Abstract
Background
No prior studies have investigated the association of QRS-T angle with cardiac structure/function and outcomes in heart failure with preserved ejection fraction (HFpEF). We hypothesized that increased frontal QRS-T angle is associated with worse cardiac function/remodeling and adverse outcomes in HFpEF.
Methods
We prospectively studied 376 patients with HFpEF (i.e. symptomatic HF with left ventricular [LV] ejection fraction >50%.) The frontal QRS-T angle was calculated from the 12-lead electrocardiogram. Patients were divided into tertiles by frontal QRS-T angle (0–26°, 27–75°, and 76–179°), and clinical, laboratory, and echocardiographic data were compared among groups. Cox proportional hazards analyses were performed to determine the association between QRS-T angle and outcomes.
Results
The mean age of the cohort was 64±13 years, 65% were women, and the mean QRS-T angle was 61±51°. Patients with increased QRS-T angle were older, had a lower body-mass index, more frequently had coronary artery disease, diabetes, chronic kidney disease, and atrial fibrillation, and had higher B-type natriuretic peptide (BNP) levels (P<0.05 for all comparisons). After multivariable adjustment, patients with increased QRS-T angle had higher BNP levels in addition to higher LV mass index, worse diastolic function parameters, more right ventricular (RV) remodeling, and worse RV systolic function (P<0.05 for all associations). QRS-T angle was independently associated with the composite outcome of cardiovascular hospitalization or death on multivariable analysis, even after adjusting for BNP (HR for the highest QRS-T tertile = 2.0, 95% CI 1.2–3.4; P=0.008).
Conclusions
In HFpEF, increased QRS-T angle is independently associated with worse left and right ventricular function/remodeling and adverse outcomes.
Keywords: QRS-T angle, diastolic heart failure, echocardiography, outcomes
INTRODUCTION
Heart failure with preserved ejection fraction (HFpEF) is associated with high morbidity and mortality similar to HF with reduced EF, and 5-year mortality rates approach a dismal 70% after hospitalization for HF [1, 2]. Abnormalities in depolarization and repolarization are common in patients with HFpEF [3], yet our understanding of their significance is limited. Identification of novel prognostic markers may detect at-risk patients early and provide new insight into therapeutic avenues in HFpEF.
The frontal QRS-T angle, a measure easily derived from the standard 12-lead electrocardiogram (ECG) that approximates the angle between the vectors of depolarization and repolarization, has prognostic utility in general and in certain clinical populations [4–8]. Abnormalities in the frontal QRS-T angle may signal electrical instability, placing patients at higher risk for malignant ventricular arrhythmias and sudden cardiac death. In addition, in diabetic patients free of cardiovascular disease, increased QRS-T angle was found to be independently associated with worse left ventricular (LV) myocardial performance index [9]. Thus, the relationship between QRS-T angle and adverse outcomes may also be related to structural and functional myocardial abnormalities.
Although an increased frontal QRS-T angle predicts mortality in HF with reduced EF [5], no prior studies have investigated the echocardiographic correlates and outcomes associated with increased frontal QRS-T angle in HFpEF. Prior studies have implicated ventricular scar or ischemia as factors that may cause an imbalance of the regulation of electrical activation and recovery of the ventricles. When there is an imbalance of electrical activation and recovery (i.e., heterogeneity/discordance of ventricular depolarization and repolarization), the QRS and T wave angles are no longer aligned and the QRS-T angle widens [10]. In patients with HFpEF, besides focal ventricular scar and overt myocardial ischemia (due to epicardial coronary artery disease), pathologic abnormalities such as ventricular hypertrophy, diffuse myocardial fibrosis, and subendocardial ischemia could all be factors which increase the discordance of ventricular depolarization and repolarization. Thus, besides relating QRS-T angle to adverse outcomes in HFpEF, we also sought to determine the association between QRS-T angle and echocardiographic markers of LV and RV structure and function.
We hypothesized that increased frontal QRS-T angle is independently associated with worse left and right ventricular function and greater left and right ventricular remodeling in HFpEF. Furthermore, we hypothesized that increased frontal QRS-T angle is associated with worse outcomes, including HF hospitalization, cardiovascular hospitalization, and all-cause mortality. We therefore conducted a prospective study of frontal QRS-T angle in HFpEF.
METHODS
Study population
Consecutive patients were prospectively recruited from the outpatient clinic of the Northwestern University HFpEF Program between March 2008 and January 2011 as part of a systematic observational study of HFpEF (ClinicalTrials.gov identifier #NCT01030991). Patients were initially identified by an automated daily query of the inpatient electronic medical record at Northwestern Memorial Hospital using the following search criteria: (1) diagnosis of HF or the words — heart failure in the hospital notes; or (2) BNP >100 pg/ml; or (3) administration of 2 or more doses of intravenous diuretics. The list of patients generated was screened daily, and only those patients who had LV ejection fraction (EF) > 50% and who meet Framingham criteria for HF [11] were offered post-discharge follow-up in a specialized HFpEF outpatient program. Once evaluated as an outpatient in the HFpEF clinic, the diagnosis of HF was confirmed by a cardiologist who specializes in HF. The diagnosis of HFpEF was based on previously published criteria [12, 13] which requires both an LVEF > 50% and an LV end-diastolic volume index < 97 ml/m2. In line with a large population-based study in HFpEF [1], patients with hemodynamically significant valvular disease (defined as greater than moderate in severity), prior cardiac transplantation, prior history of overt LV systolic dysfunction (LVEF < 40%), or a diagnosis of constrictive pericarditis were not recruited into the study. Patients were excluded in the present study if they had ventricular paced rhythms [6].
In the Northwestern HFpEF Program, all study procedures (including laboratory testing, ECG, and echocardiography) are performed in the outpatient setting. All study participants gave written, informed consent, and the institutional review board at Northwestern University approved the study.
Clinical characteristics
We collected and analyzed demographics and clinical data (including co-morbidities and medications) in all study subjects. We also documented New York Heart Association (NYHA) functional class and several laboratory parameters, including B-type natriuretic peptide (BNP). Estimated glomerular filtration rate (eGFR) was calculated using the Modified Diet in Renal Disease equation.
Electrocardiography
All subjects underwent 12-lead ECG testing (Marquette MAC 5000 Resting ECG System, GE Healthcare, Boston, MA). ECGs were analyzed by a single, trained reader blinded to all other data, including echocardiographic data and outcomes. We measured the PR interval, QRS duration, QT interval, QRS axis, and T-wave axis according to published guidelines [14]. The corrected QT interval was calculated using Bazett’s formula (QTc). We verified computer-generated axes from the ECG report by manual over-read. T-wave inversion was defined by a negative T-wave ≥ 1 mm in amplitude in two or more contiguous leads [15]. The frontal QRS-T angle was calculated as the smallest angle between the frontal plane QRS and T-wave axes (QRS-T angle = |QRS axis − T wave axis|; if |QRS-T angle| was > 180°, the complimentary angle [i.e. 180° − angle] was used) [6].
Echocardiography
All study participants underwent comprehensive 2-dimensional echocardiography with Doppler and tissue Doppler imaging. All standard echocardiographic views were obtained using commercially available ultrasound systems with harmonic imaging (Philips iE33 or 7500, Philips Medical Systems, Andover, MA; or Vivid 7, GE Healthcare, General Electric Corp., Waukesha, WI). Cardiac structure and function (including LV systolic and diastolic function and right ventricular [RV] size and function) were quantified as recommended by the American Society of Echocardiography (ASE) [16–18].
LV end-diastolic and end-systolic volumes, and left atrial volume, were measured in the apical 4- and 2-chamber views using the biplane method of discs. LV ejection fraction was calculated as (LV end-diastolic volume – LV end-systolic volume)/LV end-diastolic volume. LV mass index was calculated using the linear method, as outline in ASE guidelines.
LV diastolic function was graded according to published criteria [19] by using mitral inflow characteristics and tissue Doppler e’ velocities. Tissue Doppler e’ and s’ velocities were measured at the septal and lateral aspects of the mitral annulus and were averaged. Sample volume size and placement were optimized for all pulse-wave Doppler and tissue Doppler measurements. All Doppler and tissue Doppler measurements were averaged over 3 beats (5 beats for patients in atrial fibrillation).
Right heart parameters were measured on echocardiography according to published guidelines [17]. Specifically, we measured RV basal diameter, RV length, RV end-diastolic area, RV end-systolic area, RV wall thickness, and right atrial area. Tricuspid annular plane systolic excursion (TAPSE) was also calculated. Lastly, pulmonary artery systolic pressure was measured using the peak tricuspid regurgitation (TR) velocity (to estimate peak TR gradient) and adding that to the estimated right atrial pressure, which was based on size and collapsibility of the inferior vena cava.
All cardiac structural measurements, including right heart parameters, were indexed to body surface area. All echocardiographic measurements were made blinded to all other data by an experienced research sonographer using ProSolv 4.0 echocardiographic analysis software (ProSolv CardioVascular; Indianapolis, IN) and verified by an experienced investigator with expertise in echocardiography.
Outcome variables
All study participants were evaluated clinically after enrollment into the study as clinically indicated but no less frequently than every 6 months. At each clinic visit, inter-current hospitalizations were documented. For each reported hospitalization, chart review was performed to categorize the etiology of hospitalization as HF, cardiovascular (including HF), or non-cardiovascular. Every 6 months, participants (or their proxy) were also contacted to determine vital status. Finally, the Social Security Death Index was queried for additional verification of vital status. Enrollment date was defined as the date of first visit to the outpatient HFpEF clinic, and date of last follow-up was defined as date of death or date of last HFpEF clinic visit. Follow-up was complete in all patients.
Statistical analysis
For descriptive purposes, we divided participants into tertiles based on frontal QRS-T angle (0–26°, 27–75°, and 76–179°). Clinical characteristics, laboratory data, and echocardiographic parameters were compared between groups with one-way analysis of variance (or Kruskal-Wallis test when appropriate). Chi-squared tests (or Fisher’s exact test when appropriate) were used to compare categorical variables between groups. A two-sided p-value < 0.05 was considered statistically significant. Continuous data with a normal distribution were displayed as mean ± standard deviation. Right-skewed data were displayed as median and interquartile range.
Next, unadjusted and multivariable-adjusted linear regression analyses were performed to determine if frontal QRS-T angle was independently associated with BNP and echocardiographic markers of LV and RV remodeling (LV mass index and RV wall thickness, respectively), LV and RV systolic function (tissue Doppler s’ velocity and TAPSE, respectively), and LV diastolic function (tissue Doppler e’ velocity, E/e’ ratio, and isovolumic relaxation time). For all linear regression analyses, residuals were analyzed to confirm normality/linearity. β-coefficients are reported per 1-standard deviation increase in QRS-T angle. Echocardiographic parameters were further adjusted for BNP.
For analysis of the association between QRS-T angle and outcomes (including HF hospitalization, cardiovascular hospitalization, mortality, and a composite of these outcomes), we conducted unadjusted and multivariable-adjusted Cox proportional-hazards regression analyses. For all Cox regression models, the proportional hazard assumption was tested by visual inspection of Schoenfeld residual plots. The referent group in all Cox models was the lowest frontal QRS-T angle tertile. Kaplan-Meier curves for the composite outcome were also plotted and compared using the log-rank test. In addition, to compare the association of QRS-T angle and outcomes to QRS axis and T-wave axis alone, we constructed locally-weighted, smoothed scatterplots (LOWESS) graphs.
Candidate covariates were selected for inclusion into the multivariable models based on a combination of clinical relevance (pre-specified based on face validity) and association with QRS-T angle (either in prior studies or in our study). All multivariable models were adjusted for age, sex, body-mass index, diabetes mellitus, coronary artery disease, atrial fibrillation, eGFR, amiodarone use, diuretic use, QRS interval, QTc interval, and BNP. For Cox regression analyses, we additionally adjusted for selected echocardiographic parameters, including tissue Doppler s’ velocity, E/e’ ratio, TAPSE, LV mass, and RV wall thickness.
On sensitivity analyses, we additionally adjusted for specific types of prolonged QRS duration (i.e., left bundle branch block, right bundle branch block, and intraventricular conduction delay) and the presence of T-wave inversions to determine whether the association between QRS-T angle and outcomes was simply due to the presence of bundle branch block or abnormal repolarization pattern (i.e., T-wave inversion) causing increased QRS-T angle. We also repeated our regression analyses after excluding patients with QRS duration > 120 ms to determine whether our findings were confounded by the presence of prolonged QRS duration. All model covariates were examined for collinearity, and all statistical analyses were performed using Stata v.10.1 (StataCorp, College Station, TX).
RESULTS
Characteristics of study participants
From an initial cohort of 401 HFpEF patients, 25 were excluded for ventricular paced rhythms, leaving 376 eligible participants. Table 1 displays the demographic and clinical characteristics of the study participants, who were elderly (but somewhat younger compared to prior epidemiologic and observational studies of HFpEF [1, 2]) and predominantly female. Nearly half of all participants had NYHA functional class III or IV symptoms, and comorbidities were common. ECG analysis demonstrated a mean frontal QRS-T angle of 61±51°.
Table 1.
Clinical and Laboratory Characteristics by QRS-T Angle Tertile
| Characteristic | Tertile 1 | Tertile 2 | Tertile 3 | P-value |
|---|---|---|---|---|
| 0–26° (N=124) | 27–75° (N=125) | 76–179° (N=127) | ||
| QRS-T Angle (°) | 13±7 | 47 ±14 | 123±31 | |
|
| ||||
| Age, y | 62±11 | 66±12 | 64±14 | 0.046 |
| Female, n (%) | 86(69) | 79(63) | 78(61) | 0.39 |
| Ethnicity, n (%) | 0.87 | |||
| • White | 65(52) | 61(49) | 61(48) | |
| • Black | 48(39) | 54(43) | 52(41) | |
| • Other | 11(9) | 10(8) | 14(11) | |
| Body-mass index, kg/m2 | 33.8±9.1 | 34.0±10.1 | 30.5±9.0 | 0.005 |
| NYHA functional class, n (%) | 0.12 | |||
| • I | 13(11) | 20(16) | 10(8) | |
| • II | 56(45) | 48(38) | 46(36) | |
| • III or IV | 55(44) | 57(46) | 71(56) | |
| Comorbidities, n (%) | ||||
| • Coronary artery disease | 40(32) | 37(30) | 54(43) | 0.08 |
| • Hypertension | 92(74) | 100(80) | 99(78) | 0.54 |
| • Hyperlipidemia | 75(60) | 63(50) | 65(51) | 0.21 |
| • Diabetes mellitus | 32(26) | 46(37) | 51(40) | 0.044 |
| • Chronic kidney disease | 47(38) | 74(59) | 73(57) | 0.001 |
| • Atrial fibrillation | 18(15) | 30(24) | 40(32) | 0.006 |
| • Obesity | 75(60) | 72(58) | 56(44) | 0.021 |
| • COPD or asthma | 50(40) | 47(38) | 46(36) | 0.79 |
| • Obstructive sleep apnea | 48(39) | 48(38) | 44(35) | 0.76 |
| Medications, n (%) | ||||
| • ACE-inhibitor or ARB | 64(52) | 68(54) | 71(56) | 0.79 |
| • Aldosterone blocker | 14(11) | 16(13) | 18(14) | 0.79 |
| • Beta-blocker | 74(60) | 87(70) | 87(69) | 0.19 |
| • Calcium channel blocker | 35(28) | 44(35) | 45(35) | 0.39 |
| • Nitrate | 10(8) | 21(17) | 28(22) | 0.01 |
| • Loop diuretic | 60(48) | 73(58) | 83(65) | 0.024 |
| • Thiazide diuretic | 33(27) | 26(21) | 26(20) | 0.94 |
| • Statin | 60(48) | 59(47) | 66(52) | 0.73 |
| • Aspirin | 47(38) | 61(49) | 64(50) | 0.10 |
| • Warfarin | 25(20) | 20(16) | 38(30) | 0.024 |
| • Amiodarone | 1(1) | 3(2) | 11(9) | 0.006 |
| • Digoxin | 5(4) | 10(8) | 11(9) | 0.30 |
| Laboratory data: | ||||
| • Sodium, mEq/L | 139±3 | 139±3 | 138±3 | 0.24 |
| • Blood urea nitrogen, mg/dl | 19±13 | 25±17 | 28±17 | <0.001 |
| • Serum creatinine, mg/dl | 1.19±0.90 | 1.71±1.87 | 1.95±1.80 | <0.001 |
| • Estimated GFR, ml/min per 1.73m2 | 68±25 | 55±26 | 52±30 | <0.001 |
| • Hemoglobin, g/dl | 11.9±1.9 | 11.8±1.8 | 11.8±1.9 | 0.94 |
| • B-type natriuretic peptide, pg/ml* | 123 (40,290) | 222 (68,410) | 379 (125,838) | <0.001† |
Median (25th, 75th percentile);
Kruskal-Wallis test; ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; COPD, chronic obstructive pulmonary disease; GFR, glomerular filtration rate; NYHA, New York Heart Association.
Echocardiographic variables were consistent with expected findings in HFpEF: preserved LVEF, normal LV end-diastolic volume index, increased left atrial volume index, and increased E/e’ ratio, indicative of increased LV filling pressures. Moderate or severe diastolic dysfunction was present in the majority of patients: 7% had normal diastolic function, 11% had grade 1 (mild) diastolic dysfunction, 41% had grade 2 (moderate) diastolic dysfunction, 33% had grade 3 (severe) diastolic dysfunction, and 8% had indeterminate diastolic function.
Clinical and laboratory parameters associated with increased frontal QRS-T angle
Patients with increased frontal QRS-T angle were more likely to be older and had a lower frequency of obesity with lower body-mass index. The patients with the highest QRS-T angle values were more likely to have comorbidities such as coronary artery disease, diabetes, and atrial fibrillation, and worse renal function.
Electrocardiographic and echocardiographic characteristics associated with increased frontal QRS-T angle
On ECG, patients with the highest QRS-T angle values had longer PR and QRS intervals. Bundle branch blocks, intraventricular conduction delay, and T-wave inversions were also more common in the highest tertile of QRS-T angle. No differences in QT interval, QTc interval, or heart rate were observed. On echocardiography, patients with the highest QRS-T angle values had larger left atrial and LV volumes, higher LV mass, worse longitudinal systolic function (reduced s’ velocities), and worse diastolic function (Table 2). However, there were no observed differences in global LVEF. Patients with increased frontal QRS-T angle also demonstrated greater right heart remodeling and worse global and longitudinal RV systolic function (Table 2).
Table 2.
Electrocardiographic and Echocardiographic Parameters by QRS-T Angle Tertile
| Parameter | Tertile 1 | Tertile 2 | Tertile 3 | P-value |
|---|---|---|---|---|
| 0–26° (N=124) | 27–75° (N=125) | 76–179° (N=127) | ||
| QRS-T Angle (°) | 13±7 | 47 ±14 | 123±31 | |
|
| ||||
| Electrocardiographic parameters | ||||
| • Heart rate, bpm | 70±14 | 74±15 | 73±17 | 0.20 |
| • PR interval, ms | 167±29 | 174±40 | 183±42 | 0.01 |
| • QRS interval, ms | 86±11 | 94±19 | 109±26 | <0.001 |
| • Left bundle branch block, n (%) | 0 (0) | 2 (2) | 11 (9) | <0.001 |
| • Right bundle branch block, n (%) | 1 (1) | 6 (5) | 17 (13) | <0.001 |
| • Intraventricular conduction delay, n (%) | 1 (1) | 8 (6) | 12 (9) | 0.005 |
| • QT interval, ms | 417±44 | 411±44 | 425±50 | 0.053 |
| • QTc interval, ms | 447±37 | 450±32 | 462±40 | 0.058 |
| • QRS axis, degrees* | 31 (11,49) | 6 (−16,42) | −15 (−48,41) | <0.001† |
| • T-wave axis, degrees* | 34 (18,49) | 37 (16,56) | 80 (57,110) | <0.001† |
| • T-wave inversion, n (%) | 18 (15) | 31 (26) | 81 (68) | <0.001 |
| Echocardiographic parameters | ||||
| • LV ejection fraction, % | 62±6 | 61±6 | 61±7 | 0.32 |
| • LV end-systolic volume index, ml/m2 | 16±6 | 15±5 | 18±9 | 0.029 |
| • LV end-diastolic volume index, ml/m2 | 41±11 | 39±8 | 45±15 | 0.008 |
| • Left atrial volume index, ml/m2 | 31±12 | 33±14 | 37±16 | 0.012 |
| • LV mass index, g/m2 | 90±24 | 98±29 | 119±44 | <0.001 |
| • LV diastolic function, n (%) | 0.003 | |||
| ○ Normal | 17(14) | 9(7) | 3(2) | |
| ○ Grade 1 (impaired relaxation) | 7(6) | 16(13) | 17(14) | |
| ○ Grade 2 (pseudonormal) | 59(58) | 49(39) | 45(35) | |
| ○ Grade 3 (restrictive) | 36(29) | 38(30) | 49(39) | |
| ○ Indeterminate | 5(4) | 13(10) | 13(10) | |
| • E/A ratio | 1.3±0.6 | 1.3±0.7 | 1.5±0.9 | 0.13 |
| • E deceleration time, ms | 222±47 | 241±76 | 227±71 | 0.06 |
| • Isovolumic relaxation time, ms | 82±18 | 85±20 | 94±27 | <0.001 |
| • Tissue Doppler e’ velocity, cm/s | 7.9±2.5 | 7.1±2.6 | 6.4±2.9 | <0.001 |
| • Tissue Doppler s’ velocity, cm/s | 8.0±2.1 | 7.3±1.9 | 6.7±2.0 | <0.001 |
| • E/e’ ratio | 14.4±5.4 | 16.3±8.7 | 19.9±11.5 | <0.001 |
| • RV basal diameter, cm/m2 | 1.9±0.4 | 1.9±0.4 | 2.1±0.4 | 0.002 |
| • RV length, cm/m2 | 3.9±0.6 | 3.9±0.6 | 4.2±0.7 | <0.001 |
| • RV end-diastolic area index, cm2/m2 | 13.1±3.0 | 13.7±3.8 | 14.8±4.5 | 0.002 |
| • RV end-systolic area index, cm2/m2 | 7.4±2.1 | 7.9±2.8 | 8.8±3.3 | <0.001 |
| • RV wall thickness, cm/m2 | 0.24±0.05 | 0.26±0.06 | 0.28±0.07 | <0.001 |
| • RV fractional area change | 0.44±0.06 | 0.43±0.07 | 0.41±0.08 | 0.011 |
| • Right atrial area, cm2/m2 | 9.8±3.3 | 10.7±5.3 | 12.0±5.1 | 0.002 |
| • TAPSE, cm | 1.10±0.32 | 0.98±0.30 | 0.94±0.31 | <0.001 |
| • PA systolic pressure, mmHg | 41±16 | 47±18 | 49±18 | 0.011 |
Median (25th, 75th percentile);
Kruskal-Wallis test; LV, left ventricular; RV, right ventricular; TAPSE, tricuspid annular plane systolic excursion; PA, pulmonary artery
Independent association of frontal QRS-T angle with BNP and worse left and right ventricular structure/function
After adjusting for age, sex, comorbidities, QRS duration and QTc interval, QRS-T remained associated with BNP and echocardiographic markers of left and right ventricular remodeling, longitudinal left and right ventricular systolic function, and LV diastolic dysfunction (Table 3). Further adjustment for BNP did not eliminate any of the associations between QRS-T angle and echocardiographic parameters except for E/e’ ratio (P=0.055 after adjustment for BNP). Additional adjustment for T-wave inversions and specific types of bundle branch block did not attenuate these associations. The associations between QRS-T angle and BNP and echocardiographic parameters persisted after exclusion of patients with QRS duration > 120 ms (see online Appendix A Supplemental Table).
Table 3.
Association of Frontal QRS-T Angle with B-type Natriuretic Peptide and Echocardiographic Parameters on Unadjusted and Multivariable Adjusted Linear Regression Analysis
| Dependent variable | Unadjusted | Multivariable-adjusted* | Additional adjustment for BNP** | |||
|---|---|---|---|---|---|---|
|
| ||||||
| β-Coefficient (95% CI) | P-value | β-Coefficient (95% CI) | P-value | β-Coefficient (95% CI) | P-value | |
| • Log BNP, pg/ml | 0.46 (0.31, 0.60) | <0.001 | 0.18 (0.03, 0.33) | 0.019 | — | — |
| • Tissue Doppler s’ velocity, cm/s | −0.55 (−0.76, −0.34) | <0.001 | −0.38 (−0.62, −0.13) | 0.002 | −0.33 (−0.57, −0.08) | 0.010 |
| • Tissue Doppler e’ velocity, cm/s | −0.61 (−0.88, −0.33) | <0.001 | −0.50 (−0.82, −0.18) | 0.002 | −0.49 (−0.81, −0.17) | 0.003 |
| • E/e’ ratio | 2.29 (1.37, 3.22) | <0.001 | 1.40 (0.34, 2.44) | 0.010 | 1.09 (−0.02, 2.20) | 0.055 |
| • Isovolumic relaxation time, ms | 7.75 (5.42, 10.09) | <0.001 | 4.91 (2.20, 7.62) | <0.001 | 3.69 (0.92, 6.45) | 0.009 |
| • LV mass index, g/m2 | 10.39 (6.90, 13.87) | <0.001 | 5.95 (1.74, 10.15) | 0.006 | 4.63 (0.18, 9.07) | 0.041 |
| • TAPSE, cm | −0.16 (−0.22, −0.10) | <0.001 | −0.13 (−0.20, −0.06) | 0.001 | −0.09 (−0.17, −0.01) | 0.023 |
| • RV wall thickness, mm/m2 | 0.15 (0.09, 0.22) | <0.001 | 0.11 (0.04, 0.17) | 0.001 | 0.08 (0.01, 0.14) | 0.021 |
Multivariable model adjusted for age, sex, body-mass index, diabetes mellitus, atrial fibrillation, coronary artery disease, estimated glomerular filtration rate, amiodarone use, diuretic use, QRS interval, and QTc interval
Includes all covariates of the multivariable model plus log BNP
BNP, B-type natriuretic peptide; TAPSE, tricuspid annular plane systolic excursion; RV, right ventricular; LV, left ventricular; CI, confidence interval.
Independent association of frontal QRS-T angle with adverse outcomes
During a median follow-up period of 12 months (25th–75th percentile 4–23 months), 43% of the study participants were hospitalized for HF, hospitalized for other cardiovascular reasons, and/or died (53 deaths in total). Cause of death was most often HF-related (25%), followed by sudden cardiac death (23%), infection (21%), malignancy (15%), myocardial infarction (4%), and other causes (12%). All outcomes occurred more frequently in the highest tertile of frontal QRS-T angle (Table 4). Specific causes of death, however, did not differ among QRS-T tertiles.
Table 4.
Association of Frontal QRS-T Angle with Adverse Outcomes on Cox Proportional Hazards Analysis
| Outcome | Event rate (n/N, %) | Unadjusted | Multivariable-Adjusted* | Additional adjustment for B-type natriuretic peptide** | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | ||
| Heart failure hospitalization | ||||||||||
| • Tertile 1 | 22/124 (18) | — | — | — | — | — | — | — | — | — |
| • Tertile 2 | 29/125 (23) | 1.3 | 0.8, 2.4 | 0.30 | 1.1 | 0.6, 1.9 | 0.86 | 1.0 | 0.6, 1.9 | 0.97 |
| • Tertile 3 | 43/127 (34) | 2.2 | 1.3, 3.8 | 0.003 | 1.9 | 1.0, 3.6 | 0.047 | 1.5 | 0.8, 2.9 | 0.20 |
| • QRS-T angle (per 1-SD increase) | — | 1.3 | 1.1, 1.6 | 0.009 | 1.3 | 1.0, 1.7 | 0.052 | 1.2 | 0.9, 1.5 | 0.22 |
| Cardiovascular hospitalization | ||||||||||
| • Tertile 1 | 28/124 (23) | — | — | — | — | — | — | — | — | — |
| • Tertile 2 | 47/125 (38) | 1.7 | 1.1, 2.8 | 0.023 | 1.4 | 0.8, 2.3 | 0.21 | 1.3 | 0.8, 2.1 | 0.36 |
| • Tertile 3 | 55/127 (43) | 2.4 | 1.5, 3.8 | <0.001 | 2.2 | 1.3, 3.8 | 0.005 | 1.9 | 1.1, 3.4 | 0.022 |
| • QRS-T angle (per 1-SD increase) | — | 1.3 | 1.1, 1.5 | 0.001 | 1.3 | 1.1, 1.6 | 0.015 | 1.3 | 1.0, 1.6 | 0.046 |
| Death | ||||||||||
| • Tertile 1 | 12/124 (10) | — | — | — | — | — | — | — | — | — |
| • Tertile 2 | 16/125 (13) | 1.2 | 0.6, 2.6 | 0.61 | 0.8 | 0.4, 1.7 | 0.47 | 0.5 | 0.2, 1.3 | 0.18 |
| • Tertile 3 | 25/127 (20) | 2.1 | 1.0, 4.1 | 0.040 | 1.6 | 0.7, 3.7 | 0.28 | 1.2 | 0.5, 2.9 | 0.71 |
| • QRS-T angle (per 1-SD increase) | — | 1.3 | 1.0, 1.6 | 0.070 | 1.2 | 0.8, 1.6 | 0.39 | 1.1 | 0.8, 1.6 | 0.60 |
| Cardiovascular hospitalization or death | ||||||||||
| • Tertile 1 | 35/124 (28) | — | — | — | — | — | — | — | — | — |
| • Tertile 2 | 55/125 (44) | 1.7 | 1.1, 2.6 | 0.019 | 1.4 | 0.9, 2.1 | 0.19 | 1.2 | 0.8, 2.0 | 0.39 |
| • Tertile 3 | 71/127 (56) | 2.5 | 1.6, 3.8 | <0.001 | 2.3 | 1.4, 3.9 | 0.001 | 2.0 | 1.2, 3.4 | 0.008 |
| • QRS-T angle (per 1-SD increase) | — | 1.3 | 1.1, 1.5 | <0.001 | 1.3 | 1.1, 1.6 | 0.005 | 1.3 | 1.0, 1.6 | 0.018 |
Multivariable model adjusted for age, sex, body-mass index, diabetes mellitus, atrial fibrillation, coronary artery disease, estimated glomerular filtration rate, amiodarone use, diuretic use, QRS interval, and QTc interval;
Includes all covariates of the multivariable model plus log BNP; HR, hazard ratio; CI, confidence interval; SD, standard deviation
After adjustment for several potential confounders, including age, sex, body-mass index, diabetes, atrial fibrillation, coronary artery disease, eGFR, amiodarone use, diuretic use, QRS duration, and QTc interval, the highest tertile of QRS-T angle remained independently associated with all outcomes except for death (Table 4).
We created an additional Cox proportional hazards regression model to test whether QRS-T angle was predictive of cardiovascular death (N=28). Neither QRS-T angle quartile 2 (HR 1.1, 95% CI 0.3–3.6; P=0.86) nor quartile 3 (HR 2.2, 95% CI 0.8–6.3; P=0.14) was associated with cardiovascular death on univariate analysis, though the trend of results suggested that with a larger number of outcomes, QRS-T angle may have been associated with cardiovascular death.
Adding BNP to the model eliminated the association between QRS-T angle and HF hospitalization, but QRS-T angle remained predictive of cardiovascular hospitalization even after adjusting for BNP (Table 4). Additional adjustment for the presence of T-wave inversions did not attenuate the independent association between the highest QRS-T angle and HF hospitalization (P=0.033), cardiovascular hospitalization (P=0.001), and the combined endpoint of cardiovascular hospitalization or death (P<0.001).
After further adjustment for tissue Doppler s’ velocity, E/e’ ratio, and TAPSE, QRS-T angle was still associated with the composite outcome (P=0.002). Additional adjustment LV mass index and RV wall thickness attenuated the association but did not eliminate it (P=0.040). Findings were similar when QRS duration was replaced with the specific type of bundle branch block in the multivariable models. After excluding patients with QRS duration > 120 ms, both the second and third tertiles of frontal QRS-T angle were associated with cardiovascular hospitalization and the combined endpoint of cardiovascular hospitalization and death (see online Appendix B Supplemental Table).
Figure 1 displays Kaplan Meier curves for the composite outcome by tertile of frontal QRS-T angle over time, demonstrating that survival curves separate early and remain separated throughout the duration of follow-up (log-rank P<0.0001). Figure 2 displays the LOWESS plots for QRS axis, T-wave axis, and QRS-T angle for the composite outcome. Although all 3 ECG parameters were associated with outcomes on unadjusted analyses (P=0.034 for QRS axis; P=0.018 for T-wave axis, and P<0.0001 for QRS-T angle), only QRS-T angle remained associated after multivariable adjustment. In addition, Figure 2 shows the relative linearity of the association between QRS-T angle and outcomes up to a QRS-T angle of 105°.
Figure 1. Kaplan-Meier Survival Curves Stratified by Frontal QRS-T Angle Tertile.
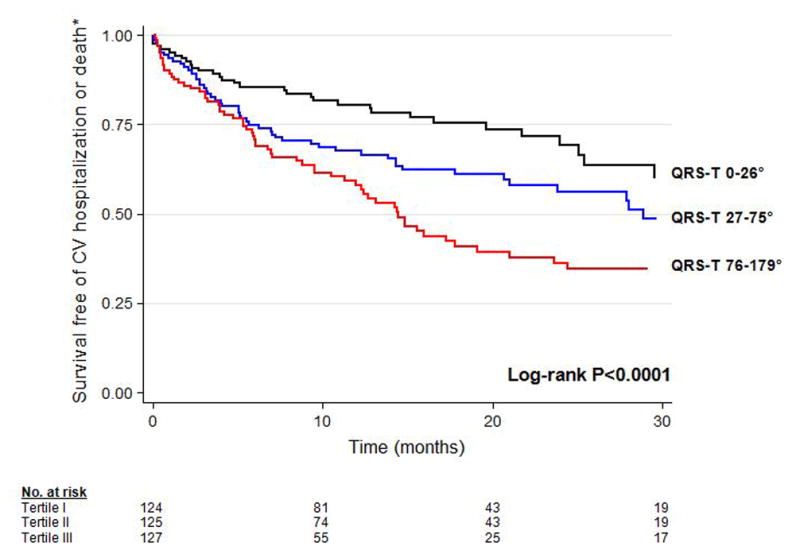
CV = cardiovascular. Increased frontal QRS-T angle is associated with greater risk for the composite outcome, cardiovascular hospitalization or mortality.
Figure 2. Relationship of QRS-T Angle, QRS Axis, and T-wave Axis with the Composite Outcome of Cardiovascular Hospitalization and Death.
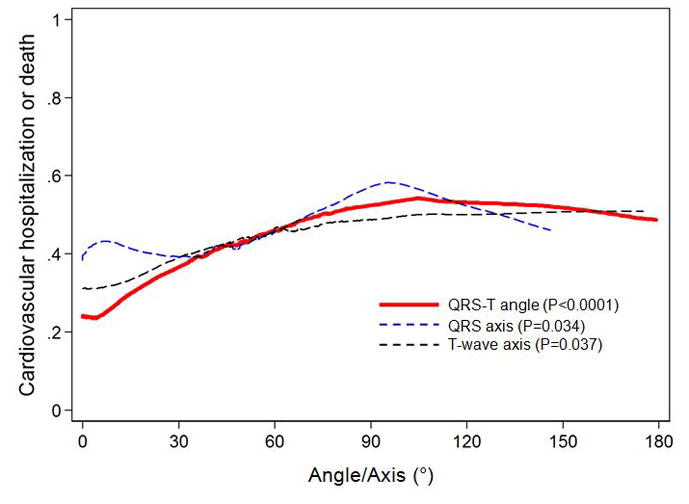
Locally-weighted, smoothed scatterplot (LOWESS) curves. P-values represent significance for each electrocardiographic parameter and the composite outcome on unadjusted linear regression analyses.
DISCUSSION
In a prospective study of patients with HFpEF, we found that increased frontal QRS-T angle, a measure easily calculated from the standard 12-lead ECG, is independently associated with worse left and right ventricular function and greater left and right ventricular remodeling. In addition, increased frontal QRS-T angle is independently associated with worse outcomes, even after adjustment for several potential confounders including BNP and abnormal RV and LV structure and function. Exclusion of patients with prolonged QRS duration did not alter the significance of our findings.
Ours is the first study of its kind on QRS-T angle in HFpEF, and complements prior work that demonstrated the prognostic utility of the frontal QRS-T angle in HF with reduced EF [5]. Our study is also the first investigation of QRS-T angle which has featured comprehensive quantitation of cardiac structure and function. Based on our results, increased QRS-T angle is a marker of greater left and right ventricular remodeling and associated dysfunction in patients with HF, even in the setting of a preserved LVEF. The relationship observed in previous studies in different patient populations between the QRS-T and adverse outcomes may be mediated by its relationship to the abnormal echocardiographic indices observed here. Thus, future studies of the QRS-T in different patient populations may benefit from a similar echocardiographic analysis, as wider QRS-T angles appear to indicate greater myocardial remodeling and dysfunction.
It is unknown why increased QRS-T angle is associated with left and right ventricular remodeling and dysfunction. Abnormal cardiac remodeling likely physically alters electrical conduction in the heart [20] and subsequently shifts the direction of the vectors of depolarization and repolarization, contributing to increased QRS-T angle. Cardiac remodeling also causes electrical alterations at the molecular level by modulating ion channel activity. Repolarizing potassium currents are impaired in remodeled and failing myocardium. Further, the expression and function of the sodium/potassium-ATPase is decreased in failing hearts, which likewise impairs repolarization, but importantly also alters intracellular calcium homeostasis, which may worsen cardiac diastolic function [21].
Several mechanisms may explain the association between increased QRS-T angle and worse outcomes in HFpEF. An increased frontal QRS-T angle may indicate abnormal ventricular repolarization, the ECG counterpart of myocardial relaxation. Thus, an increased angle may be a surrogate marker for impaired relaxation and worse diastolic function, placing patients at higher risk for adverse events. This hypothesis is supported by our results, given the independent association of increased frontal QRS-T angle with worse parameters of diastolic function. Our finding is consistent with other measures of ventricular repolarization, specifically the QTc and T-peak to T-end, which are also independently associated with worse diastolic function [22].
It is particularly interesting that the relationship between QRS-T angle and adverse outcomes persists after adjustment for BNP, which has strong prognostic utility in HFpEF [23]. Thus, risk stratification formulas in HFpEF may be better served with the addition of the QRS-T angle. If confirmed by additional studies, the QRS-T angle may also have clinical utility in the diagnosis of HFpEF. In patients who have equivocal symptoms of HF and a preserved LVEF seen on echocardiography, an abnormal QRS-T angle may be helpful in identifying and separating patients with true HFpEF from patients with non-cardiac symptomatology.
There are two notable differences between our study and other studies that examine the QRS-T angle in relation to adverse outcomes. First, our study uses the frontal QRS-T angle as opposed to the spatial QRS-T angle, an analogous parameter that requires computerized algorithms to derive quasi-orthogonal vectors. Its utility in predicting mortality in general and clinical populations is well established [4, 7, 8, 24]. However, the spatial QRS-T angle is more cumbersome to derive and not routinely reported, reducing its clinical utility. Further, the frontal QRS-T angle is equivalent in predicting mortality [6]. Second, we used different angle thresholds (derived by dividing our study population into tertiles) than previous studies. Previous studies have defined the lower limit for abnormal frontal QRS-T angles between 69 and 100° whereas the lower limit for the third tertile in our study, which was independently associated with adverse outcomes, was 76° [5, 6, 24]. However, as shown in Figure 2, the association between QRS-T angle and outcomes is relatively linear with no apparent threshold effect.
Our study findings should be interpreted in the context of several limitations. Our results are limited to this single center analysis, and would be strengthened by a larger, multicenter study. However, the clinical characteristics of our HFpEF patients are similar to prior epidemiologic studies [1, 2]. Furthermore, the proportion of deaths due to cardiac causes in our study (52%) is very similar to data on mode of death in large-scale HFpEF clinical trials (e.g., 52% of the deaths in CHARM-Preserved and 45% of the deaths in I-PRESERVE were due to cardiac causes) [25]. Our study is also limited by lack of data on serial QRS-T angle measurements in the study participants. It is unclear whether changes in QRS-T angle over time would be associated with lower risk for adverse events, and future studies investigating the association between temporal changes in QRS-T angle and adverse events would be beneficial. Finally, we were underpowered to detect associations between QRS-T angle and death (and specifically sudden cardiac death) because of the relatively small number of deaths in our study. Longer-term follow-up and a larger sample size and/or higher number of deaths may be necessary to fully examine the association between QRS-T angle and mortality in HFpEF.
In summary, the frontal QRS-T angle, easily measured on a standard 12-lead ECG, is independently associated with worse left and right ventricular remodeling and function and a higher frequency of adverse outcomes in HFpEF.
Supplementary Material
Acknowledgments
Funding: This work was supported by grants from the American Heart Association (Scientist Development Grant #0835488N) and the National Institutes of Health (R01 HL107557) (both to S.J.S.).
Abbreviations
- BNP
B-type natriuretic peptide
- ECG
electrocardiogram
- eGFR
estimated glomerular filtration rate
- HFpEF
heart failure with preserved ejection fraction
- LOWESS
locally weighted scatterplot smoothing
- LV
left ventricular
- NYHA
New York Heart Association
- RV
right ventricular
- TAPSE
tricuspid annular plane systolic excursion
Footnotes
Industry Relationships: None
DISCLOSURES
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. New England Journal of Medicine. 2006;355:260–9. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 2.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. New England Journal of Medicine. 2006;355:251–9. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 3.Karaye KM, Sani MU. Electrocardiographic abnormalities in patients with heart failure. Cardiovasc J Afr. 2008;19:22–5. [PMC free article] [PubMed] [Google Scholar]
- 4.Kardys I, Kors JA, van der Meer IM, Hofman A, van der Kuip DA, Witteman JC. Spatial QRS-T angle predicts cardiac death in a general population. Eur Heart J. 2003;24:1357–64. doi: 10.1016/s0195-668x(03)00203-3. [DOI] [PubMed] [Google Scholar]
- 5.Pavri BB, Hillis MB, Subacius H, Brumberg GE, Schaechter A, Levine JH, et al. Prognostic value and temporal behavior of the planar QRS-T angle in patients with nonischemic cardiomyopathy. Circulation. 2008;117:3181–6. doi: 10.1161/CIRCULATIONAHA.107.733451. [DOI] [PubMed] [Google Scholar]
- 6.Zhang ZM, Prineas RJ, Case D, Soliman EZ, Rautaharju PM, Group AR. Comparison of the prognostic significance of the electrocardiographic QRS/T angles in predicting incident coronary heart disease and total mortality (from the atherosclerosis risk in communities study) Am J Cardiol. 2007;100:844–9. doi: 10.1016/j.amjcard.2007.03.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamazaki T, Froelicher VF, Myers J, Chun S, Wang P. Spatial QRS-T angle predicts cardiac death in a clinical population. Heart Rhythm. 2005;2:73–8. doi: 10.1016/j.hrthm.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 8.Rautaharju PM, Kooperberg C, Larson JC, LaCroix A. Electrocardiographic predictors of incident congestive heart failure and all-cause mortality in postmenopausal women: the Women’s Health Initiative. Circulation. 2006;113:481–9. doi: 10.1161/CIRCULATIONAHA.105.537415. [DOI] [PubMed] [Google Scholar]
- 9.Voulgari C, Tentolouris N, Moyssakis I, Dilaveris P, Gialafos E, Papadogiannis D, et al. Spatial QRS-T angle: association with diabetes and left ventricular performance. Eur J Clin Invest. 2006;36:608–13. doi: 10.1111/j.1365-2362.2006.01697.x. [DOI] [PubMed] [Google Scholar]
- 10.Borleffs CJ, Scherptong RW, Man SC, van Welsenes GH, Bax JJ, van Erven L, et al. Predicting ventricular arrhythmias in patients with ischemic heart disease: clinical application of the ECG-derived QRS-T angle. Circ Arrhythm Electrophysiol. 2009;2:548–54. doi: 10.1161/CIRCEP.109.859108. [DOI] [PubMed] [Google Scholar]
- 11.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–6. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 12.Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–50. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 13.Paulus WJ, van Ballegoij JJM. Treatment of heart failure with normal ejection fraction: an inconvenient truth! J Am Coll Cardiol. 2010;55:526–37. doi: 10.1016/j.jacc.2009.06.067. [DOI] [PubMed] [Google Scholar]
- 14.Surawicz B, Childers R, Deal BJ, Gettes LS, Bailey JJ, Gorgels A, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part III: intraventricular conduction disturbances: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation. 2009;119:e235–40. doi: 10.1161/CIRCULATIONAHA.108.191095. [DOI] [PubMed] [Google Scholar]
- 15.Migliore F, Zorzi A, Michieli P, Perazzolo Marra M, Siciliano M, Rigato I, et al. Prevalence of cardiomyopathy in Italian asymptomatic children with electrocardiographic T-wave inversion at preparticipation screening. Circulation. 2012;125:529–38. doi: 10.1161/CIRCULATIONAHA.111.055673. [DOI] [PubMed] [Google Scholar]
- 16.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. Journal of the American Society of Echocardiography. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. Journal of the American Society of Echocardiography. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. quiz 86–8. [DOI] [PubMed] [Google Scholar]
- 18.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–33. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 19.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. Jama. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 20.Madias JE. Pseudo-T-wave alternans with atrio-ventricular nodal reentrant tachycardia and group beating: the proof is in the QRS complexes. Europace. 2011;13:1662. doi: 10.1093/europace/eur180. [DOI] [PubMed] [Google Scholar]
- 21.Tomaselli GF, Marban E. Electrophysiological remodeling in hypertrophy and heart failure. Cardiovasc Res. 1999;42:270–83. doi: 10.1016/s0008-6363(99)00017-6. [DOI] [PubMed] [Google Scholar]
- 22.Sauer A, Wilcox JE, Andrei AC, Passman R, Goldberger JJ, Shah SJ. Diastolic electromechanical coupling: association of the ECG T-peak to T-end interval with echocardiographic markers of diastolic dysfunction. Circ Arrhythm Electrophysiol. 2012;5:537–43. doi: 10.1161/CIRCEP.111.969717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Veldhuisen DJ, Linssen GC, Jaarsma T, van Gilst WH, Hoes AW, Tijssen JG, et al. B-type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol. 2013;61:1498–506. doi: 10.1016/j.jacc.2012.12.044. [DOI] [PubMed] [Google Scholar]
- 24.Anjan VY, Loftus TM, Burke MA, Akhter N, Fonarow GC, Gheorghiade M, et al. Prevalence, Clinical Phenotype, and Outcomes Associated With Normal B-Type Natriuretic Peptide Levels in Heart Failure With Preserved Ejection Fraction. Am J Cardiol. 2012 doi: 10.1016/j.amjcard.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zile MR, Gaasch WH, Anand IS, Haass M, Little WC, Miller AB, et al. Mode of death in patients with heart failure and a preserved ejection fraction: results from the Irbesartan in Heart Failure With Preserved Ejection Fraction Study (I-Preserve) trial. Circulation. 2010;121:1393–405. doi: 10.1161/CIRCULATIONAHA.109.909614. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


