies on Alcohol. 1993;54:600–613. doi: 10.15288/jsa.1993.54.600. [DOI] [PubMed] [Google Scholar]
- Donovan JE, Jessor R. Structure of problem behavior in adolescence and young adulthood. Journal of Consulting and Clinical Psychology. 1985;53:890–904. doi: 10.1037//0022-006x.53.6.890. [DOI] [PubMed] [Google Scholar]
- Giancola PR, Josephs RA, Parrott DJ, Duke AA. Alcohol myopia revisited: Clarifying aggression and other acts of disinhibition through a distorted lens. Perspectives on Psychological Science. 2010;5:265–278. doi: 10.1177/1745691610369467. [DOI] [PubMed] [Google Scholar]
- Guttman L. A new approach to factor analysis: The radex. In: Lazerfield PF, editor. Mathematical thinking in the social sciences. Glencoe, IL: Free Press; 1954. [Google Scholar]
- Harden KP, Mendle J. Gene-environment interplay in the association between pubertal timing and delinquency in adolescent girls. Journal of Abnormal Psychology. 2012;121:73–87. doi: 10.1037/a0024160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harford TC, Grant BF, Yi H-y, Chen CM. Patterns of DSM-IV Alcohol Abuse and Dependence criteria among adolescents and adults: Results from the 2001 National Household Survey on Drug Abuse. Alcoholism: Clinical and Experimental Research. 2005;29:810–828. doi: 10.1097/01.alc.0000164381.67723.76. [DOI] [PubMed] [Google Scholar]
- Harris KM. Design features of Add Health. Chapel Hill: Carolina Population Center, University of North Carolina at Chapel Hill; 2011. Retrieved January 2013 from http://www.cpc.unc.edu/projects/addhealth/data/guides/design%20paper%20WI-IV.pdf. [Google Scholar]
- Harris KM, Halpern CT, Smolen A, Haberstick BC. The National Longitudinal Study of Adolescent Health (Add Health) Twin Data. Twin Research and Human Genetics. 2006;9:988–997. doi: 10.1375/183242706779462787. [DOI] [PubMed] [Google Scholar]
- Hasin D, Paykin A. DSM-IV alcohol abuse: Investigation in a sample of at-risk drinkers in the community. Journal of Studies on Alcohol. 1999;60:181–187. doi: 10.15288/jsa.1999.60.181. [DOI] [PubMed] [Google Scholar]
- Hasin D, Paykin A, Endicott J, Grant B. The validity of DSM-IV alcohol abuse: Drunk drivers versus all others. Journal of Studies on Alcohol. 1999;60:746–755. doi: 10.15288/jsa.1999.60.746. [DOI] [PubMed] [Google Scholar]
- Hicks BM, Krueger RF, Iacono WG, McGue M, Patrick CJ. Family transmission and heritability of externalizing disorders: A twin-family study. Archives of General Psychiatry. 2004;61:922–928. doi: 10.1001/archpsyc.61.9.922. [DOI] [PubMed] [Google Scholar]
- Hicks BM, South SC, DiRago AC, Iacono WG, McGue M. Environmental adversity and increasing genetic risk for externalizing disorders. Arch Gen Psychiatry. 2009;66:640–648. doi: 10.1001/archgenpsychiatry.2008.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson R, Heeren T, Levenson S, Jamanka A, Voas R. Age of drinking onset, driving after drinking, and involvement in alcohol related motor-vehicle crashes. Accident Analysis and Prevention. 2002;34:85–92. doi: 10.1016/s0001-4575(01)00002-1. [DOI] [PubMed] [Google Scholar]
- Hingson R, Zha W, Weitzman ER. Magnitude of and trends in alcohol-related mortality and morbidity among U.S. college students ages 18–24, 1998–2005. Journal of Studies on Alcohol and Drugs. 2009;(Supplement No. 16):12–20. doi: 10.15288/jsads.2009.s16.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennrich RI, Bentler PM. Exploratory bi-factor analysis. Psychometrika. 2011;76:537–549. doi: 10.1007/s11336-011-9218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessor R, Jessor SL. Adolescent development and the onset of drinking: A longitudinal study. Journal of Studies on Alcohol. 1975;36:27–51. doi: 10.15288/jsa.1975.36.27. [DOI] [PubMed] [Google Scholar]
- Johnson W, Turkheimer E, Gottesman II, Bouchard TJ., Jr Beyond heritability: Twin studies in behavioral research. Current Directions in Psychological Science. 2009;18:217–220. doi: 10.1111/j.1467-8721.2009.01639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Hasin DS. Socio-economic status and problem alcohol use: the positive relationship between income and the DSM-IV alcohol abuse diagnosis. Addiction. 2008;103:1120–1130. doi: 10.1111/j.1360-0443.2008.02218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior and personality: Modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111:411–424. [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Benning SD, Kramer MD. Linking antisocial behavior, substance use, and personality: An integrative quantitative model of the adult externalizing spectrum. Journal of Abnormal Psychology. 2007;116:645–666. doi: 10.1037/0021-843X.116.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Iacono WG. Externalizing psychopathology in adulthood: A dimensional-spectrum conceptualization and its implications for DSM-V. Journal of Abnormal Psychology. 2005;114:537–550. doi: 10.1037/0021-843X.114.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynskey MT, Bucholz KK, Madden PAF, Heath AC. Early-onset alcohol-use behaviors and subsequent alcohol-related driving risks in young women: A twin study. Journal of Studies on Alcohol and Drugs. 2007;68:798–804. doi: 10.15288/jsad.2007.68.798. [DOI] [PubMed] [Google Scholar]
- Maraun MD. Appearance and reality: Is the Big Five the structure of trait descriptors? Personality and Individual Differences. 1997;22:629–647. [Google Scholar]
- Martin CS, Chung T, Langenbucher JW. How should we revise diagnostic criteria for substance use disorders in the DSM-V? Journal of Abnormal Psychology. 2008;117:561–575. doi: 10.1037/0021-843X.117.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CS, Sher KJ, Chung T. Hazardous use should not be a diagnostic criterion for Substance Use Disorders in DSM-5. Journal of Studies on Alcohol and Drugs. 2011;72:685–686. doi: 10.15288/jsad.2011.72.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Elkins I. Origins and consequences of age at first drink. II: Familial risk and heritability. Alcoholism: Clinical and Experimental Research. 2001;25:1166–1173. [PubMed] [Google Scholar]
- Mewton L, Slade T, Memedovic S, Teesson M. Alcohol use in hazardous situations: Implications for DSM-IV and DSM-5 Alcohol Use Disorders. Alcoholism: Clinical and Experimental Research. 2012;37:E228–E236. doi: 10.1111/j.1530-0277.2012.01881.x. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. MPlus user’s guide. 5. Los Angeles: Muthén & Muthén; 1998–2007. [Google Scholar]
- National Highway Traffic Safety Administration. Traffic Safety Facts 2011. Washington, DC: Department of Transportation; 2013. (No. DOT HS 811 754) [Google Scholar]
- Neale MC, Maes HHM. Methodology for genetic studies of twins and families. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2004. [Google Scholar]
- O’Malley PM, Johnston LD. Drugs and driving by American high school seniors, 2001–2006. Journal of Studies on Alcohol and Drugs. 2007;68:834–842. doi: 10.15288/jsad.2007.68.834. [DOI] [PubMed] [Google Scholar]
- Prescott CA. Using the Mplus computer program to estimate models for continuous and categorical data from twins. Behavior Genetics. 2004;34:17–40. doi: 10.1023/B:BEGE.0000009474.97649.2f. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Age at first drink and risk for alcoholism: A noncausal association. Alcoholism: Clinical and Experimental Research. 1999;23:101–107. [PubMed] [Google Scholar]
- Quinn PD, Fromme K. Personal and contextual factors in the escalation of driving after drinking across the college years. Psychology of Addictive Behaviors. 2012;26:714–723. doi: 10.1037/a0026819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Development Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2009. http://www.R-project.org. [Google Scholar]
- Sartor CE, Grant JD, Bucholz KK, Madden PAF, Heath AC, Agrawal A, et al. Common genetic contributions to alcohol and cannabis use and dependence symptomatology. Alcoholism: Clinical and Experimental Research. 2010;34:545–554. doi: 10.1111/j.1530-0277.2009.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sass DA, Schmitt TA. A comparative investigation of rotation criteria within exploratory factor analysis. Multivariate Behavioral Research. 2010;45:73–103. doi: 10.1080/00273170903504810. [DOI] [PubMed] [Google Scholar]
- Schmitt TA. Current methodological considerations in exploratory and confirmatory factor analysis. Journal of Psychoeducational Assessment. 2011;29:304–321. [Google Scholar]
- Shope JT, Bingham CR. Drinking-driving as a component of problem driving and problem behavior in young adults. Journal of Studies on Alcohol. 2002;63:24–33. [PubMed] [Google Scholar]
- Slutske WS, True WR, Scherrer JF, Heath AC, Bucholz KK, Eisen SA, et al. The heritability of alcoholism symptoms: “Indicators of genetic and environmental influence in alcohol-dependent individuals” revisited. Alcoholism: Clinical and Experimental Research. 1999;23:759–769. doi: 10.1111/j.1530-0277.1999.tb04181.x. [DOI] [PubMed] [Google Scholar]
- Snow RE, Kyllonen PC, Marshalek B. The topography of ability and learning correlation. In: Sternberg RJ, editor. Advances in the psychology of human intelligence. Hillsdale, NJ: Erlbaum; 1984. pp. 47–103. [Google Scholar]
- Thombs DL, O’Mara RJ, Hou W, Wagenaar AC, Dong HJ, Merves ML, et al. 5-HTTLPR genotype and associations with intoxication and intention to drive: results from a field study of bar patrons. Addiction Biology. 2011;16:133–141. doi: 10.1111/j.1369-1600.2010.00225.x. [DOI] [PubMed] [Google Scholar]
- Thurstone LL. Multiple-factor analysis. Chicago: University of Chicago Press; 1947. [Google Scholar]
- Tucker LR, Lewis C. A reliability coefficient for maximum likelihood factor analysis. Psychometrika. 1973;38:1–10. [Google Scholar]
- Tucker-Drob E, Salthouse TA. Confirmatory factor analysis and multidimensional scaling for construct validation of cognitive abilities. International Journal of Behavioral Development. 2009;33:277–285. doi: 10.1177/0165025409104489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkheimer E, Ford DC, Oltmanns TF. Regional analysis of self-reported personality disorder criteria. Journal of Personality. 2008;76:1587–1622. doi: 10.1111/j.1467-6494.2008.00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassallo S, Smart D, Sanson A, Cockfield S, Harris A, McIntyre A, Harrison W. Risky driving among young Australian drivers II: Co-occurrence with other problem behaviours. Accident Analysis and Prevention. 2008;40:376–386. doi: 10.1016/j.aap.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Venables WN, Ripley BD. Modern applied statistics with S. 4. New York: Springer; 2002. [Google Scholar]
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, Hewitt JK. Behavioral disinhibition: Liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. Journal of Abnormal Psychology. 2009;118:117–130. doi: 10.1037/a0014657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wieczorek WF, Welte JW, Colder C, Nochajski TH. Delinquency and alcohol-impaired driving among young males: A longitudinal study. Journal of Criminal Justice. 2010;38:439–445. doi: 10.1016/j.jcrimjus.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Rotating image of the three-dimensional multidimensional scaling map of non-shared environmental associations among drunk driving and other externalizing behaviors. Light Blue = Drunk Driving, Black = Alcohol Dependence Symptoms, Pink = Early Age at First Drink, Yellow = Adolescent Alcohol Use, Orange = Adolescent Binge Drinking, Red = Adolescent Rule-Breaking Delinquency, Blue = Adult Alcohol Use, Green = Adult Binge Drinking, Gray = Adult Rule-Breaking Delinquency.