Abstract
Objectives
Modulation detection thresholds (MDTs) vary across stimulation sites in a cochlear implant electrode array in a manner that is subject and ear specific. Previous studies have demonstrated that speech recognition with a cochlear implant can be improved by site-selection strategies, where selected stimulation sites with poor modulation sensitivity are removed from a subject’s processor MAP1. Limitations of site-selection strategies are that they can compromise spectral resolution and distort frequency-place mapping since the frequencies assigned to the removed sites are usually reallocated to other sites and site bandwidths are broadened. The objective of the current study was to test an alternative approach for rehabilitation that aimed at improving the across-site mean (ASM) MDTs by adjusting stimulation parameters at the poorly-performing sites. Based on previous findings that modulation detection contributes to speech recognition and improves significantly with stimulus level, we hypothesized that modulation sensitivity at the poor sites can be improved by artificially increasing stimulation levels at those sites in the speech processor, which then leads to improved speech recognition.
Design
Nine postlingually deafened ears implanted with Nucleus cochlear implants were evaluated for MDTs, absolute-detection threshold levels (T levels) and the maximum loudness levels (C levels) on each of the available stimulation sites. For each ear, the minimum stimulation level settings in the speech processor MAP were raised by 5%, and alternatively by 10%, of the dynamic range (DR) from true thresholds on 5 stimulation sites with the poorest MDTs. For comparison, a 5% level raise was globally applied to all stimulation sites. The C levels were fixed during these level manipulations. MDTs at the 5 poorest stimulation sites were compared at 20% DR before and after the level adjustments. Speech reception thresholds (SRTs), i.e., signal to noise ratios (SNRs) required for 50% correct speech recognition, were evaluated for these MAPs using CUNY sentences. The site-specific level-adjusted MAPs were compared to the globally level-adjusted MAP and the MAP without level adjustment. The effects on speech recognition of adjusting the minimal stimulation level settings on the 5 poorest stimulation sites were also compared with effects of removing these sites from the speech-processor MAP.
Results
The 5% level increase on the 5 electrodes with the worst MDTs resulted in an improvement in the group-mean SRT of 2.36 dB SNR relative to the MAP without level adjustment. The magnitude of level increase that resulted in the greatest SRT improvement for individuals varied across ears. MDTs measured at 20% DR significantly improved on the poor sites after the level adjustment that resulted in the best SRT for that ear was applied. Increasing the minimal stimulation levels on all stimulation sites or removing sites selected for rehabilitation, the parsimonious approaches, did not improve speech reception thresholds.
Conclusions
The site-specific adjustments of the T level settings improved modulation sensitivity at low levels and significantly improved subjects’ speech reception thresholds. Thus, this site-rehabilitation strategy was an effective alternative to site-selection strategies for improving speech recognition in cochlear implant users.
Introduction
Functional responses to electrical stimulation with a cochlear implant vary across stimulation sites along the electrode array in human ears. The across-site patterns have been demonstrated for various psychophysical measures that assess stimulus detection, loudness, and temporal or spatial acuity in humans (Zwolan et al., 1997; Donaldson and1 Nelson, 2000; Pfingst et al., 2004; Pfingst and Xu, 2005; Bierer, 2007; Bierer and Faulkner, 2010; Pfingst et al., 2008; Zhou et al., 2011; Garadat et al., 2012). The across-site patterns are specific to individual ears, indicating that the variation is not due to normal organization of the auditory system. A number of factors could contribute to the variation across stimulation sites including electrode-neuron distance, the impedances of the current path, or placement of the electrodes, collectively referred to as the electrode-neuron interface (Bierer and Faulkner, 2010). There is also evidence from animal studies to indicate that various psychophysical and physiological responses to electrical stimulation are related, at least in part, to the localized conditions in the inner ear (Pfingst et al., 1981; Shepherd et al., 1993; Shepherd and Javel, 1997; Shepherd et al., 2004; Chikar et al., 2008; Kang et al., 2010; Pfingst et al., 2011b). Data from guinea pigs indicate that the slopes of threshold versus pulse rate functions, for example, are correlated with sensory and neural survival status across animals with a large range of cochlear health (Kang et al., 2010; Pfingst et al., 2011b).
The processor MAP of a cochlear implant can be optimized for human listeners by identifying and turning off the sub-optimal stimulation sites, an approach referred to as site selection (Zwolan et al., 1997; Garadat et al., Reference note 1). Removal of the sub-optimal stimulation sites improves the across-site mean (ASM) psychophysical acuity for the implanted ear and reduces perceptual variation along the tonotopic axis. Variation in perceptual acuity across sites can be a detrimental factor for speech recognition using a cochlear implant. Large across-site variance in psychophysical detection thresholds for example has been shown to correlate with poor speech recognition performance (Pfingst et al., 2004; Pfingst and Xu, 2005; Bierer, 2007). Uneven acuity attributable to either localized pathology or differences in electrode-neuron interface could affect perception with the whole array.
Zwolan et al. (1997) showed that speech recognition improved in some of their tested subjects after the stimulation sites that were not discriminable from neighboring sites were excluded from the processor MAP. Selecting stimulation sites based on a measure of temporal acuity has also resulted in significant improvements in speech recognition. Garadat and colleagues (Reference note 1) compared implant listener’s every-day use MAP with an experimental MAP that removed 5 stimulation sites with poor masked modulation detection thresholds (masked MDTs) and reallocated the frequencies to the remainder of the array. The strategy improved the subjects’ speech reception thresholds (SRTs) in noise for CUNY sentences by approximately 2 dB signal to noise ratio (SNR) and consonant recognition by a few percentage points. Different from Zwolan et al., the rule of selection was that the 5 sites must spread across the array with one chosen from each of 5 segments along the tonotopic axis. Removal of multiple sites in one tonotopic location was avoided in order to minimize the potential disruption of the tonotopic map (e.g., see Shannon et al., 1998; 2002).
However in implanted ears the poor stimulation are not always distributed evenly across the electrode array, but often are clustered in one or two tonotopic areas. The rule that the removed sites be distributed across the tonotopic axis would then miss the poorest sites. In addition, removal of stimulation sites can result in compromised spectral resolution, because bandwidths of the remaining channels have to be widened to cover the full speech spectrum. Given these limitations for the site-selection strategies, the present study explores an alternative procedure for optimizing the processor MAP. Instead of removing the sub-optimal sites, they might be rehabilitated by adjusting the sites’ stimulation parameters. This alternative is not restricted by any distribution rules such as that described in Garadat et al. (Reference note 1) and does not require reallocation of frequencies, but has similar advantages as site removal that it improves the ASM acuity and reduces across-site variation. In the present study, we also tested the effects of violating the distribution rule applied in Garadat et al. (Reference note 1) by creating a processor MAP in which the same stimulation sites chosen for rehabilitation, often clustered, were instead removed from the speech processor MAP.
The site selection studies showed that speech recognition, particularly perception of the temporal dynamics of the speech signal, benefited from choosing sites that were based on measures of modulation sensitivity (Garadat et al., 2012; Zhou and Pfingst et al., 2012; Garadat et al., Reference note 1). Given the nature of the envelope-based speech processing strategies that the modern implant devices use, modulation perception is presumably one of the most essential mechanisms on which implant users rely for understanding speech signals (Wilson et al., 1993; 1995). Previous studies have shown 2 that temporal processing capacities vary greatly across cochlear implant users (Cazals et al., 1994; Shannon, 1992; Busby et al., 1993), but demonstrate low-pass characteristics and slopes of high-frequency rejection in the temporal modulation transfer functions (TMTF) similar to those found in normal-hearing listeners (Cazals et al., 1994). Low frequency MDTs have been identified as a psychophysical predictor for speech recognition performance across subjects (Cazals et al., 1994; Fu, 2002; Colletti and Shannon, 2005; Luo et al., 2008). In the current study, we report an alternative approach for improving the ASM modulation sensitivity in cochlear implant listeners.
Sensitivity to amplitude modulation with electrical stimulation is affected by several stimulus parameters including stimulation rate, mode and level (e.g., Galvin and Fu, 2005; 2009; Pfingst et al., 2007). Modulation detection increases significantly with increase of loudness or percent of DR (Fu, 2002; Pfingst et al., 2008). MDTs saturate at 30% of DR for some cases, while for others MDTs gradually improve throughout the whole DR (Fu, 2002; Pfingst et al., 2008). For most cases, performance seems to saturate by 70% of the DR, beyond which increases in stimulation level result in no further improvement (Pfingst et al., 2008). Given the dependence of MDTs on stimulus level, we hypothesized that stimulation sites with poor modulation sensitivity would be improved by increasing stimulation levels.
In the present study, we explored raising the minimum stimulation level from the true threshold level (T level) for sites with poor modulation sensitivity. With fixed maximum comfortable levels, stimulation level in microamps increased throughout the artificially reduced DR, but more so at lower levels than at the upper levels. Assuming modulation sensitivity is dependent on stimulation level and is important for speech recognition, we hypothesized that raising the minimal stimulation levels would improve MDTs at low levels, which in turn would benefit speech recognition. We call this a site-rehabilitation approach. The effects of various level manipulations on modulation detection at the lower portion of the DR and on speech reception thresholds in noise were examined.
Materials and methods
1. Ears Tested
Seven postlingually-deafened cochlear implant users recruited from the University of Michigan, Ann Arbor Cochlear Implant clinic and surrounding areas participated in the study. Two of the seven subjects were sequentially implanted bilaterally. The nine implanted ears from these seven individuals were treated as independent cases. The ears were implanted with Nucleus CI24R or CI24RE devices and all were programmed with an ACE speech-processing strategy. The demographic information for the subjects (nine ears) is shown in Table 1. The use of human subjects in the study was reviewed and approved by the University of Michigan Medical School Institutional Review Board.
Table 1.
Demographics for the 9 ears
| Subject | Gender | Ear | Age | Duration of deafness prior to implantation (yrs) |
Duration of implant use (yrs) |
Device | Processor | Pulse rate (pps) |
Phase duration (us) |
Etiology |
|---|---|---|---|---|---|---|---|---|---|---|
| S60 | M | L | 72 | 0.2 | 8.9 | CI24R(CS) | CP810 | 900 | 25 | Hereditary |
| S60 | M | R | 72 | 6.2 | 3.2 | CI24RE(CA) | CP810 | 900 | 25 | Hereditary |
| S83 | F | L | 67 | 46.8 | 7.4 | CI24RE(CA) | Freedom | 900 | 25 | Otosclerosis |
| S88 | M | L | 61 | 10.8 | 3.1 | CI24RE(CA) | CP810 | 900 | 25 | Unknown |
| S88 | M | R | 61 | 33.5 | 9.3 | CI24R(CS) | CP810 | 900 | 25 | Unknown |
| S89 | M | L | 67 | 0.1 | 3.2 | CI24RE(CA) | Freedom | 900 | 25 | Hereditary |
| S92 | F | L | 28 | 0.4 | 6.8 | CI24RE(CA) | CP810 | 900 | 25 | Unknown |
| S93 | F | L | 64 | 0.8 | 5.3 | CI24RE(CA) | Freedom | 900 | 25 | Hereditary |
| S94 | F | R | 69 | 1.7 | 5.7 | CI24RE(CA) | Freedom | 1200 | 25 | Unknown |
2. Psychophysical testing
For the psychophysical tests, stimuli were 500 ms trains of symmetric-biphasic pulses with a mean phase duration of 50 μs and an inter-phase interval of 8 μs. The pulse trains were presented using a monopolar (MP1+2) electrode configuration at a rate of 900 pps. Measurements were taken at electrodes that were activated and functioning in the clinical everyday-use MAPs. The number of electrodes tested ranged from 18 to 22 per ear. A laboratory-owned Cochlear Freedom speech processor (Cochlear Corporation, Englewood, CO) was used for the psychophysical tests.
a. T and C levels
For each tested ear, T levels and the maximum comfortable loudness levels (C levels) for each of the functioning stimulation sites were measured using the Cochlear Custom Sound Suite 3.1 software. T levels were obtained using the method of adjustment. The “counting method” was used, where the subjects reported if they heard beeps and how many beeps there were, as the level of the stimulus was varied up and down by the experimenter. The number of beeps (500 ms pulse trains) for each presentation was varied between 1 and 3, at the experimenter’s choice, with a fixed presentation rate. Threshold was taken as the level at which the subject could accurately report the number of beeps heard. To measure C levels, the experimenter raised the stimulus level of a single beep to the maximum point where the subjects judged that they could tolerate the sound for a long period of time. T and C levels for each electrode starting with electrode 21 were loudness matched to the immediately-apical adjacent electrode. The T and C levels measured in clinical units were converted to dB re 1 mA peak amplitude. DR was calculated as the difference between the T and C levels.
b. Amplitude-modulation detection
MDTs were first measured at each stimulation site across the array at 50% of the respective DRs for the purpose of identifying the worst-performing sites. Stimuli were presented through a research interface (Nucleus Implant Communicator II, Cochlear Corporation) implemented in Matlab. The pulse duration was modulated by a 10 Hz sinusoid that started and ended at zero phase. Durations of the positive and negative phases of the pulse were modulated while the interphase interval was held constant. Pulse duration rather than amplitude was modulated because stimulus charge could be controlled in finer steps. A four-alternative forced-choice (4AFC) paradigm was used, where four time intervals contained four pulse trains. One of the four pulse trains, chosen at random on each trial, was amplitude modulated. The four 500 ms stimulus intervals were separated by 500 ms silent intervals. Subjects were instructed to choose the one out of four intervals that sounded different from the other three. Modulation depth started at 50% and adapted depending on subject’s responses following a two-down, one-up adaptive-tracking procedure (Levitt, 1971). The test stopped after 14 reversals occurred. Step size started at 6 dB for the first reversal, reduced to 4 dB for the next two reversals, and 1 dB for the remaining reversals. The MDT was calculated as the averaged modulation depths at the last 6 reversal points. Visual feedback was given on the user interface after each trial. The MDT measurements were then repeated using a different randomization. The two resulting MDTs were averaged.
To construct the experimental processor MAPs, as described in Materials and Methods Section 3 below, 5 stimulation sites with the poorest averaged MDTs were identified for each ear (Fig. 1). To determine if the loudness at these sites differed from that at the other sites in the array, subjects’ were instructed to compare loudness at the level MDTs were measured (i.e., 50% DR) across the array. Stimulus presentations at each electrode were first swept from the apical direction then from the basal. Then, stimuli were swept across segments of four electrodes at a time in both directions with one electrode overlapping with the previous segment. Subjects were instructed to report any electrodes that sounded softer than the rest of the electrodes in the sweep.
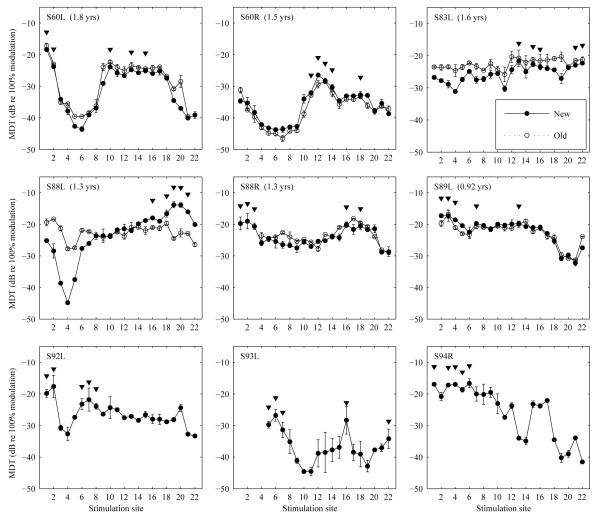
Figure 1.
Mean modulation detection thresholds as a function of stimulation site measured for 9 ears as indicated in the upper left corner of each panel. Error bars represent range of the data for 2 repeated measures. The 5 stimulation sites with the poorest MDTs chosen for level adjustments or removal are indicated by arrows. The older MDT data measured on average 1.4 years prior to the experiments are shown in open symbols for 6 ears. Time span between the two sets of the measurements is indicated in the parenthesis next to the subject number for each ear. Electrodes 1-3 for S88R were deactivated in the subject’s clinical daily MAP at the time MDT was tested the first time.
For 6 ears, MDTs had already been tested on average 1.4 years prior to the present experiment. For these ears, MDTs were replicated at the time of this experiment to examine the stability of MDTs over time. Since MDTs are proposed as a measure to determine sites for rehabilitation, it is important particularly from a clinical perspective to know the stability of this measure over time. For the 6 ears that were tested twice, selection of the 5 poorest electrodes for the current experiment was based on the more recent MDT measurements.
3. Speech processor MAPs
To test the effects of site rehabilitation, four processor MAPs were created for each ear, including one control MAP and three that involved level adjustments. These were all open MAPs that did not activate any SmartSound settings. The control MAP was configured in the monopolar mode (MP1+2) using the T and C levels measured with a phase duration of 50 μs and a pulse rate of 900 pps as described in the section above. The control MAP utilized true T levels as minimum stimulation levels. Two experimental MAPs were constructed based on the 5 sites with the worst MDTs (see Fig. 1). For these MAPs, the minimum stimulation level settings in the speech processer were experimentally raised by 5% and 10% of the DR respectively on the 5 sites with the worst MDTs (e.g., Programmed T = true T + 0.05 × DR). The magnitude of level change was chosen based on results of a pilot study, where a level raise larger than 10% DR created worsened performance and unfavorable perception. In the third experimental MAP, a level raise corresponding to 5% of the DR was applied to all electrodes to test if a global level boost, which does not require assessing modulation detection on each site, is equivalent or superior to the effect of site-specific level adjustment.
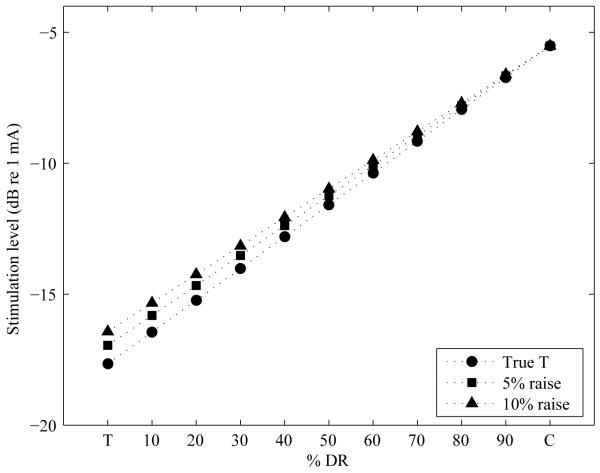
For all three MAPs with modified T levels, the C levels remained unchanged from the control MAP. This resulted in reduced DRs. The artificially raised T levels resulted in increased current level throughout the DR with the increase being the largest at the minimal stimulation level and decreasing in magnitude as a function of percent of the DR (see Figure 2 for an example). The artificial raise in T level settings of 5% DR is shown in dB for each stimulation site for all ears in Table 2.
Figure 2.
Stimulation current level in dB re 1 mA as a function of % of the DR (DR) before and after artificial current increase of 5% or 10% of the pre-adjustment DR was applied to the minimal stimulation level (T level setting) in the speech processor.
Table 2.
Magnitude of current increase in dB corresponding to 5% DR with the 5 poorest sites underlined. The 10% raise would be twice these values
| Stimulation sites/Ears | S60L | S60R | S83L | S88L | S88R | S89L | S92L | S93L | S94R |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.39 | 0.27 | 0.55 | 0.35 | 0.35 | nan | 0.20 | nan | 0.10 |
| 2 | 0.43 | 0.33 | 0.52 | 0.37 | 0.39 | 0.14 | 0.31 | nan | 0.08 |
| 3 | 0.49 | 0.33 | 0.52 | 0.36 | 0.50 | 0.19 | 0.35 | nan | 0.20 |
| 4 | 0.67 | 0.35 | 0.51 | 0.41 | 0.59 | 0.17 | 0.37 | nan | 0.28 |
| 5 | 0.68 | 0.39 | 0.55 | 0.41 | 0.67 | 0.26 | 0.42 | 0.13 | 0.29 |
| 6 | 0.66 | 0.50 | 0.55 | 0.38 | 0.63 | 0.22 | 0.44 | 0.19 | 0.39 |
| 7 | 0.58 | 0.57 | 0.51 | 0.39 | 0.64 | 0.31 | 0.46 | 0.20 | 0.35 |
| 8 | 0.62 | 0.63 | 0.52 | 0.36 | 0.62 | 0.37 | 0.47 | 0.21 | 0.33 |
| 9 | 0.66 | 0.67 | 0.56 | 0.38 | 0.63 | 0.31 | 0.47 | 0.20 | 0.34 |
| 10 | 0.67 | 0.58 | 0.58 | 0.36 | 0.64 | 0.38 | 0.50 | 0.20 | 0.36 |
| 11 | 0.63 | 0.54 | 0.59 | 0.39 | 0.60 | 0.39 | 0.51 | 0.19 | 0.31 |
| 12 | 0.64 | 0.57 | 0.67 | 0.30 | 0.68 | 0.34 | 0.53 | 0.21 | 0.32 |
| 13 | 0.67 | 0.57 | 0.69 | 0.28 | 0.55 | 0.31 | 0.51 | 0.18 | 0.24 |
| 14 | 0.69 | 0.60 | 0.68 | 0.32 | 0.55 | 0.40 | 0.50 | 0.18 | 0.20 |
| 15 | 0.61 | 0.56 | 0.72 | 0.34 | 0.55 | 0.35 | 0.53 | 0.22 | 0.29 |
| 16 | 0.65 | 0.55 | 0.68 | 0.33 | 0.54 | 0.35 | 0.51 | 0.28 | 0.26 |
| 17 | 0.64 | 0.53 | 0.73 | 0.24 | 0.47 | 0.30 | 0.48 | 0.23 | 0.21 |
| 18 | 0.64 | 0.53 | 0.68 | 0.31 | 0.48 | 0.28 | 0.45 | 0.24 | 0.15 |
| 19 | 0.60 | 0.53 | 0.69 | 0.23 | 0.47 | 0.22 | 0.45 | 0.25 | 0.20 |
| 20 | 0.58 | 0.49 | 0.64 | 0.15 | 0.47 | 0.25 | 0.42 | 0.28 | 0.25 |
| 21 | 0.55 | 0.49 | 0.65 | 0.10 | 0.44 | 0.21 | 0.36 | 0.30 | 0.31 |
| 22 | 0.56 | 0.45 | 0.56 | 0.19 | 0.46 | 0.22 | 0.34 | 0.27 | 0.24 |
All four MAPs were set at volume number 5 and a sensitivity level of 9. These settings were fixed in the programs such that the subjects were not able to adjust them on the processor during testing. Although level changes had been made to three MAPs, subjects did not spontaneously report noticeable loudness differences among the four MAPs. Note that the control MAP, although utilizing true Ts, was programmed with 50 μs phase duration and 900 pps and thus it was different from the subject’s clinical everyday-use MAP (see Table 1). In addition, SmartSound options were turned off. The subjects therefore were considered to have no previous experience with any of the four MAPs.
4. Speech reception threshold test
The four MAPs were tested in a random order for speech reception thresholds (SRTs) using CUNY sentences (Boothroyd et al., 1985) presented in a modulated-noise background. Four sentence lists (each containing 12 sentences) randomly chosen from a total of 72 lists were used for measuring one SRT. The CUNY sentences are meaningful utterances with contextual cues spoken by a male speaker. The target CUNY sentences were presented in white noise amplitude modulated at 100% modulation depth with a 4 Hz sinusoid, a frequency similar to the low frequency components of the envelope of the speech waveform. The amplitude-modulated noise was presented alone for 1.5 sec before the target, during presentation of the target, and for 0.5 sec alone after the target. Raised cosine ramps were applied at the onset and offset of the stimulus with the onset and offset each measuring 5% of the entire stimulus length. SNR was calculated for the time period when the target and noise overlapped. The mixed signal (target + noise) was normalized to its peak amplitude therefore level of the masker plus sentence was similar from trial to trial. SNR started at 20 dB at the beginning of the test and adapted in a one-down one-up procedure using a step size of 2 dB. The subject was presented with the sentence in the noise background for one time and was instructed to repeat the sentence to the experimenter. The experimenter lowered SNR by 2 dB if the subject repeated all words in the sentence correctly, or increased SNR by 2 dB for an incorrect response. The one-down one-up procedure estimated a 50% correct point on the psychometric function. The SRT was taken as the mean of the SNRs at the last 6 reversals out of a total of 12 reversals.
Speech recognition was measured using a laboratory-owned processor that was the same type as the processor which the subject wore daily for their clinical everyday-use MAP (Freedom or CP810). For the two bilateral implant users, speech recognition was measured for the left and right ear alone. An ear plug was used in the ear contralateral to the testing ear for subjects who had residual acoustic hearing indicated by the unaided acoustic thresholds for narrow-band noise stimuli (Table 3). The speech tests were administered in a double-walled sound-attenuated booth (Acoustic Systems Model RE 242 S). The test was administered via a graphic user interface programmed in MATLAB (Mathworks, Natick, MA). Speech materials were delivered from the computer to a Rane ME60 graphic equalizer, a Rolls RA235 35W power amplifier, and presented via a loudspeaker positioned 1 m from subject at 0° azimuth. The mixed stimuli (signal + noise) were calibrated at 60 dB (A) SPL with a sound-level meter (Bruel & Kjær, Naerum, Denmark, type 2231) in a slow time setting. During calibration sessions, the sound-level meter was also positioned 1 m away from the loudspeaker at 0° azimuth.
Table 3.
Unaided acoustic thresholds for narrow-band noise stimuli.
| Subjects | Center Frequencies (Hz) | 125 | 250 | 500 | 1000 | 2000 | 4000 | 8000 |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Limit (dB HL) | 70 | 90 | 100 | 100 | 100 | 100 | 80 | |
| S60 L (implanted) | n/a* | n/a | n/a | n/a | n/a | n/a | n/a | |
| S60 R (implanted) | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| S83 L (implanted) | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| S83 R (non-implanted) | n/a | 80 | 90 | n/a | n/a | n/a | n/a | |
| S88 L (implanted) | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| S88 R (implanted) | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| Hearing thresholds (dB HL) | ||||||||
| S92 L (implanted) | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| S92 R (non-implanted) | n/a | 80 | 50 | n/a | n/a | n/a | n/a | |
| S93 L (implanted) | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| S93 R (non-implanted) | n/a | n/a | 80 | 60 | 90 | 90 | n/a | |
| S94 L (non-implanted) | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| S94 R (implanted) | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
Thresholds not measurable.
Each MAP was tested for SRT three times with a different random order of testing used each time. Testing time for one MAP ranged from 15 to 20 minutes.
5. Measuring the effects of raised T levels on MDTs
The rationale of modifying stimulation levels on the poor sites is that it can improve modulation sensitivity on these sites. This was explicitly tested by comparing MDTs at adjusted and un-adjusted levels for these sites. Since the modification of the T level settings increases primarily the stimulation levels at the lower end of the DR, MDTs were first measured for the 5 identified poor sites at 20% of the DR derived from the true thresholds and C levels, and then tested again on those sites at 20% of the adjusted DR derived from artificially raised T levels. The ears tested (N = 7) were those that showed improved SRTs using the site-specific level-adjusted MAPs. DRs were adjusted based on a magnitude of T level increase (either 5% or 10%) that produced the best SRT for a given ear (see Fig. 3 or text in panels of Fig. 4). MDTs were measured twice at adjusted and non-adjusted levels following the procedure described in Materials and Methods Section 2 above and the mean of the two measurements were taken.
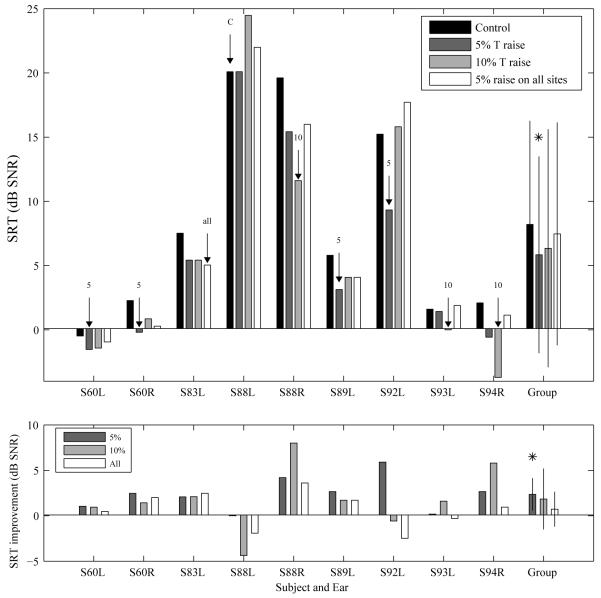
Figure 3.
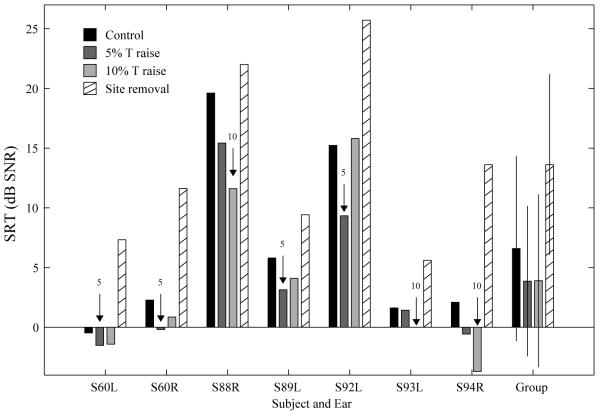
Speech reception thresholds. Upper panel: SRTs measured for the control 3 MAP (no level adjustment) and the MAPs that involved an increase in the minimum stimulation level of 5% and 10% of DR on 5 sites with the poorest MDTs and an increase in the minimum stimulation level of 5% of DR on all sites. The best MAP is indicated by a labeled arrow for each tested ear (C: control map with no level increase; 5: 5% DR; 10: 7 10% DR; all: 5% DR on all sites). Lower panel: SRT improvement relative to the control MAP for the three MAPs that involved level adjustments. For the group data in both panels, the MAP that produced significantly better (lower) mean SRT over the control MAP (p < 0.05) is indicated by an asterisk.
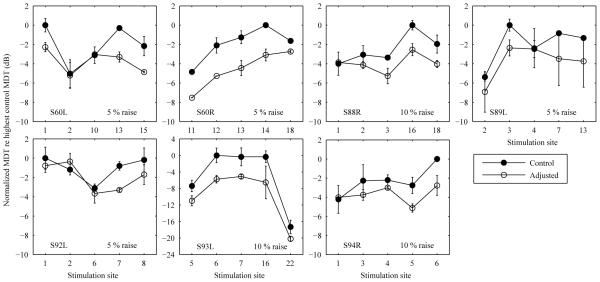
Figure 4.
Modulation detection thresholds before and after level adjustments measured for 7 ears. Data were normalized against the highest un-adjusted MDT. Mean modulation detection thresholds measured before and after the level adjustments were applied to the 5 worst sites (indicated by x-axis labels) at 20% DR. The magnitude of level adjustment evaluated for each ear (indicated in the right lower corner of each panel) was the percentage DR that produced the best SRT performance. Error bars represent range of the data.
6. Comparison of site rehabilitation versus site removal
The site-selection strategy used in Garadat et al. (Reference note 1) followed a rule that distributed the sites for removal across the tonotopic axis to avoid frequency-place distortion. The distribution rule often misses the poorest sites if they are clustered. This is one of the limitations that motivated a site rehabilitation strategy. In the present study, we also demonstrate the effects of violating the distribution rule described in Garadat et al. by removing the same sites chosen for rehabilitation, often clustered, and reallocating frequencies. For each of the 7 ears that showed the greatest benefit from site-specific level adjustment on the poor sites, an open MAP that excluded these sites was created and evaluated for SRTs following the procedure described above in Materials and Methods Section 4. Subjects’ SRTs for the MAP with removed sites were compared to those for the control MAP with no level manipulation, and the two MAPs with site-specific level adjustments.
Results
1. Across-site patterns and stability of MDTs
Figure 1 shows the across-site patterns of MDTs measured for all ears at the time of the experiments and for 6 ears averaging 1.4 years prior to the experiments. The across-site patterns of MDTs are unique for each ear.
The across-site patterns of MDTs were stable over time in most of the 6 cases where two measurements were obtained. Large fluctuations of MDTs over time were observed in limited instances such as S88L in the basal and apical ends. ANOVA revealed that, overall, the more recent mean MDTs were not significantly different from the older measurements in the six ears [F (1, 5) = 0.71, p = 0.43]. Absolute MDT change across sites and ears was 2.31 ± 1.40 dB.
The 5 sites with the poorest MDTs for each ear are indicated by arrows in Figure 1. These sites are based on the more recent data. Subjects reported that loudness was not even across the array at 50% DR except for one ear (S83 L) where all sites were perceived to be equally loud. The loudness dips identified in the ears did not always correspond to the 5 identified sites with the poorest MDTs.
2. Effect of level adjustment on SRTs
SRTs measured with and without level adjustments are shown in Figure 3 (upper panel). Note that lower values indicate better performance (sentence recognition at lower SNRs). All tested ears, except for S88L, showed improved SRT using one or more level-adjusted MAPs. The MAP that resulted in the best SRT for each ear is indicated by a labeled arrow. The improvements in SRTs for the various MAPs relative to the control MAP that did not involve level adjustment are shown in the lower panel of Figure 4. Positive dB values indicate improved tolerance of noise while negative values indicate decreased performance in noise, relative to performance with the control MAP.
Four ears achieved the lowest (best) SRT with a 5% increase on the selected sites, and a larger increase on these sites either did not provide further improvement and in one case (S92L) had a negative effect on performance showing a worsened SRT relative to the control MAP. Three ears showed the best SRT with a 10% increase on the selected sites. One of the tested ears (S83L) showed comparable SRT improvement with a 5% and a 10% increase, but performance improved further although in a small magnitude when the minimum stimulation levels on all electrodes were boosted by 5% DR.
Improvement in signal to noise ratio using the MAP that produced the best SRT relative to that using the control MAP was on average 3.33 dB (± 2.64 dB). Mean SRT improvements for the 5%, 10% site-specific adjustment and a 5% adjustment on all electrodes were 2.36, 1.85 and 0.73 dB, respectively, relative to the control MAP. The site-specific 5% increase significantly improved group mean SRT [t (8) = 3.76, p < 0.0167, Bonferroni corrected], but the other two level-adjusted MAPs did not produce a statistically significant improvement.
3. Effect of level adjustment on MDTs
The effects of level adjustment on modulation sensitivity at 20% of DR are shown in Figure 4 for 7 ears. The magnitudes of the level adjustment were those that produced the best SRT for each ear as indicated by text in each panel of Figure 4. For clarity of presentation and comparison, MDTs were normalized against the worst (highest) MDT measured at un-adjusted levels. Results show that MDTs improved at the adjusted levels for most poor sites. Averaging across sites, MDT improvement ranged from 0.90 to 4.63 dB among the 7 ears with a mean of 2.07 dB. The improvement in MDTs was significantly different than zero for each ear tested (all p < 0.05).
4. Comparison of site rehabilitation versus site removal
Figure 5 shows the effects of modifying the minimal stimulation levels on the 5 selected sites (data taken from Fig. 4) in comparison with the effects of removing these sites from the processor in the 7 ears. None of the 7 tested ears benefited from removal of the 5 worst sites. With these sites turned off, SRTs were elevated relative to the control MAP without level adjustment and those with site-specific level adjustments. Repeated-measures T tests show that SRTs after site removal was significantly poorer than those tested with the control MAP [t (6) = 5.07, p < 0.0167, Bonferroni corrected], the MAP with 5% level raise [t (6) = 5.72, p < 0.0167, Bonferroni corrected], and the MAP with 10% level raise [t (6) = 6.41, p < 0.0167, Bonferroni corrected].
Figure 5.
Speech reception thresholds. SRTs measured for the control MAP (no level adjustment), the two site-specific level-adjusted MAPs, and the MAP that removed the 5 sites without the poorest MDTs. The best MAP is indicated by a labeled arrow for each tested ear.
5. Correlation between MDTs and speech recognition
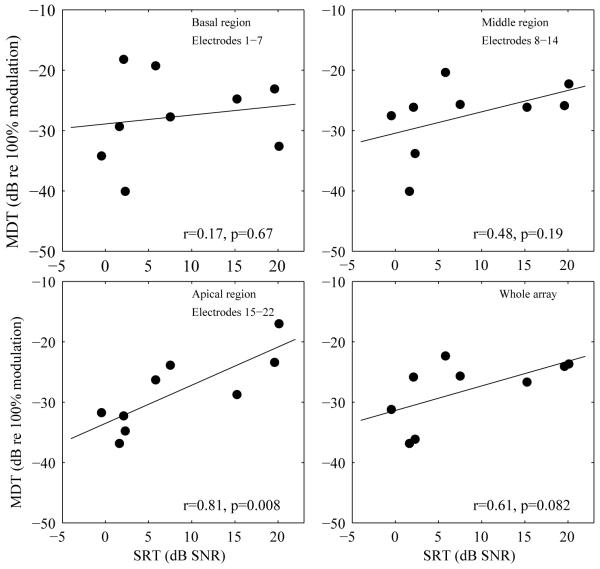
The relationship between speech recognition and MDTs at specific tonotopic regions was examined across ears. MDTs were averaged from the basal region (sites 1-7), the middle region (sites 8-14), the apical region (sites 15-22), and the whole electrode array. Corner frequencies assigned to the basal, middle and apical regions were 7938-3063 Hz, 3063-1188 Hz, and 1188-188 Hz, respectively. Correlational analysis was conducted for the averaged MDTs from each region and from the whole array with the subject’s SRTs using the control MAP that did not involve level adjustment. Correlations between SRTs and the ASM MDTs are shown in Figure 6. A significant correlation between MDT averaged from the apical region and SRTs was found (r = 0.81, p < 0.0125, Bonferroni corrected). There was no significant correlation between the averaged MDTs for the whole array and SRTs (r = 0.61, p > 0.0125, Bonferroni corrected).
Figure 6.
Correlations between modulation detection thresholds (MDTs) and speech reception thresholds (SRTs). MDTs averaged from three tonotopic locations (electrodes covered in each region are specified in the corresponding panels) and from the whole array were plotted against the subjects’ SRTs. Correlation coefficients and p values are shown in each panel. The lines represent linear fits to the data.
Discussion
The present study investigated the effects of modifying the minimal stimulation levels on improving MDTs at stimulation sites with poor modulation sensitivity and on subjects’ speech reception thresholds. The results were consistent with the hypothesis that modulation sensitivity can be improved by site-specific increases in current level and the across-site mean improvement in modulation sensitivity benefits speech recognition.
1. Stability of MDTs
Identification of the poor sites for rehabilitation or removal was based on modulation sensitivity. It is therefore important to examine if this sensitivity remains stable over time. Our results showed that the across-site patterns of MDTs were rather stable over an average time of 1.4 years for most ears and therefore can be used as the basis for optimization. One of the tested ears (S88L) did show substantial MDT changes over 1.3 years particularly at the basal and the apical regions (Fig. 1). In contrast, this bilaterally implanted listener’s right ear (S88R) showed minimal changes in MDTs. The difference between the two ears in the same listener suggests that the functional changes over time did not originate in the central auditory system. The changes rather seem to result from altered conditions in the auditory periphery.
2. The effects of level adjustments on MDTs and SRTs
Level adjustments of at least one kind that involve either artificial raises of the T levels on specific sites or on all sites resulted in improved SRTs for all ears with one exception, i.e., S88L (Fig. 4). This ear also showed the largest changes in MDTs over time. The five electrodes in that ear identified as requiring level adjustment based on the latest MDTs therefore may not be the true sub-optimal sites due to test-retest variance. For 7 of the 8 ears that benefited from artificial raises of Ts, the best SRT was achieved with level adjustments applied to the 5 sites with the poorest MDTs. Results further suggest that in these 7 ears, improvements in SRTs can be attributed to significantly improved modulation sensitivity at the lower end of the level-adjusted DRs (Fig. 5).
The site-specific level adjustment procedure was tested with two magnitudes, i.e., 5% and 10% of the DR. The 5% increase almost always produced benefit, reflected in an improved group-mean SRT that was statistically significant. For four ears, a 5% level increase produced the best performance and a larger increase created weaker or negative effects. For other cases, the best performance was not seen until a 10% increase was applied (Fig. 4). Improvement in MDTs with a given magnitude of level increase could be subject to individual variation in MDT versus level functions (Fu, 2002; 2005; Pfingst et al., 2008). From Figure 4, it can be seen that within ears, MDTs did not improve equally for the 5 stimulation sites even though the same magnitude of level adjustments in percent of DR was applied to these sites. Perhaps for the same reason, the improvement in MDTs with a 10% level increase in one ear was not necessarily larger than that with a 5% level increase in another ear. Further, even with the same amount of MDT improvement, speech recognition would not necessarily improve to the same extent across ears because subjects differ in their ability to use the improved temporal cues in speech recognition. These considerations might explain the variation in the increment size required to produce the ears’ best performance.
It should be noted that the raise of the minimum stimulation level could cause factors other than modulation detection to vary. For example, it could alter channel interaction on the adjusted electrodes (McKay et al., 1999; Pfingst et al., 1999). It could possibly explain the lack of benefit or negative effects associated with the 10% increase or 5% increase on all sites in some ears (Fig. 4). The weaker effect of modifying levels on all sites could also be due to the fact that it did not reduce across-site variation whereas site-specific rehabilitation did. In addition, after level adjustment, the soft acoustic sounds were mapped to higher points on the electrical DR, thus becoming more audible, compensating for the slow loudness growth at low current levels (Shannon, 1985). It is unlikely though that the poor MDTs were just a result of poor loudness growth. As described in the Results section, the 5 selected poor sites were not necessarily those that sounded softer than the rest of the array. Increasing levels on these sites, that did not need compensation for loudness, still improved speech recognition. Moreover, the lack of benefits of modifying levels on all stimulation sites, which probably compensated for audibility to the greatest extent, also supports the notion that the increased loudness was not the cause for improved speech recognition. A previous study that used a similar approach but a larger level increase (15 clinical units/ 1.9 dB per electrode) reported that the method was only effective when CNC words and CUNY sentences were tested at soft levels for listeners programmed with the SPEAK strategy (Skinner et al., 1999). Since the increase of the minimal stimulation levels primarily modifies the lower portion of the DR, it would be interesting to test in future studies if soft level phonemic features (e.g., manner of labial-dental non-sibilant sounds or periodicity in voiced fricatives) are improved in particular and if there is an advantage of raising stimulation levels at the upper levels of the DR.
3. Advantages of site rehabilitation over site removal
Rehabilitation for poor stimulation sites described in the present study was compared to removing these sites. However, site removal in these cases, where the removed sites were often clustered in one or two tonotopic regions, was detrimental for all ears tested, producing elevated SRTs relative to not only the MAPs with level adjustment but also the control MAP (Fig. 6). Comparisons with findings from Garadat et al. (Reference note 1) where site removal has been shown to be effective is not valid because in that study site removal followed a rule of distribution. In contrast, in the present study, the 5 poorest among all sites along the array were turned off regardless of their tonotopic distribution. When these sites were bundled, as they were in most cases, site removal could cause large frequency-place distortions that probably offset the benefit of increased psychophysical acuity. Scattering sites for removal as described in Garadat and colleagues however would miss the poorest sites. The site-rehabilitation approach described in the present study thus has a potential advantage over a site-selection strategy in that it is less limited by the location of the sites selected for rehabilitation.
4. Relationship between MDTs and speech recognition
Across ears, the strength of correlation between averaged MDTs and speech recognition in noise appeared to be weaker in the 9 ears tested compared to those reported previously (e.g., Fu, 2002). The strength of correlation between the regional MDTs and speech recognition increased from base to apex (Fig. 6), with the only statistically significant correlation seen for the apical region. Given the large variability in results typically found across subjects with cochlear implants, a larger sample is recommended to examine if modulation sensitivity at the low frequency region is more important for speech recognition than sensitivity at other regions. The weaker relationship compared to findings from previous studies might be due to differences in the extent to which the tested speech materials require temporal coding and/or the extent to which they involve central processes such as the subjects’ ability to use contextual cues. Findings from a previous study (Garadat et al., 2012) that used a within-subject design clearly demonstrated that modulation sensitivity does contribute to speech recognition with cochlear implants. Garadat and colleagues showed that for the same implant listener, speech recognition using 10 stimulation sites from the electrode array with the best modulation sensitivity was superior to that using the 10 sites with the poorest modulation sensitivity. Findings from the present study showing that improved ASM MDTs contribute to improved speech recognition in noise is also consistent with the notion that modulation sensitivity is important for speech recognition in electrical hearing.
5. Clinical implications
Site rehabilitation has important clinical implications. Results of the present study suggest that for ears that have one or two large tonotopic regions with poor modulation sensitivity, site rehabilitation could be a more advantageous method than site removal. The parsimonious and less time-consuming approach of applying a global level raise on all electrodes was not effective compared to the site-specific level adjustment approach. In terms of magnitude, a 5% DR increase of the minimal stimulation level appears to be an effective and safe size to apply without risking detrimental effects. Future studies are warranted to examine the long-term effects of the site-rehabilitation method and the effects of training.
The site-specific level adjustments as used here resulted in a few dB improvement in SNR at which subjects could understand sentences, i.e., SRTs. The SRT improvement shown in Figure 3B are relatively small compared to the across-subject differences in SRTs shown in Figure 3A. Nevertheless, benefits of the changes were noticeable by the subjects and some subjects have spontaneously asked to take the experimental processor MAPs for their everyday use.
Short summary.
Based on previous findings that MDT improves with stimulus level and the hypothesis that modulation sensitivity contributes to speech recognition, the present study presents a site-rehabilitation strategy that increases the minimum stimulation level from true threshold on sites with the poorest MDTs. The modification of the stimulation levels improved modulation sensitivity at the lower end of the dynamic range on the poorly-performing sites. The site-specific level manipulation with a magnitude of 5% of the dynamic range significantly improved the subjects’ speech reception thresholds relative to the control condition without level manipulation.
Acknowledgements
We thank the dedicated cochlear implant subjects who participated in this research and Jennifer Benson and Soha Garadat for their assistance with data collection. The work was supported by NIH-NIDCD grants R01 DC010786, T32 DC00011, and P30 DC05188.
Conflicts of Interest and Source of Funding: Work is supported by NIH-NIDCD grants R01 DC010786, T32 DC00011, and P30 DC05188.
Footnotes
“MAP” refers to the specific stimulation parameters including electrodes (sites) to be stimulated and their allocated frequencies, stimulation rate, and level settings etc.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bierer JA. Threshold and channel interaction in cochlear implant users: evaluation of the tripolar electrode configuration. J Acoust Soc Am. 2007;121:1642–1653. doi: 10.1121/1.2436712. [DOI] [PubMed] [Google Scholar]
- Bierer JA, Faulkner KF. Identifying cochlear implant channels with poor electrode-neuron interface: partial tripolar, single-channel thresholds and psychophysical tuning curves. Ear Hear. 2010;31:247–258. doi: 10.1097/AUD.0b013e3181c7daf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothroyd A, Hanin L, Hnath T. A Sentence Test of Speech Perception: Reliability, Set Equivalence, and Short Term Learning. Speech and Hearing Sciences Research Center, City University of New York City; New York: 1985. [Google Scholar]
- Busby PA, Tong YC, Clark GM. The perception of temporal modulations by cochlear implant patients. J Acoust Soc Am. 1993;94:124–131. doi: 10.1121/1.408212. [DOI] [PubMed] [Google Scholar]
- Cazals Y, Pelizzone M, Saudan O, et al. Low-pass filtering in amplitude modulation detection associated with vowel and consonant identification in subjects with cochlear implants. J Acoust Soc Am. 1994;96:2048–2054. doi: 10.1121/1.410146. [DOI] [PubMed] [Google Scholar]
- Chikar JA, Colesa DJ, Swiderski DL, et al. Over-expression of BDNF by adenovirus with concurrent electrical stimulation improves cochlear implant thresholds and survival of auditory neurons. Hear Res. 2008;245:24–34. doi: 10.1016/j.heares.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colletti V, Shannon RV. Open set speech perception with auditory brainstem implant? Laryngoscope. 2005;115:1974–1978. doi: 10.1097/01.mlg.0000178327.42926.ec. [DOI] [PubMed] [Google Scholar]
- Donaldson GS, Nelson DA. Place-pitch sensitivity and its relation to consonant recognition by cochlear implant listeners using the MPEAK and SPEAK speech processing strategies. J Acoust Soc Am. 2000;107:1645–1658. doi: 10.1121/1.428449. [DOI] [PubMed] [Google Scholar]
- Fu Q-J. Temporal processing and speech recognition in cochlear implant users. Neuroreport. 2002;13:1635–1639. doi: 10.1097/00001756-200209160-00013. [DOI] [PubMed] [Google Scholar]
- Fu Q-J. Loudness growth in cochlear implants: effect of stimulation rate and electrode configuration. Hear Res. 2005;202:55–62. doi: 10.1016/j.heares.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Galvin JJ, III., Fu Q-J. Effects of stimulation rate, mode and level on modulation detection by cochlear implant users. J Assoc Res Otolaryngol. 2005;6:269–279. doi: 10.1007/s10162-005-0007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin JJ, III., Fu Q-J. Influence of stimulation rate and loudness growth on modulation detection and intensity discrimination in cochlear implant users. Hear Res. 2009;250:46–54. doi: 10.1016/j.heares.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garadat SN, Zwolan TA, Pfingst BE. Across-site patterns of modulation detection: Relation to speech recognition. J Acoust Soc Am. 2012;131:4030–4041. doi: 10.1121/1.3701879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SY, Colesa DJ, Swiderski DL, et al. Effects of hearing preservation on psychophysical responses to cochlear implant stimulation. J Assoc Res Otolaryngol. 2010;11:245–265. doi: 10.1007/s10162-009-0194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. J Acoust Soc Am. 1971;49:467–477. [PubMed] [Google Scholar]
- Luo X, Fu Q-J, Wei CG, et al. Speech recognition and temporal amplitude modulation processing by Mandarin-speaking cochlear implant users. Ear Hear. 2008;29:957–970. doi: 10.1097/AUD.0b013e3181888f61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay CM, O’Brien A, James CJ. Effect of current level on electrode discrimination in electrical stimulation. Hear Res. 1999;136:159–164. doi: 10.1016/s0378-5955(99)00121-5. [DOI] [PubMed] [Google Scholar]
- Pfingst BE, Burkholder-Juhasz RA, Xu L, et al. Across-site patterns of modulation detection in listeners with cochlear implants. J Acoust Soc Am. 2008;123:1054–1062. doi: 10.1121/1.2828051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst BE, Colesa DJ, Hembrador S, et al. Detection of pulse trains in the electrically stimulated cochlea: effects of cochlear health. J Acoust Soc Am. 2011b;130:3954–3968. doi: 10.1121/1.3651820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst BE, Holloway LA, Zwolan TA, et al. Effects of stimulus level on electrode-place discrimination in human subjects with cochlear implants. Hear Res. 1999;134:105–115. doi: 10.1016/s0378-5955(99)00079-9. [DOI] [PubMed] [Google Scholar]
- Pfingst BE, Sutton D, Miller JM, et al. Relation of psychophysical data to histopathology in monkeys with cochlear implants. Acta Otolaryngol. 1981;92:1–13. doi: 10.3109/00016488109133232. [DOI] [PubMed] [Google Scholar]
- Pfingst BE, Xu L. Psychophysical metrics and speech recognition in cochlear implant users. Audiol Neurotol. 2005;10:331–341. doi: 10.1159/000087350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst BE, Xu L, Thompson CS. Across-site threshold variation in cochlear implants: relation to speech recognition. Audiol Neurotol. 2004;9:341–352. doi: 10.1159/000081283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst BE, Xu L, Thompson CS. Effects of carrier pulse rate on modulation detection in subjects with cochlear implants. J Acoust Soc Am. 2007;121:2236–2246. doi: 10.1121/1.2537501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon RV. Threshold and loudness functions for pulsatile stimulation of cochlear implants. Hear Res. 1985;18:135–143. doi: 10.1016/0378-5955(85)90005-x. [DOI] [PubMed] [Google Scholar]
- Shannon RV. Temporal modulation transfer functions in patients with cochlear implants. J Acoust Soc Am. 1992;91:2156–2164. doi: 10.1121/1.403807. [DOI] [PubMed] [Google Scholar]
- Shannon RV, Galvin JJ, III, Baskent D. Holes in hearing. J Assoc Res Otolaryngol. 2002;3:185–199. doi: 10.1007/s101620020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon RV, Zeng F-G, Wygonski J. Speech recognition with altered spectral distribution of envelope cues. J Acoust Soc Am. 1998;104:2467–2476. doi: 10.1121/1.423774. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Hatsushika S, Clark GM. Electrical stimulation of the auditory nerve: the effect of electrode position on neural excitation. Hear Res. 1993;66:108–120. doi: 10.1016/0378-5955(93)90265-3. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Javel E. Electrical stimulation of the auditory nerve. I. Correlation of physiological responses with cochlear status. Hear Res. 1997;108:112–144. doi: 10.1016/s0378-5955(97)00046-4. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Roberts LA, Paolini AG. Long-term sensorineural hearing loss induces functional changes in the rat auditory nerve. Eur J Neurosci. 2004;20:3131–3140. doi: 10.1111/j.1460-9568.2004.03809.x. [DOI] [PubMed] [Google Scholar]
- Skinner MW, Holden LK, Holden TA, et al. Comparison of two methods for selecting minimum stimulation levels used in programming the Nucleus 22 cochlear implant. J Speech Lang Hear Res. 1999;42:814–828. doi: 10.1044/jslhr.4204.814. [DOI] [PubMed] [Google Scholar]
- Wilson BS, Finley CC, Lawson DT, et al. Design and evaluation of a continuous interleaved sampling (CIS) processing strategy for multichannel cochlear implants. J Rehabil Res Dev. 1993;30:110–116. [PubMed] [Google Scholar]
- Wilson BS, Lawson DT, Zerbi M, et al. New processing strategies in cochlear implantation. Am J Otol. 1995;16:669–675. [PubMed] [Google Scholar]
- Zhou N, Xu L, Pfingst BE. Characteristics of detection thresholds and maximum comfortable loudness levels as a function of pulse rate in human cochlear implant users. Hear Res. 2011;284:25–32. doi: 10.1016/j.heares.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N, Pfingst BE. Psychophysically-based site selection coupled with dichotic stimulation improves speech recognition in noise with bilateral cochlear implants. J Acoust Soc Am. 2012;132:994–1008. doi: 10.1121/1.4730907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwolan TA, Collins LM, Wakefield GH. Electrode discrimination and speech recognition in postlingually deafened adult cochlear implant subjects. J Acoust Soc Am. 1997;102:3673–3685. doi: 10.1121/1.420401. [DOI] [PubMed] [Google Scholar]
Reference note
- 1.Garadat SN, Zwolan TA, Pfingst BE. Speech recognition in cochlear implant users: selection of sites of stimulation based on temporal modulation sensitivity. CIAP meeting abs. 2011:12. [Google Scholar]