Abstract
Background:
Burn injuries are one of the main causes of mortality and morbidity throughout the world and burn patients have higher chances for infection due to their decreased immune resistance. Levamisole, as an immunomodulation agent, stimulates the immune response against infection.
Materials and Methods:
This randomized clinical trial was conducted in Motahari Burn Center, Tehran, Iran. Patients who had second- or third-degree burn with involvement of more than 50% of total body surface area (TBSA) were studied. The levamisole group received levamisole tablet, 100 mg per day. Meantime, both the levamisole and control groups received the standard therapy of the Burn Center, based on a standard protocol. Then, the outcome of the patients was evaluated.
Results:
237 patients entered the study. After excluding 42 patients with inhalation injury, electrical and chemical burns, and the patients who died in the first 72 h, 195 patients remained in the study, including 110 patients in the control group and 85 in the treatment group. The mean age of all patients (between 13 to 64 years) was 33.29 ± 11.39 years (Mean ± SD), and it was 33.86 ± 11.45 years in the control group and 32.57 ± 11.32 years in the treatment group. The mean percentage of TBSA burn was 64.50 ± 14.34 and 68.58 ± 14.55 for the levamisole and control groups, respectively, with the range of 50-100% and 50-95% TBSA. The mortality rate was 68 (61.8%) patients in the control group and 50 (58.8%) patients in the treatment group (P = 0.8).
Conclusion:
According to this study, there was no significant relationship between improvement of mortality and levamisole consumption.
Keywords: Burn, immunomodulation agent, infection, levamisole
INTRODUCTION
Treatment of patients with extensive burns remains a major challenge, even with advances in burn care over recent decades.[1] Burn injuries are one of the main causes of mortality and morbidity throughout the world, and infections and inhalation injuries are the major causes of death following these injuries.[2,3,4,5,6,7,8,9]
Burns greater than 30% of the total body surface area (TBSA) particularly affect the immune system. Both the innate and adaptive, especially the cellular, immune systems are influenced by the thermal injury,[10,11,12,13] and effector mechanisms of nonspecific and specific host defenses are impaired (phagocytosis, chemotaxis, lymphocyte proliferation, antibody production).[14,15,16,17,18,19] Severe burn injury also alters the T cell by inducing an imbalance in T helper (Th) cell functions, caused by a phenotypic imbalance in the regulation of Th1 and Th2 immune response.[20,21,22,23]
Therefore, some ways have been developed to improve the immune response and host resistance to septic challenge in thermally injured patients. It has been indicated that consumption of polymyxin B and interleukin-2 can improve resistance against infections in the burned animal models.[24,25]
Levamisole is a derivative of levo isomer of tetramisole, a potent broad-spectrum anthelmintic.[26,27] In 1972, its immunomodulation effect was observed. It has been used in many clinical trials to treat many diseases including colon carcinomas, breast cancer, advanced malignant diseases, aphthous stomatitis, childhood nephrotic syndrome, chronic idiopathic urticarial, bacterial infections, and HIV infection with different results.-[28,29,30,31,32,33,34] Levamisole stimulates the polymorpho-nuclear lymphocytes (PNL), macrophages, and T lymphocytes, and increases the chemotaxis and proliferation of these cells. Levamisole has an immunostimulant effect by proliferating natural killer cells, which kill T lymphocytes, virus-infected cells, and tumor cells.[24,30,35,36,37,38,39] Some studies have also shown that its consumption is almost effective in increasing immunity in burned human and animal models.[40,41,42,43,44]
Because of this modulatory effect of levamisole on immune system and the impairment of this system in burned patients, a study was designed to evaluate the effect of this drug on the mortality of severe second- and third-degree burns over 50% TBSA.
MATERIALS AND METHODS
This randomized parallel-group clinical trial study was conducted in Burn Center, Motahari Hospital, Iran University of Medical Sciences (IUMS), Iran from 7 July 2010 to 7 September 2011. The study was confirmed by the ethical committee of IUMS and was submitted in Iran clinical trial website (www.irct.ir). Written informed consent was obtained from all the participants (and/or their parents).
The study population included the patients who referred within 24 h of injuries with second- and/or third-degree thermal burns with more than 50% of TBSA. Patients with chemical burn, electrical burn, inhalation injury, underlying disease, and also the patients who expired within 72 h after hospitalization were excluded from the study. All of the admitted burn patients with 50% and more of TBSA who accepted to participate in the study were enrolled; thus, 237 patients entered the study. At the time of admission, they were randomly assigned to two groups of treatment and control. The levamisole group received levamisole tablet (Poorsina, Tehran, Iran) 100 mg/day until discharge from the hospital or death. Except this part of treatment, patients were treated separately based on their condition by surgeon's decisions.
Age, sex, etiology, percentage of the burned area, and outcome of each patient were registered.
The data were analyzed using the independent sample t-test, chi-square test, and analysis of variance (ANOVA) by means of SPSS software, with P < 0.05 considered as the level of significance.
RESULTS
From 7 July 2010 until 7 September 2011, 237 patients aged 13 years and over were enrolled in the study. Data were collected from 133 patients (78.9% males and 21.1% females) in the control group and 104 patients in the treatment group (76.9% males and 23.1% females).
The mean age of all patients (between 13 to 64 years) was 33.29 ± 11.39 years (Mean ± SD). It was 33.86 ± 11.45 years in the control group and 32.57 ± 11.32 years in the treatment group.
The major causes of burn were gas explosion (38%), flame (39.7%), self-immolation (17.3%), electrical burn (1.7%), and some others. All of them had second- and/or third-degree burn and more than 90% of them had head and neck, trunk, and extremities injury simultaneously.
After excluding 42 patients with inhalation injury, electrical and chemical burns, and the patients who died in the first 72 h, 195 patients remained in the study, including 110 patients in the control group (81.8% males and 18.2% females) and 85 in the treatment group (77.6% males and 22.4% females).
The mean percentage of TBSA burn was 64.50 ± 14.34 and 68.58 ± 14.55 for the levamisole and control groups, respectively, with the range of 50-100% and 50-95% TBSA.
Mortality among patients was 77 in the control group and 60 of the treatment group. There was no significant difference in mortality in the two groups (P = 0.929) [Table 1].
Table 1.
Mortality in levamisole and control groups

In both groups, mortality was significantly more common in female patients (P = 0.009 and 0.031, respectively), but mortality was not significantly different in males and females of levamisole and control groups (P = 0.83 and 0.61, respectively).
Levamisole had no effect in reducing mortality in subgroups of different ages (P value between 0.479 and 1) [Table 2].
Table 2.
Mortality in age subgroups of levamisole and control patients
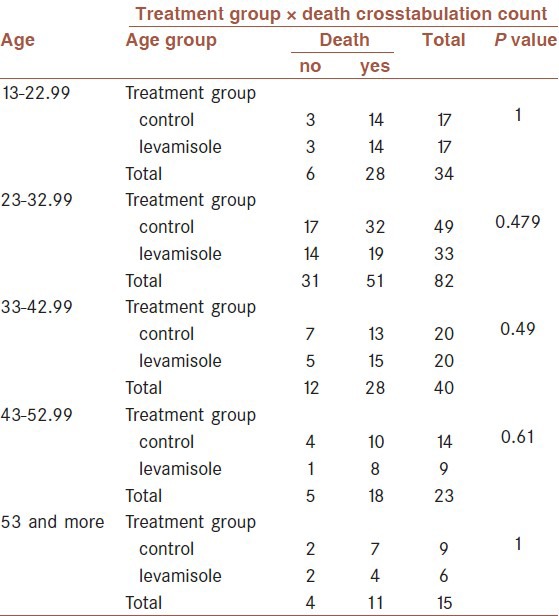
Also, the mortality rate was not significantly different between the two groups at different percentages of burns (P value between 0.66 and 1) [Table 3].
Table 3.
Mortality in different burn percentage of levamisole and control patients
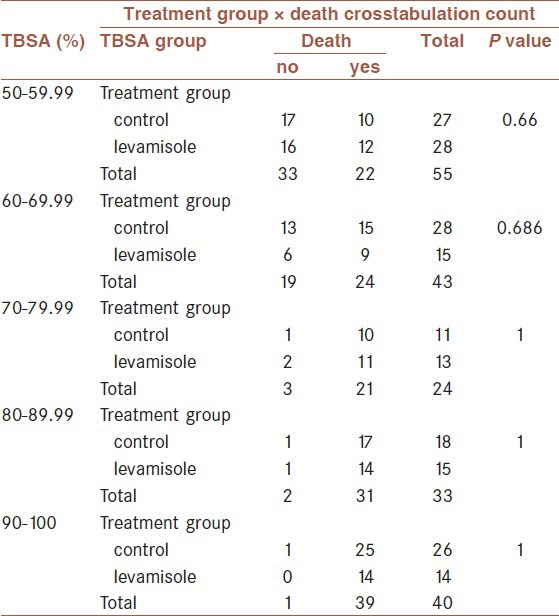
There were significant differences between the two groups in plasma transfusion (P = 0.03) and escharotomy surgery (P = 0.04). The transfusion and escharotomy indications and need were lower in levamisole group.
The differences in the debridement number (P = 0.2) and graft surgery (P = 0.11) were not significant.
DISCUSSION
Even with advances in burn care over recent decades, the treatment of patients with extensive burns remains a major challenge.[1,45] Recent data from the United States indicate 69% mortality among the patients with burns over 70% of TBSA.[45]
The immune system is altered after thermal injury. The severity of immune suppression correlates with the severity of injury. Depressed immune system is one of the major causes of the susceptibility of these patients to infection and sepsis. The humoral immunity is altered, as seen by the decreased levels of immunoglobulins, activation of complement, release of anaphylatoxins, and formation of membrane-attacking complexes. Also, the specific immune response is altered after burn, which includes a depressed ability to produce active rosette-forming cells, depressed stimulation of lymphocyte proliferation, as well as the mixed lymphocyte response. These effects are modulated by the release of kinins, prostaglandins, anaphylatoxins, superoxides, and leukotrienes, all of which can influence the inflammatory response following thermal injury. The immune response is also influenced by some drugs used for other reasons such as steroids, chemotherapeutic agents, and topical agents used for burn wound care.[46,47,48]
Any modulation that can improve the host defense mechanism has a beneficial effect on the rate of sepsis and infection and can decrease the mortality rate of burned patients. The study results of Stinnett et al. showed that immunomodulators could be of benefit in burns; however, not all agents are effective.[40]
Levamisole, a synthetic phenylimidazolthiazole, is a potent antihelmintic agent that was first introduced in 1966.[1,49]
After a few years, it was found that this drug had immunotropic properties and seemingly restored inefficient host defense mechanisms.[50]
In many clinical trials and experimental studies, it has been used as an immunomodulation agent for the treatment of cancers, viral and bacterial infections, and aphthous lesions. Moreover, there are many studies about the mechanism of its action over immune system.[1,2,3,6,9,18,51,52] It is also useful in the treatment of leprosy, based on an Indian clinical trial.[2,49]
Levamisole has been found effective in the improvement of immune system in some studies and had no effects in some other studies.
Niedworok et al. found that the survival time of the transplants was prolonged significantly by levamisole in healthy rabbits and was diminished in rabbits with burn disease.[42]
In a study on burned animals, it was found that continuous infusion of levamisole treatment significantly increased the inflammatory response.[41] The study by Matchin showed that levamisole, antioxidants, and hyperbaric oxygenation facilitated most rapid recovery of the immunological status in burned patients.[43]
In Stinnett et al.'s study in animal model, oral administration of levamisole did not reduce mortality in burned guinea pig, which was infected with Pseudomonas microorganism.[40]
The price of levamisole is low and has a few side effects if it is used for a short period of time. The plasma elimination half-life of levamisole is between 3 and 4 h. Levamisole is metabolized by the liver and the metabolites are excreted mainly by the kidneys (70% over 3 days). The elimination half-life of metabolite excretion is approximately 16 h, and 5% is excreted in the feces.[53]
The complications of levamisole include nausea, vomiting, fever, dizziness, headache, skin eruptions, mild anemia, and elevation of liver enzymes. Rare cases of side effects include agranulocytosis, thrombocytopenia, convulsion, and leukoencephalopathy. We did not evaluate the complications of the medication in our patients because the same complications are common in severely burned patients.[31,32,54,55,56,57]
This study indicated no significant relationship between mortality and improvement outcome with levamisole consumption in the patients with third-degree burn with more than 50% of TBSA. This meant that the levamisole group had an equivalent chance for improvement in comparison with the control group.
Our study has some limitations. First of all, we did not measure any immunological response such as immunoglobulin or immune cellular functions. We only evaluated the mortality as an index of better immunity of the patients and it is not enough.
Extensively burned patients were selected for this study. These patients had a high mortality rate and the immune modulation may be insufficient for reducing mortality in these patients. Also, the gastrointestinal absorption of the medication was not predictable in the victims of extensive burns and the blood level of levamisole was not measured.
Although this study showed no beneficial effect of levamisole in extensively burned patients, repetition of the study for less-extensively burned patients and evaluation of blood level of levamizole during the study can clarify the immunomodulation and infection-prevention effects of this medication in thermal injury.
CONCLUSION
Based on these findings, it can be suggested that levamisole consumption in second- and third-degree burn patients with the TBSA of more than 50% had no effect on mortality rate. It appears that more randomized trials with less-extensively burned patients are required to evaluate the exact blood level of levamisole in order to fully establish the efficacy of levamisole on the improvement and mortality rate of these patients.
Footnotes
Source of Support: The study received funding for research from Iran University of Medical Sciences
Conflict of Interest: None declared.
REFERENCES
- 1.Brusselaers N, Hoste EA, Monstrey S, Colpaert KE, De Waele JJ, Vandewoude KH, et al. Outcome and changes over time in survival following severe burns from 1985 to 2004. Intensive Care Med. 2005;31:1648–53. doi: 10.1007/s00134-005-2819-6. [DOI] [PubMed] [Google Scholar]
- 2.Olaitan PB, Olaitan JO. Burns and scalds — epidemiology and prevention in a developing country. Niger J Med. 2005;14:9–16. doi: 10.4314/njm.v14i1.37128. [DOI] [PubMed] [Google Scholar]
- 3.O’Keefe GE, Hunt JL, Purdue GF. An evaluation of risk factors for mortality after burn trauma and the identification of gender-dependent differences in outcomes. J Am Coll Surg. 2001;192:153–60. doi: 10.1016/s1072-7515(00)00785-7. [DOI] [PubMed] [Google Scholar]
- 4.Angus DC, Wax RS. Epidemiology of sepsis: An update. Crit Care Med. 2001;29(7 Suppl):S109–16. doi: 10.1097/00003246-200107001-00035. [DOI] [PubMed] [Google Scholar]
- 5.Abraham E, Matthay MA, Dinarello CA, Vincent JL, Cohen J, Opal SM, et al. Consensus conference definitions for sepsis, septic shock, acute lung injury, and acute respiratory distress syndrome: Time for a reevaluation. Crit Care Med. 2000;28:232–5. doi: 10.1097/00003246-200001000-00039. [DOI] [PubMed] [Google Scholar]
- 6.Ekenna O, Sherertz RJ, Bingham H. Natural history of bloodstream infections in a burn patient population: The importance of candidemia. Am J Infect Control. 1993;21:189–95. doi: 10.1016/0196-6553(93)90030-8. [DOI] [PubMed] [Google Scholar]
- 7.Bruitt BA., Jr The diagnosis and treatment of infection in burn patients. Burns. 1984;11:79–91. doi: 10.1016/0305-4179(84)90129-3. [DOI] [PubMed] [Google Scholar]
- 8.Howes RM, Allen RC, Su CT, Hoopes JE. Altered polymorphonuclear leukocyte bioenergetics in patients with thermal injury. Surg Forum. 1976;27:558–60. [PubMed] [Google Scholar]
- 9.Howard RJ, Simmons RL. Acquired immunologic deficiencies after trauma and surgical procedures. Surg Gynecol Obstet. 1974;139:771–82. [PubMed] [Google Scholar]
- 10.Edwards-Jones V, Greenwood JE Manchester Burns Research Group. What's new in burn microbiology? Burns. 2003;29:15–24. doi: 10.1016/s0305-4179(02)00203-6. [DOI] [PubMed] [Google Scholar]
- 11.O’Sullivan ST, O’Connor TP. Immunosuppression following thermal injury: The pathogenesis of immunodysfunction. Br J Plast Surg. 1997;50:615–23. doi: 10.1016/s0007-1226(97)90507-5. [DOI] [PubMed] [Google Scholar]
- 12.Lederer JA, Rodrick ML, Mannick JA. The effects of injury on the adaptive immune response. Shock. 1999;11:153–9. doi: 10.1097/00024382-199903000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Gennari R, Alexander JW. Anti-interleukin-6 antibody treatment improves survival during gut-derived sepsis in a time-dependent manner by enhancing host defense. Crit Care Med Crit Care Med. 1995;23:1945–53. doi: 10.1097/00003246-199512000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Schlüter B, König W, Köller M, Erbs G, Müller FE. Differential regulation of T- and B-lymphocyte activation in severely burned patients. J Trauma. 1991;31:239–46. [PubMed] [Google Scholar]
- 15.Schlüter B, König W, Köller M, Erbs G, Müller FE. Studies on B-lymphocyte dysfunctions in severely burned patients. J Trauma. 1990;30:1380–9. doi: 10.1097/00005373-199011000-00011. [DOI] [PubMed] [Google Scholar]
- 16.McIrvine AJ, O’Mahony JB, Saporoschetz I, Mannick JA. Depressed immune response in burn patients: Use of monoclonal antibodies and functional assay to define the role of suppressor cells. Ann Surg. 1982;196:297–304. doi: 10.1097/00000658-198209000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakai H, Daniels JC, Beathard GA, Lewis SR, Lynch JB, Ritzmann SE. Mixed lymphocyte culture reaction in patients with acute thermal Burns. J Trauma. 1974;14:53–7. doi: 10.1097/00005373-197401000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Calvano SE, deRiesthal HF, Marano MA, Antonacci AC. The decrease in peripheral blood CD4 and T-cell following thermal injury in human can be accounted for by a concomitant decrease in suppressor-inducer CD4 and T cells as assessed using anti CD4. Clin Immunol Immunopathol. 1988;47:164–73. doi: 10.1016/0090-1229(88)90069-4. [DOI] [PubMed] [Google Scholar]
- 19.Ninnemann JL. Trauma, Sepsis and the immune response. J Burn Care Rehabil. 1987;8:462–8. [PubMed] [Google Scholar]
- 20.Guo Z, Kavanagh E, Zang Y, Dolan SM, Krignovich SJ, Mannick JA, et al. Burn injury promotes antigen-driven Th2-type responses in vivo. J Immunol. 2003;171:3983–90. doi: 10.4049/jimmunol.171.8.3983. [DOI] [PubMed] [Google Scholar]
- 21.Kelly JL, O’Suilleabhain CB, Soberg CC, Mannick JA, Lederer JA. Severe injury triggers antigen-specific T-helper cell dysfunction. Shock. 1999;12:39–45. doi: 10.1097/00024382-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 22.O’Sullivan ST, Lederer JA, Horgan AF, Chin DH, Mannick JA, Rodrick ML. Major injury leads to predominance of T helper-2 lymphocyte phenotype and diminished interleukin-12 production associated with decreased resistance to infection. Ann Surg. 1995;222:482–90. doi: 10.1097/00000658-199522240-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyons A, Kelly JL, Rodrick ML, Mannick JA, Lederer JA. Major injury induces increased production of interleukin-10 by cells of the immune system with a negative impact on resistance to infection. Ann. Ann Surg. 1997;226:450–8. doi: 10.1097/00000658-199710000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gough DB, Moss NM, Jordan A, Grbic JT, Rodrick ML, Mannick JA. Recombinant Interleukin-2 improves immune response and host resistance to septic challenge in thermally injured mice. Surgery. 1988;104:292–300. [PubMed] [Google Scholar]
- 25.Alexander JW. Texas: Alteration of opsonic activity after burn injury, proceeding of the 40th Anniversary symposium U.S. Army institute or surgical research. Fortsam Houston; 1989. p. 126. [Google Scholar]
- 26.Renoux G, Renoux M. Modulation of immune reactivity by phenylimidothiazole salts in mice immunized by sheep red blood cells. J Immunol. 1974;113:779–90. [PubMed] [Google Scholar]
- 27.Brunner CJ, Muscoplat CC. Immunomodulatory effects of Levamisole. J Am Vet Med Assoc. 1980;176:1159–62. [PubMed] [Google Scholar]
- 28.Symoens J, Rosenthal M. Levamisole in the modulation of the immune response: The current experimental and clinical state. J Reticuloendothel Soc. 1977;21:175–221. [PubMed] [Google Scholar]
- 29.Szeto C, Gillespie KM, Mathieson PW. Levamisole induces interleukin-18 and shifts type 1/type2 cytokine balance. Immunology. 2000;100:217–24. doi: 10.1046/j.1365-2567.2000.00042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bozić F, Bilić V, Valpotić I. Modulating by Levamisole of CD45RA and CD45RC isoforms expression in gut of weaned pigs vaccinated against colibacillosis. J Vet Pharmacol Ther. 2002;25:69–72. doi: 10.1046/j.1365-2885.2002.00379.x. [DOI] [PubMed] [Google Scholar]
- 31.Hibi D, Imazawa T, Kijima A, Suzuki Y, Ishii Y, Jin M, et al. Investigation of carcinogenicity for levamisole administered in the diet to F344 rats. Food Chem Toxicol. 2010;48:3321–6. doi: 10.1016/j.fct.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 32.Boyer O, Moulder JK, Grandin L, Somers MJ. Short- and long-term efficacy of levamisole as adjunctive therapy in childhood nephrotic syndrome. Pediatr Nephrol. 2008;23:575–80. doi: 10.1007/s00467-007-0708-7. [DOI] [PubMed] [Google Scholar]
- 33.Ejlertsen B, Mouridsen HT, Jensen MB, Andersen J, Andersson M, Kamby C, et al. Cyclophosphamide, methotrexate, and fluorouracil; Oral cyclophosphamide; Levamisole; or no adjusant therapy for patients with high-risk, premenopausal breast cancer. Cancer. 2010;116:2081–9. doi: 10.1002/cncr.24969. [DOI] [PubMed] [Google Scholar]
- 34.Sayad B, Alavian SM, Najafi F, Soltani B, Shirvani M, Janbakhsh A, et al. Effects of oral levamisole as an adjuvant to hepatitis b vaccine in HIV/ AIDS patients: A randomized controlled trial. Hepat Mon. 2012;12:e6234. doi: 10.5812/hepatmon.6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bozić F, Lacković G, Kovsca-Janjatović A, Smolec O, Valpotić I. Levamisole synergizes experimental F4ac+ Escherchiacoli oral vaccine in stimulating ileal peyer's patch T cell in weaned pigs. J Vet Pharmacol Ther. 2006;29:199–204. doi: 10.1111/j.1365-2885.2006.00731.x. [DOI] [PubMed] [Google Scholar]
- 36.Janjatović AK, Lacković G, Bozić F, Popović M, Valpotić I. Levamisole synergizes proliferation of intestinal IgA+ cells in weaned pigs immunized with vaccine candidate F4ac+ nonenterotoxigenic Esherchia coli strain. J Vet Pharmacol Ther. 2008;31:328–33. doi: 10.1111/j.1365-2885.2008.00961.x. [DOI] [PubMed] [Google Scholar]
- 37.Fata F, Mirza A, Craig G, Nair S, Law A, Gallagher J, et al. Efficacy and toxicity of adjuvant therapy in elderly patients with colon carcinoma: A 10 years experience of the Geisinger Medical center. Cancer. 2002;94:1931–8. doi: 10.1002/cncr.10430. [DOI] [PubMed] [Google Scholar]
- 38.Grinev MV. Cytoreductive surgery as an alternative to palliative operation in oncology. Vestn Khir Im I I Grek. 2002;161:100–3. [PubMed] [Google Scholar]
- 39.Karaoglan M, Demirci F, Coskun Y, Karaoglan L, Bayraktaroglu Z, Okan V, et al. Immunomodulation therapy in children with chronic hepatitis B. J Natl Med Assoc. 2006;98:143–7. [PMC free article] [PubMed] [Google Scholar]
- 40.Stinnett JD, Loose LD, Miskell P, Tenney CL, Gonce SJ, Alexander JW. Synthetic immunomodulators for prevention of fatal infection in a burned guiea pig model. Ann Surg. 1983;198:53–7. doi: 10.1097/00000658-198307000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McManus AT. Examination of neutrophil function in a rat model of decreased host resistance following burn trauma. Rev Infect Dis. 1983;5(Suppl 5):S898–907. doi: 10.1093/clinids/5.supplement_5.s898. [DOI] [PubMed] [Google Scholar]
- 42.Niedworok J, Offierska M, Lambrecht W. Effect of Levamisole on the survival time of skin Transplants in burn. Z Exp Chir. 1980;13:151–4. [PubMed] [Google Scholar]
- 43.Matchin EN, Atiasov NI, Zuev PD, Vinogradova GV. Methods of correction of humeral and cellular immunity in patients with burns and erosive-ulcerative Levamisole lesions of the gastrointestinal tract. Vestn Khir Im I I Grek. 1990;144:44–9. [PubMed] [Google Scholar]
- 44.Wang Y, Tang HT, Xia ZF, Zhu SH, Ma B, Wei W, et al. Factors affecting survival in adult patients with massive burns. Burns. 2010;36:57–64. doi: 10.1016/j.burns.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 45.American Burn Association. National Burn Repository 2005 report. Available from: http://WWW.ameriburn.org/NBR2005.pdf] 08/03/2013 .
- 46.Alexander JW. Serum and Leukocyte lysosomal enzymes: Derangements following severe thermal injury. Arch Surg. 1967;95:482–91. doi: 10.1001/archsurg.1967.01330150158020. [DOI] [PubMed] [Google Scholar]
- 47.Schwacha MG, Somers SD. Thermal injury-induced immunosuppression in mice. The role of macrophage-derived reactive nitrogen intermediates. J Leukoc Biol. 1998;63:51–8. doi: 10.1002/jlb.63.1.51. [DOI] [PubMed] [Google Scholar]
- 48.Heideman M, Bengtsson A. The immunologic response to thermal injury. World J Surg. 1992;16:53–6. doi: 10.1007/BF02067115. [DOI] [PubMed] [Google Scholar]
- 49.Chen LY, Lin YL, Chiang BL. Levamisole enhances immune response by affecting the activation and maturation of human monocyte-derived dendritic cells. British Society for Immunology, Clin Exp Immunol. 2007;151:174–81. doi: 10.1111/j.1365-2249.2007.03541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swierenga J, Gooszen HC, Vanderschueren RG, Cosemans J, Louwagie A, Stam J, et al. Immunopotentiation with levamisole in resectable bronchogenic carcinoma: A double-blind controlled trial, study group for bronchogenic carcinoma. Br Med J. 1975;3:461–4. doi: 10.1136/bmj.3.5981.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hedge M, Karki SS, Thomas E, Kumar S, Panjamurthy K, Ranganatha SR, et al. Novel levamisole derivative induces extrinsic pathway of apoptosis in cancer cell and inhabits tumor progression in mice. Plos One. 2012;7:1–13. doi: 10.1371/journal.pone.0043632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Al-Ibrahim AA, Al-kharraz SM, Al-Sadoon DM, Al-Madani AJ, Al-Musallam SA. Levamisole therapy as a second — line immunosuppressive agent in corticosteroid — sensitive nephritic Syndrome in children. Saudi J Kidney Dis Transplant. 2003;14:153–7. [PubMed] [Google Scholar]
- 53.Argani H, Akhtarishojaie E. Levamizole enhances immune responsiveness to intra-dermal and intra-muscular hepatitis B vaccination in chronic hemodialysis patients. J Immune Based Ther Vaccines. 2006;4:3. doi: 10.1186/1476-8518-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ching JA, Smith DJ., Jr Levamisole-induced necrosis of skin, soft tissue, and bone: Case report and review of literature. J Burn Care Res. 2012;33:e1–5. doi: 10.1097/BCR.0b013e318233fc64. [DOI] [PubMed] [Google Scholar]
- 55.Won TH, Park SY, Kim BS, Seo PS, Park SD. Levamisole monotherapy for oral lichen planus. Ann Dermatol. 2009;21:250–4. doi: 10.5021/ad.2009.21.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu N, Zhou W, Li S, Zhou G, Zhang N, Liang J. Clinical and MRI characteristics of levamisole-induced leukoencephalopathy in 16 patients. J Neuroimaging. 2009;19:326–31. doi: 10.1111/j.1552-6569.2008.00344.x. [DOI] [PubMed] [Google Scholar]
- 57.Lee KC, Ladizinski B, Federman DG. Complications associated with use of levamisole-contaminated cocaine: An emerging public health challenge. Mayo Clin Proc. 2012;87:581–6. doi: 10.1016/j.mayocp.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


