Abstract
Background
A number of noninvasive diagnostic tests are available to detect Helicobacter pylori infection. Data on serologic testing of children are lacking, however, and thus it remains unclear whether the serology cutoff points used for adults are appropriate for children.
Methods
Serum and stool samples were obtained from 73 children who visited 5 hospitals in Japan between March 1993 and December 2009. Analysis of stool samples was carried out using an H pylori stool antigen enzyme-linked immunosorbent assay (HpSA ELISA), and serum antibodies to H pylori were examined using an antibody determination kit (E-Plate Eiken H pylori antibody). The validity of the serologic test was evaluated based on its sensitivity, specificity, and receiver operating characteristics curve.
Results
Of the 73 children included in this study, 34 were HpSA-positive and 39 were negative. Among the 34 HpSA-positive patients, 32 were IgG-positive and 2 were IgG-negative. Of the 39 patients who were HpSA-negative, 38 were IgG-negative and 1 was IgG-positive. The sensitivity, specificity, and positive likelihood ratio for IgG antibody testing were 91.2%, 97.4%, and 35.6, respectively, based on the recommended adult cutoff point of 10 U/ml. Among children, use of cutoff points in the range of 7 to 9 U/ml yielded optimal values for sensitivity and specificity, as well as a positive likelihood ratio.
Conclusions
The performance of the E-plate anti-H pylori IgG antibody test was comparable to that of the stool antigen test and is therefore suitable for epidemiologic studies of H pylori infection in large samples.
Key words: Helicobacter pylori, serologic test, validity, stool antigen test
INTRODUCTION
Helicobacter pylori (H pylori) causes gastrointestinal diseases such as gastritis and peptic ulcer in adults and children.1,2 In addition, previous reports have linked H pylori infection with iron deficiency anemia and thrombocytopenic purpura in children.3,4 Although the prevalence of H pylori infection remains high among Japanese adults, a marked decrease has been observed among Japanese children.5–7 The exact route of H pylori transmission remains to be clarified; however, a widely held view is that the vast majority of new infections are acquired during early childhood.8
Numerous noninvasive diagnostic tests are available to detect H pylori infection, including serologic, stool antigen (HpSA), and 13C-urea breath testing.9–11 Each test has strengths and weaknesses in terms of diagnostic accuracy, performance characteristics for the various samples collected in epidemiologic studies, and rapidity as a bedside diagnostic test. The serologic test for H pylori infection can be easily done using stored sera in epidemiologic studies involving large samples; however, concerns regarding validity have been raised due to its lower sensitivity and specificity as compared with the stool antigen and urea breath tests.12 Furthermore, there are few data on serologic tests for children, and thus it remains unclear whether the serology cutoffs used for adults are applicable to children. Here, we used a commercially available ELISA kit (E-plate) to assess the utility of serologic testing for H pylori infection among Japanese children.
METHODS
Study population
Serum and stool samples were collected from 73 consecutive patients with dyspepsia (mean [SD] age, 6.3 [4.3] years) who visited 5 hospitals in the Kinki area of Japan between March 1993 and December 2009. Informed consent was obtained from the parents of the children. The study was approved by the Ethics Committee of Aichi Medical University.
HpSA ELISA
The presence of H pylori was determined according to the result of a stool antigen test using HpSA ELISA (Meridian HpSA, TFB, Meridian, USA). Stool samples were analyzed using spectrophotometry (450/630). A value less than 0.10 indicated a negative result, 0.10 to 0.119 indicated an indeterminable result, and 0.12 or higher indicated a positive result, as specified in the manufacturer’s instructions.
Microplate enzyme immunoassay
Serum and stool samples were stored at −80°C until the laboratory assay was performed. Serum antibodies to H pylori were examined using a microplate enzyme immunoassay (EIA) and an antibody determination kit (E-Plate Eiken H pylori antibody, Eiken Chemical Co., Ltd., Tokyo, Japan). All samples were analyzed according to the manufacturer’s instructions, and the cutoff point was set at 10 U/ml. All assays were performed by experimenters blinded to the clinical status of the patients.
Statistical analysis
Logistic regression analysis was performed to examine the possible effects of sex and age on the serologic test. To assess the criterion validity of the serologic test, sensitivities, specificities, positive likelihood ratios, and negative likelihood ratios were estimated relative to the HpSA assay (the gold standard), across all possible cutoff values for the serologic test. To exclude the possible effects of maternal IgG antibody, we conducted additional analysis that excluded children younger than 1 year.
Receiver operating characteristics (ROC) analysis was also conducted using the HpSA assay as the gold standard. The 95% CI of the area under the ROC curve (AUC) was calculated using the bootstrap method with 10 000 bootstrap samples.
To compute the AUC with the bootstrap 95% CI, the R package pROC was used. To estimate the validity indices, eg, sensitivity and specificity, the R package Diagnosis Med was used. All analyses, except those noted above, were performed using R version 2.13.0 for Windows.13
RESULTS
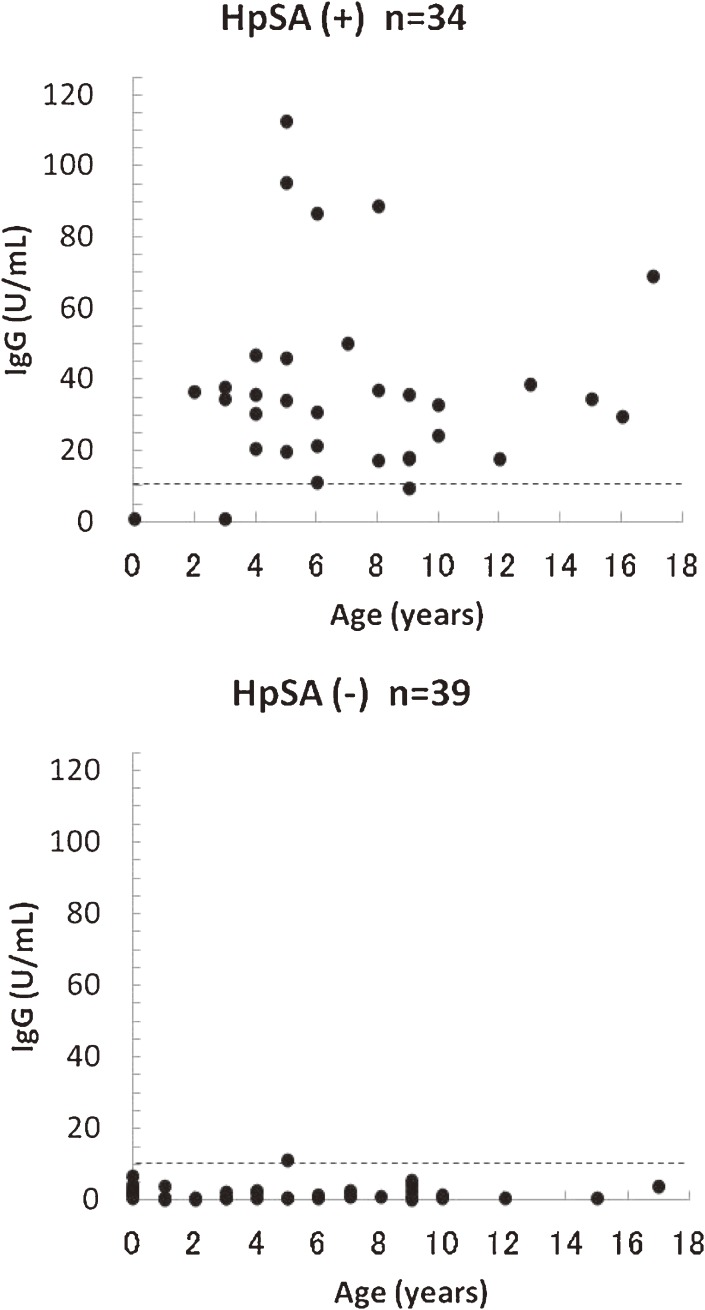
Of the 73 children included in this study, 34 were HpSA-positive and 39 were HpSA-negative (Figure 1). Of the 34 HpSA-positive patients, 32 were IgG-positive and 2 were IgG-negative. Of the 39 patients who were HpSA-negative, 38 were IgG-negative and 1 was IgG-positive.
Figure 1. Results of the H pylori stool antigen (HpSA) assay and anti-H pylori IgG antibody test. (- - - -), cutoff value.
Table 1 shows the age and sex distributions of the participants and the number of individuals and the test results of the HpSA and IgG antibody tests. Of the 9 children who were younger than 1 year, only 1 was HpSA-positive and IgG-negative.
Table 1. Characteristics of the study subjects.
| HpSA(+) | HpSA(−) | ||||
| IgG(+) | IgG(−) | IgG(+) | IgG(−) | ||
| Sex | Male | 17 | 2 | 1 | 17 |
| Female | 15 | 0 | 0 | 21 | |
| Age group | 0 | 0 | 1 | 0 | 8 |
| 1–5 | 12 | 1 | 1 | 14 | |
| 6–10 | 14 | 0 | 0 | 13 | |
| 10–17 | 6 | 0 | 0 | 3 | |
HpSA: Stool antigen test; IgG; anti-H pylori IgG antibody test.
As shown in Table 2, when the cutoff point recommended by the manufacturer was used, the sensitivity, specificity, positive likelihood ratio, and negative likelihood ratio were 91.2%, 97.4%, 35.6, and 0.09, respectively. Using cutoff points in the range of 7 to 9 U/ml yielded optimal values for sensitivity, specificity, and positive likelihood ratio. In additional analysis that excluded the 9 children younger than 1 year, the results were similar, but the sensitivity was 97.0% when the cutoff point was in the range of 7 to 9 U/ml.
Table 2. Sensitivity and specificity of anti-H pylori IgG antibody test for Japanese children, by cutoff point.
| Cutoff | Sen (95% CI) | Spec (95% CI) | LR+ (95% CI) | LR− (95% CI) |
| 3 | 94.12 (80.91–98.37) | 79.49 (64.47–89.22) | 4.59 (2.46–8.57) | 0.07 (0.02–0.29) |
| 4 | 94.12 (80.91–98.37) | 87.18 (73.29–94.40) | 7.34 (3.22–16.72) | 0.07 (0.02–0.26) |
| 5 | 94.12 (80.91–98.37) | 92.31 (79.68–97.35) | 12.24 (4.11–36.41) | 0.06 (0.02–0.25) |
| 6 | 94.12 (80.91–98.37) | 94.87 (83.11–98.58) | 18.35 (4.75–70.97) | 0.06 (0.02–0.24) |
| 7–9 | 94.12 (80.91–98.37) | 97.44 (86.82–99.55) | 36.71 (5.29–254.52) | 0.06 (0.02–0.23) |
| 10 | 91.18 (77.04–96.95) | 97.44 (86.82–99.55) | 35.56 (5.12–246.78) | 0.09 (0.03–0.27) |
| 11 | 88.24 (73.38–95.33) | 97.44 (86.82–99.55) | 34.41 (4.95–239.03) | 0.12 (0.05–0.30) |
Sen, sensitivity; Spec, specificity; LR, likelihood ratio.
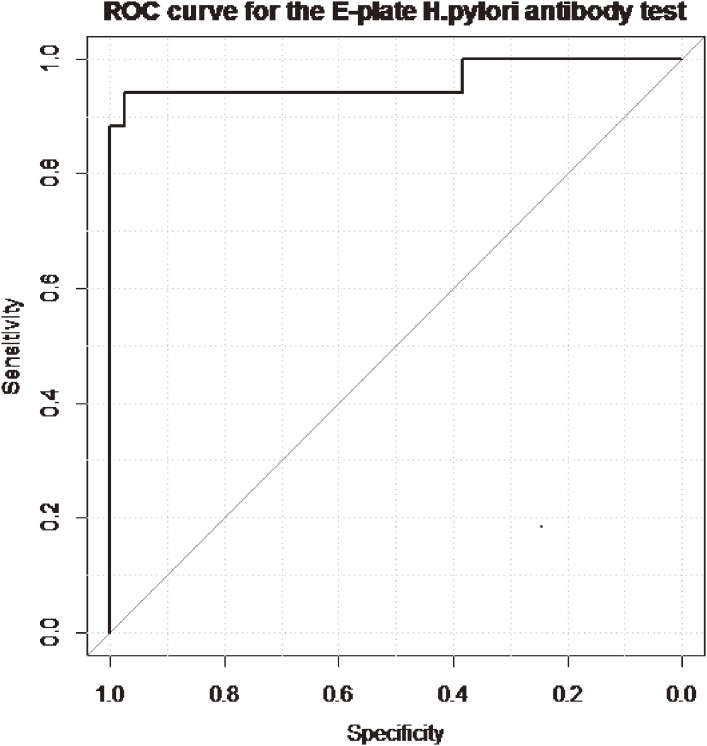
Logistic regression analysis of the results of the HpSA assay revealed that neither sex (OR, 0.86; 95% CI, 0.07–10.3) nor age (OR, 0.94; 95% CI, 0.65–1.23) were significantly associated with presence of H pylori. Thus, all patients were included in the ROC analysis. The AUC for the anti-H pylori IgG antibody test was 0.96 (95% CI, 0.91–1.00; Figure 2).
Figure 2. Receiver operating characteristics (ROC) curve for anti-H pylori IgG antibody test, with the HpSA assay as the gold standard.
DISCUSSION
We compared the results of a serologic test and HpSA assay for H pylori infection in 73 Japanese children and found that the serologic test yielded excellent sensitivity and specificity, with the HpSA assay as the gold standard.
Previous studies reported mixed results regarding the utility of serologic tests for H pylori infection in children.12,14–16 A 2008 meta-analysis of 42 studies of children showed a sensitivity of 79.2% (95% CI, 77.3–81.0) and a specificity of 92.4% (95% CI, 91.6–93.3) for a serologic IgG antibody test.11 In general, serologic tests have high specificity but low sensitivity, especially among Japanese children younger than 10 years. One explanation for the low sensitivity in children is that production of antibodies to H pylori in children may differ from that in adults because the immunologic response is immature in children.17 Another explanation is that transfer of maternal IgG antibodies influences production of antibodies in response to H pylori infection in children.18
Because of the low sensitivity observed in serologic tests, clinical guidelines have recommended that tests based on the detection of serum antibodies against H pylori are not reliable in clinical settings.19 Despite the varied results of serologic tests, sensitivity and specificity were excellent for an ELISA kit using antigens derived from Japanese individuals. In addition, this laboratory-based serologic test is relatively inexpensive, and its accuracy is not affected by medication use.20 Our results indicate that this laboratory-based serologic test can be used to analyze collected sera in epidemiologic studies of H pylori infection in children, when its performance has been locally validated.
Several factors may be responsible for the excellent performance of the serologic test in this study. First, results may vary in relation to the gold standard. We used the stool antigen test as the gold standard because it has excellent sensitivity and specificity for children, as compared with gastric biopsy and the rapid urease test.9 Second, differences in the performance of ELISA kits are partly due to strain variations. A previous study comparing the performance of the JHM-CAP EIA (an EIA test based on antigens derived from a Japanese strain) and HM-CAP EIA (an EIA test based on antigens derived from a strain in the United States) found that the JHM-CAP EIA had a similarly high specificity and a much higher sensitivity than the HM-CAP EIA.15 The higher sensitivity of the JHM-CAP EIA was explained by the presence of a 100-kDa antigen in the Japanese strains, which might be recognized by the host’s immune system at an early stage of infection.15 Because the E-Plate EIA also uses strains derived from Japanese patients, we speculate that the sera of Japanese children may exclusively react to the presence of certain antigens in the Japanese strains, such as CagA and VacA. Further studies are needed to explore genetic differences among populations and their effects on immune responses. Third, the validity of the serologic test may be affected by the cutoff points used, given that titers to IgG increase with age in response to H pylori infection.21 A previous study recommended that, in studies using serologic tests, researchers should reexamine the test results and determine if it is necessary to adopt specialized cutoff points in children.22 To address this issue, we evaluated the performance of the serologic test by using various cutoff points and found that cutoff points in the range of 7 to 9 U/ml yielded optimal sensitivity, specificity, and positive likelihood ratio. Although the recommended cutoff point is 10 for adults, cutoff points in the range of 6 to 10 U/ml yield similar values for sensitivity and specificity, according to results provided by the manufacturer. Therefore, our results indicate that no adjustment is needed for Japanese children when the E-plate EIA test is used to detect H pylori infection.
Our study has several limitations. First, the samples were collected over a long period, which might have affected the study results. To address this issue, we compared sensitivity and specificity between the earlier (1993–2000) and later periods (2001–2009). Unfortunately, there were too few subjects in the later period, and we could not calculate these indexes. However, the results for the earlier period were similar to those reported for the whole period. Second, because only a subset of children for whom both blood and stool samples were available were included in this study, our results need to be replicated in other, larger samples of consecutive patients. Third, the HpSA test detects infection and current presence of H pylori; however, IgG antibodies can be detected approximately 3 weeks after H pylori infection.22 Therefore, the latent period between H pylori infection and antibody production may be a source of misclassification. However, no children under 1 year were found to be positive for IgG antibodies in this study, suggesting that any misclassification derived from the latent period did not bias our results. Finally, we did not collect information, such as number of siblings and birth order, that may be related to transmission of H pylori infection among children.
In conclusion, the performance of the E-plate anti-H pylori IgG antibody test was comparable to that of the stool antigen test, indicating that it might be useful in epidemiologic studies involving large numbers of participants.
CONFLICTS OF INTEREST
SK received a research grant from Eiken Chemical Co, LTD to develop the novel serologic kit. Eiken Chemical Co, LTD had no role in the design or conduct of this study; in the collection, analysis, or interpretation of the data; or in the preparation, review, or approval of this manuscript. The other authors report no competing interests.
REFERENCES
- 1.Ertem D Clinical practice: Helicobacter pylori infection in childhood. Eur J Pediatr. 2012. (in press). [DOI] [PubMed] [Google Scholar]
- 2.McColl KE Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010;362:1597–604 10.1056/NEJMcp1001110 [DOI] [PubMed] [Google Scholar]
- 3.Tan HJ, Goh KL. Extragastrointestinal manifestations of Helicobacter pylori infection: Facts or myth? A critical review. J Dig Dis. 2012;13:342–9 10.1111/j.1751-2980.2012.00599.x [DOI] [PubMed] [Google Scholar]
- 4.Emilia G, Longo G, Luppi M, Gandini G, Morselli M, Ferrara L, et al. Helicobacter pylori eradication can induce platelet recovery in idiopathic thrombocytopenic purpura. Blood. 2001;97:812–4 10.1182/blood.V97.3.812 [DOI] [PubMed] [Google Scholar]
- 5.Kato S, Okamoto H, Nishino Y, Oyake Y, Nakazato Y, Okuda M, et al. Helicobacter pylori and TT virus prevalence in Japanese children. J Gastroenterol. 2003;38:1126–30 10.1007/s00535-003-1218-4 [DOI] [PubMed] [Google Scholar]
- 6.Okuda M, Miyashiro E, Booka M, Tsuji T, Nakazawa T. Helicobacter pylori colonization in the first 3 years of life in Japanese children. Helicobacter. 2007;12:324–7 10.1111/j.1523-5378.2007.00510.x [DOI] [PubMed] [Google Scholar]
- 7.Naito Y, Shimizu T, Haruna H, Fujii T, Kudo T, Shoji H, et al. Changes in the presence of urine Helicobacter pylori antibody in Japanese children in three different age groups. Pediatr Int. 2008;50:291–4 10.1111/j.1442-200X.2008.02587.x [DOI] [PubMed] [Google Scholar]
- 8.Weyermann M, Rothenbacher D, Brenner H. Acquisition of Helicobacter pylori infection in early childhood: Independent contributions of infected mothers, fathers, and siblings. Am J Gastroenterol. 2009;104:182–9 10.1038/ajg.2008.61 [DOI] [PubMed] [Google Scholar]
- 9.Gisbert JP, de la Morena F, Abraira V. Accuracy of monoclonal stool antigen test for the diagnosis of H. pylori infection: A systematic review and meta-analysis. Am J Gastroenterol. 2006;101:1921–30 10.1111/j.1572-0241.2006.00668.x [DOI] [PubMed] [Google Scholar]
- 10.Leal YA, Flores LL, Fuentes-Pananá EM, Cedillo-Rivera R, Torres J. 13C-urea breath test for the diagnosis of Helicobacter pylori infection in children: A systematic review and meta-analysis. Helicobacter. 2011;16:327–37 10.1111/j.1523-5378.2011.00863.x [DOI] [PubMed] [Google Scholar]
- 11.Leal YA, Flores LL, García-Cortés LB, Cedillo-Rivera R, Torres J. Antibody-based detection tests for the diagnosis of Helicobacter pylori infection in children: A meta-analysis. PLoS One. 2008;3:e3751 10.1371/journal.pone.0003751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okuda M, Miyashiro E, Koike M, Tanaka T, Bouoka M, Okuda S, et al. Serodiagnosis of Helicobacter pylori infection is not accurate for children aged below 10. Pediatr Int. 2002;44:387–90 10.1046/j.1442-200X.2002.01585.x [DOI] [PubMed] [Google Scholar]
- 13.R Development CoreTeam. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. [Google Scholar]
- 14.Corvaglia L, Bontems P, Devaster JM, Heimann P, Glupczynski Y, Keppens E, et al. Accuracy of serology and 13C-urea breath test for detection of Helicobacter pylori in children. Pediatr Infect Dis J. 1999;18:976–9 10.1097/00006454-199911000-00008 [DOI] [PubMed] [Google Scholar]
- 15.Okuda M, Sugiyama T, Fukunaga K, Kondou M, Miyashiro E, Nakazawa T. A strain-specific antigen in Japanese Helicobacter pylori recognized in sera of Japanese children. Clin Diagn Lab Immunol. 2005;12:1280–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frenck RW Jr, Fathy HM, Sherif M, Mohran Z, El Mohammedy H, Francis W, et al. Sensitivity and specificity of various tests for the diagnosis of Helicobacter pylori in Egyptian children. Pediatrics. 2006;118:e1195–202 10.1542/peds.2005-2925 [DOI] [PubMed] [Google Scholar]
- 17.Portal-Celhay C, Perez-Perez GI. Immune responses to Helicobacter pylori colonization: Mechanisms and clinical outcomes. Clin Sci (Lond). 2006;110:305–14 10.1042/CS20050232 [DOI] [PubMed] [Google Scholar]
- 18.Gold BD, Khanna B, Huang LM, Lee CY, Banatvala N. Helicobacter pylori acquisition in infancy after decline of maternal passive immunity. Pediatr Res. 1997;41:641–6 10.1203/00006450-199705000-00007 [DOI] [PubMed] [Google Scholar]
- 19.Koletzko S, Jones NL, Goodman KJ, Gold B, Rowland M, Cadranel S, et al. ; H pylori Working Groups of ESPGHAN and NASPGHAN . Evidence-based guidelines from ESPGHAN and NASPGHAN for Helicobacter pylori infection in children. J Pediatr Gastroenterol Nutr. 2011;53:230–43 [DOI] [PubMed] [Google Scholar]
- 20.Howden CW, Hunt RH. Guidelines for the management of Helicobacter pylori infection. Am J Gastroenterol. 1998;93:2330–8 10.1111/j.1572-0241.1998.00684.x [DOI] [PubMed] [Google Scholar]
- 21.She RC, Wilson AR, Litwin CM. Evaluation of Helicobacter pylori immunoglobulin G (IgG), IgA, and IgM serologic testing compared to stool antigen testing. Clin Vaccine Immunol. 2009;16:1253–5 10.1128/CVI.00149-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho B, Marshall BJ. Accurate diagnosis of Helicobacter pylori. Serologic testing. Gastroenterol Clin North Am. 2000;29:853–62 10.1016/S0889-8553(05)70152-7 [DOI] [PubMed] [Google Scholar]