Abstract
Objective
The high expression of cell division cycle 42 protein (CDC42) may be involved in the occurrence and progression of several tumors. However, the expression and function of CDC42 in cervical squamous cell carcinoma remains unclear. This study aimed to investigate the expression of CDC42 in cervical squamous cell carcinoma and its correlation with clinicopathologic characteristics.
Methods
The expression of CDC42 in 162 cervical squamous cell carcinoma tissue samples and 33 normal cervical tissue samples was investigated by immunohistochemistry. The CDC42 mRNA expression was detected by reverse transcription-polymerase chain reaction (RT-PCR).
Results
The cervical squamous cell carcinoma group showed a significantly higher CDC42 positive rate, compared to the normal cervical tissues (P<0.05). Furthermore, the tissues of stage II-IV carcinoma patients showed higher CDC42 expression levels compared to stage I patients (P=0.05). In addition, the expression of CDC42 was not correlated to age of patients, differentiation degree of cancer cells, or lymph node metastasis (P>0.05). Furthermore, compare with normal cervical tissues, the CDC42 mRNA expression in cervical cancer had no significant difference.
Conclusions
CDC42 was up-regulated at protein level, but not mRNA level, in cervical squamous cell carcinoma. The high expression of CDC42 was correlated to the clinical stage of the patients, indicating that CDC42 might contribute to the progression of cervical squamous cell carcinoma.
Keywords: Cell division cycle 42 protein (CDC42), cervical squamous cell carcinoma, expression
Introduction
Cervical cancer is one of the most common malignancies of the female reproductive tract, of which squamous cell carcinoma accounts for 90-95%. It has been suggested that cervical squamous cell carcinoma has a high recurrence rate and poor prognosis. Hence, cervical squamous cell carcinoma has been a serious threat to women health. Cell division cycle 42 protein (CDC42), an important member of the Rho family, is a 25 kD guanosine triphosphate binding protein and shows GTP activity. CDC42 plays key roles in regulation of cell polarity, cytoskeleton and cell cycle. The high expression of CDC42 may be involved in progression of several tumors (1-3). However, the expression and function of CDC42 in cervical squamous cell carcinoma remains unclear. In the present study, we investigated the protein and mRNA expression of CDC42 in cervical squamous cell carcinoma tissue samples by immunohistochemistry and reverse transcription-polymerase chain reaction (RT-PCR), to explore the expression of CDC42 in cervical squamous cell carcinoma and its correlation with clinicopathologic factors.
Materials and methods
Clinical data
Chips of normal cervical tissues (including chronic cervical inflammation) and cervical carcinoma were purchased from Chaoying Biotech Firm (Shanxi, China). The patients from whom the normal cervical tissues were obtained had an average age of 43.09±2.58 years and the patients from whom the 162 cervical squamous cell carcinoma tissues were obtained had an average age of 46.18±0.74 years. All tissues were verified by pathologists under a microscope. The cervical squamous cell carcinoma included 62 cases of International Federation of Gynecology and Obstetrics (FIGO) stage I, 82 cases of stage II, 10 cases of stage III and 1 case of stage IV (7 cases had no relevant information). For cancer differentiation degree, the cervical squamous cell carcinoma included 96 cases of high differentiation, 29 cases of moderate differentiation, and 35 cases of low differentiation (2 cases had no differentiation information). In addition, 9 of the 153 patients with cervical squamous cell carcinoma showed lymph node metastasis. None of the patients received chemotherapy, radiotherapy, or biological therapy.
Detection of CDC42 expression at protein level
Immunohistochemistry was conducted using PV6000 (Zhongshanjingqiao Biotechnical Co., Ltd., Beijing, China) according to the manufacturer’s instruction. Briefly, following dewaxing and hydration, the sections were rinsed using ddH2O, then incubated in 3% H2O2 for 12 min, and incubated in ethylene diamine tetraacetic acid (EDTA) at 90 °C for 10 min. After cooling down, the sections were blocked at 37 °C for 1 h, and then incubated with CDC42 primary antibody (Zhongshanjingqiao Biotechnical Co., Ltd., Beijing, China), and blocked at 37 °C for 1 h, followed by incubation at 4 °C overnight. The next day, the sections were rinsed with PBS and incubated with horseradish peroxidase (HRP)-conjugated Fab IgG at 37 °C for 1 h. After washed with PBS, the sections were stained with 3,3'-diaminobenzidine (DAB) and washed with PBS again. The sections were then re-stained, dehydrated, and mounted. Malignant melanoma sections were used as the positive control, and PBS was used as the negative control to replace the primary antibody.
Detection of CDC42 expression at RNA level
Total RNA extraction
Total RNA was extracted from two sets of tissue samples using Trizol reagent and purified using phenol/chloroform extraction and precipitation/washing according to instructions provided by the manufacturer. The obtained RNA was dissolved in RNase-free water and stored at –80 °C. The mRNAs were reversed into cDNA, and then amplified by PCR.
PCR amplification
The amplification product of CDC42 fragment was 574 bp with forward primer: ATGCAGACAATTAAGTGTGTTGTTGTGGGCGA, and reverse primer: TCATAGCAGCACACACCTGCGGCTCTTCTT. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal reference to determine the adequate quality of extracted mRNA, and the amplification product was 146 bp with forward primer: TGTGCTGCCATATCACCTAGAAAC, and reverse primer: ACTAGACCAAAACCAGTCCTCCA. The reaction system (20 µL) included: 10 µL 2× Premix TaqTMHot Start Version, 1 µL forward primer, 1 µL reverse primer, 6 µL H2O, and 2 µL DNA template. Reaction conditions were: 94 °C 5 min, 35 cycles of 94 °C 30 s, 60 °C 45 s and 72 °C 45 s, and 72 °C 10 min. Products were detached by electrophoresis with 2% agarose gel (120 V, 30 min). Gel was staining by Genefinder observed in a UV gel imager, and the size of amplified fragments was identified compared with the molecular weight marker. Image J software was applied to quantitatively analyze mRNA strip from agarose gel electrophoresis.
Statistical analysis
Data analysis was performed with SPSS13.0 software (SPSS Inc., Chicago, IL, USA). The comparison of CDC42 expression rate was analyzed by chi-square test, and P≤0.05 was considered statistically significant.
Results
Expression of CDC42 at protein level
Immunohistochemistry results
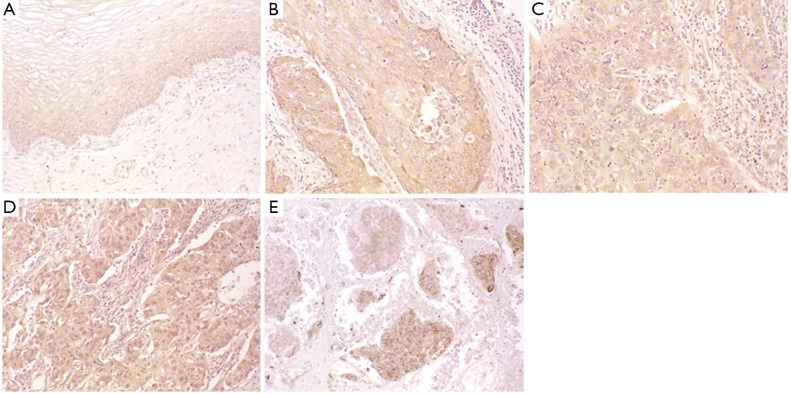
As indicated by the yellow/brown particles, CDC42 was highly expressed in the cytosol. Compared to the normal cervical tissues (60.6%), the cervical squamous cell carcinoma showed a much higher CDC42 positive rate of 77.8% (P<0.05) (Figure 1, Table 1).
Figure 1.
CDC42 expression in normal cervical tissues and cervical squamous cell carcinoma tissues. (A) Normal cervical tissue; (B) Highly-differentiated cervical squamous cell carcinoma tissue; (C) Moderately-differentiated cervical squamous cell carcinoma tissue; (D) Lowly-differentiated cervical squamous cell carcinoma tissue; (E) Malignant melanoma (positive control) (200×).
Table 1. CDC42 expression in normal cervical tissues and cervical squamous cell carcinoma tissues.
| CDC42 | NCS% [n] | SCC% [n] | P |
|---|---|---|---|
| Negative | 42.4 [14] | 22.2 [36] | 0.038* |
| Positive | 57.6 [19] | 77.8 [126] | |
| Total | 33 | 162 |
NCS, normal cervical epithelium tissue; SCC, cervical squamous cell carcinoma; *, P≤0.05.
Correlation between CDC42 expression and clinicopathological factors
The expression of CDC42 was correlated to the clinical stage of the cervical squamous cell carcinoma (P=0.05), but not to age of patients, cancer cell differentiation degree, or lymph node metastasis (P>0.05) (Table 2).
Table 2. Correlation between CDC42 expression and patients’ characteristics.
| Factors | Total [n=162] | CDC42 positive % [n=126] | CDC42 negative % [n=36] | P |
|---|---|---|---|---|
| Age (year) | ||||
| ≤35 | 18 | 83.3 [15] | 16.7 [3] | 0.764 |
| >35 | 144 | 77.1 [111] | 22.9 [33] | |
| Clinical stage | ||||
| I | 62 | 69.4 [43] | 30.6 [19] | 0.050* |
| II, III, IV | 93 | 82.8 [77] | 17.2 [16] | |
| Differentiation | ||||
| High | 96 | 77.1 [74] | 22.9 [22] | 0.713 |
| Moderate | 29 | 82.8 [24] | 17.2 [5] | |
| Low | 35 | 74.3 [26] | 25.7 [9] | |
| LN metastasis | ||||
| No | 153 | 77.1 [118] | 22.9 [35] | 0.680 |
| Yes | 9 | 88.9 [8] | 11.1 [1] |
LN, lymph node; *, P≤ Pmph.
Expression of CDC42 at RNA level
To determine whether the up-regulation of CDC42 in cervical squamous cell carcinoma is from the increase of CDC42 mRNA, the expression of CDC42 mRNA was assayed by RT-PCR in cervical squamous cell carcinoma and normal cervical tissue with GAPDH as an internal reference. The results showed that there was no significant difference (P=0.21) in CDC42 mRNA expression between cervical squamous cell carcinoma and normal cervical tissue (Figures 2,3).
Figure 2.
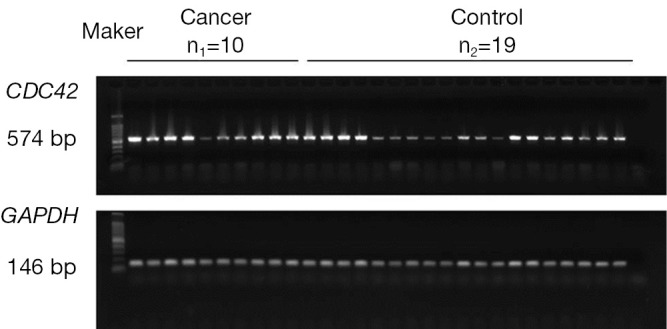
The expression of CDC42 mRNA in cervical squamous cell carcinoma and normal cervical tissue with GAPDH as an internal reference.
Figure 3.
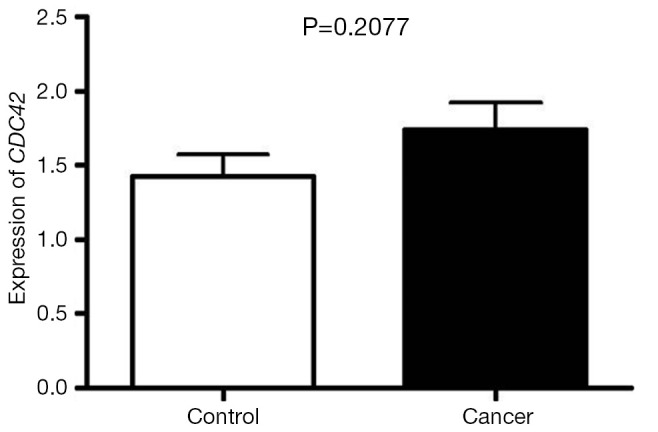
Statistical results of CDC42 mRNA expression in the cervical squamous cell carcinoma and normal cervical tissue.
Discussion
CDC42 was firstly identified in yeasts, and its mutations could reduce the budding rate of yeasts. CDC42 plays an important role of signal converter or molecular switch in the regulation of cell polarity, cytoskeleton and cell cycle. In the present study, we analyzed the expression of CDC42 in cervical squamous cell carcinoma by immunohistochemistry, and our results indicated CDC42 showed a much higher expression level in cervical squamous cell carcinoma tissues than in normal cervical tissues (P<0.05). Our observation suggested that the high expression of CDC42 might contribute to the malignant transformation of cervical epithelial cells. Thus there might be a significant correlation between CDC42 overexpression and the occurrence of cervical squamous cell carcinoma. Mendoza-Catalán et al. determined the expression of CDC42 in cervical pre-malignant lesion samples. The results showed that CDC42 reactivity was moderate/strong in 40.0% of samples without squamous intraepithelial lesions (SIL), 41.2% low-grade SIL (L-SIL), and 61.3% high-grade SIL (H-SIL) (4). Their results also indicated that CDC42 reactivity might be associated with the progression of cervical carcinoma. Overexpression of CDC42 has also been reported in several human cancers, including hepatocellular carcinoma, gastric cancer, head and neck squamous cell carcinoma, testicular cancer, breast cancer, malignant melanoma, colorectal adenocarcinoma, non-small cell lung cancer, and urinary tract cancer (5-12). Studies also have shown that the role of CDC42 in tumor progression is just like an oncogene, which can induce the neoplastic transformation of healthy cells into cancerous ones (13).
In the present study, we found that the tissues from stage II-IV patients showed higher protein expression levels of CDC42, compared to that of stage I patients (P=0.05), indicating that the abnormal protein expression of CDC42 might be related to the progression of cervical squamous cell carcinoma. Furthermore, we found that the up-regulation of CDC42 expression in patients’ specimens with cervical cancer is only at protein expression level, but not mRNA expression level. This result is consistent with our study about mRNA expression microarrays showing CDC42 mRNA expression has no significant difference between normal and cancer cervical tissues (14). Our present study suggested that posttranscriptional regulation of CDC42 could be involved in the development of cervical cancer. It was reported that the overexpression of CDC42 can enhance the activity of Jnk/P38 signaling pathway to promote the growth of yeasts (15). Furthermore, CDC42 can induce the transition of cells from G1 phase to S phase during the process of cell proliferation and subsequently plays an important role in apoptosis (16). In leiomyosarcoma cell lines, active CDC42 can promote the cell cycle of L6 myoblasts, and the dominant-negative mutant of CDC42 can inhibit the proliferation of leiomyosarcoma cells (17). In addition, Olson et al. found that the fibroblasts transitioned from G1 phase to S phase after CDC42 and RAC or RHO were microinjected into quiescent fibroblasts. In contrast, the injection of CDC42 and RAC inhibitors or RHO inhibitor could prevent the transition of serum-induced fibroblasts from G1 phase to S phase (18). The growth suppression of bladder cancer cells was also observed after silencing CDC42 by reducing the transition of cells from G1 phase to S phase (18).
In recent years, trend of incidence age of patients with cervical cancer generally gets much younger. Cervical cancer in ≤35-year-old women is defined as cervical carcinoma in young women (or young cervical carcinoma). In the present study, we found that the expression of CDC42 was similar between ≤35-year-old patients and >35-year-old patients. The reason is probably that cervical adenocarcinoma and other types of rare diseases more frequently occur in women, while our study focused on cervical squamous cell carcinoma, a small number of cases of cervical carcinoma in young women (19). It has been suggested that CDC42 is related to the lymph node metastasis in esophageal squamous cell carcinomas (20), and can promote the invasion and metastasis of cervical cancer by regulating cytoskeletal rearrangements and the maintenance of cell polarity. CDC42 can regulate the integrin signaling pathway and induce the directional migration of the white cells (21). Lai et al. found that the expression levels of RAC1 and CDC42 rapidly reduced when the cell changed the direction of movement (22). Filopodia formation in the process of cell motility was enhanced by activating RAC and CDC42 (23). However, CDC42 knockdown by siRNA can reduce the EGF-stimulated filopodia formation, which may further decrease the ability of cell motility (24).
In conclusion, our study showed that the higher positive rate of CDC42 was observed in cervical squamous cell carcinoma tissues compared to the normal cervical tissues, and the overexpression of CDC42 was associated with the clinical stage of the cervical squamous cell carcinoma, indicating that CDC42 might play an important role in the progression of cervical cancer.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No.11072006, No.10772007 and No.81070078) and National Basic Research Program of China (973 Program, 2013CB933702).
Disclosure: The authors declare no conflict of interest.
References
- 1.Bhoopathi P, Gondi CS, Gujrati M, et al. SPARC mediates Src-induced disruption of actin cytoskeleton via inactivation of small GTPases Rho-Rac-CDC42. Cell Signal 2011;23:1978-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stengel K, Zheng Y.CDC42 in oncogenic transformation, invasion, and tumorigenesis. Cell Signal 2011;23:1415-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calvo F, Sanz-Moreno V, Agudo-Ibanez L, et al. RasGRF suppresses CDC42-mediated tumour cell movement, cytoskeletal dynamics and transformation. Nat Cell Biol 2011;13:819-26 [DOI] [PubMed] [Google Scholar]
- 4.Mendoza-Catalán MA, Cristóbal-Mondragón GR, Adame-Gómez J, et al. Nuclear expression of Rac1 in cervical premalignant lesions and cervical cancer cells. BMC Cancer 2012;12:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 1998;4:844-7 [DOI] [PubMed] [Google Scholar]
- 6.Lang N, Liu M, Tang QL, et al. Effects of microRNA-29 family members on proliferation and invasion of gastric cancer cell lines. Chin J Cancer 2010;29:603-10 [DOI] [PubMed] [Google Scholar]
- 7.Gómez Del Pulgar T, Valdés-Mora F, Bandrés E, et al. CDC42 is highly expressed in colorectal adenocarcinoma and downregulates ID4 through an epigenetic mechanism. Int J Oncol 2008;33:185-93 [PubMed] [Google Scholar]
- 8.Fritz G, Just I, Kaina B.Rho GTPases are over-expressed in human tumors. Int J Cancer 1999;81:682-7 [DOI] [PubMed] [Google Scholar]
- 9.Fritz G, Brachetti C, Bahlmann F, et al. Rho GTPases in human breast tumours: expression and mutation analyses and correlation with clinical parameters. Br J Cancer 2002;87:635-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamai T, Yamanishi T, Shirataki H, et al. Overexpression of RhoA, Rac1, and CDC42 GTPases is associated with progression in testicular cancer. Clin Cancer Res 2004;10:4799-805 [DOI] [PubMed] [Google Scholar]
- 11.Cuiyan Z, Jie H, Fang Z, Kezhi Z, et al. Overexpression of RhoE in Non-small Cell Lung Cancer (NSCLC) is associated with smoking and correlates with DNA copy number changes. Cancer Biol Ther 2007;6:335-42 [DOI] [PubMed] [Google Scholar]
- 12.Kamai T, Tsujii T, Arai K, et al. Significant association of Rho/ROCK pathway with invasion and metastasis of bladder cancer. Clin Cancer Res 2003;9:2632-41 [PubMed] [Google Scholar]
- 13.Stengel KR, Zheng Y. Essential role of CDC42 in Ras-induced transformation revealed by gene targeting. PLoS One 2012;7:e37317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma D, Zhang YY, Guo YL, et al. Profiling of microRNA-mRNA reveals roles of microRNAs in cervical cancer. Chin Med J (Engl) 2012;125:4270-6 [PubMed] [Google Scholar]
- 15.Coso OA, Chiariello M, Yu JC, et al. The small GTP-binding proteins Rac1 and CDC42 regulate the activity of the JNK/SAPK signaling pathway. Cell 1995;81:1137-46 [DOI] [PubMed] [Google Scholar]
- 16.Aznar S, Fernandez-Valeron P, Espina C, et al. Rho GTPases: potential candidates for anticancer therapy. Cancer Lett 2004;206:181-91 [DOI] [PubMed] [Google Scholar]
- 17.Meriane M, Charrasse S, Comunale F, et al. Participation of small GTPases Rac1 and CDC42Hs in myoblast transformation. Oncogene 2002;21:2901-7 [DOI] [PubMed] [Google Scholar]
- 18.Olson MF, Ashworth A, Hall A. An essential role for Rho, Rac, and CDC42 GTPases in cell cycle progression through G1. Science 1995;269:1270-2 [DOI] [PubMed] [Google Scholar]
- 19.Levi F, Lucchini F, Negri E, et al. Cervical cancer mortality in young women in Europe: patterns and trends. Eur J Cancer 2000;36:2266-71 [DOI] [PubMed] [Google Scholar]
- 20.Feng JG, Liu Q, Qin X, et al. Clinicopathological pattern and Annexin A2 and CDC42 status in patients presenting with differentiation and lymphnode metastasis of esophageal squamous cell carcinomas. Mol Biol Rep 2012;39:1267-74 [DOI] [PubMed] [Google Scholar]
- 21.Szczur K, Zheng Y, Filippi MD. The small Rho GTPase CDC42 regulates neutrophil polarity via CD11b integrin signaling. Blood 2009;114:4527-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai SY, Ziober AF, Lee MN, et al. Activated Vav2 modulates cellular invasion through Rac1 and CDC42 in oral squamous cell carcinoma. Oral Oncol 2008;44:683-8 [DOI] [PubMed] [Google Scholar]
- 23.El-Sibai M, Nalbant P, Pang H, et al. CDC42 is required for EGF-stimulated protrusion and motility in MTLn3 carcinoma cells. J Cell Sci 2007;120:3465-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takabayashi T, Takahashi N, Okamoto M, et al. Lipopolysaccharides increase the amount of CXCR4, and modulate the morphology and invasive activity of oral cancer cells in a CXCL12-dependent manner. Oral Oncol 2009;45:968-73 [DOI] [PubMed] [Google Scholar]