Abstract
Background:
The objective of this study was to investigate whether the sex hormone binding globulin (SHBG) levels before conception are predictive of gestational diabetes mellitus (GDM) in women with polycystic ovarian syndrome (PCOS).
Materials and Methods:
A total of 180 women with PCOS were enrolled and followed up during pregnancy. Diagnosis of GDM was based on a 2-hour, 75 g oral glucose tolerance test (GTT) performed at 24-28 weeks of gestational age. SHBG levels were measured from serum samples that had collected before conception. We examined the incidence of GDM and plotted a receiver operating characteristic (ROC) curve to assess discrimination.
Results:
Of the 180 women, 50 (27.8%) were diagnosed with GDM. Those with lower levels of SHBG before conception were more likely to develop GDM than those with higher SHBG (44.4 ± 14.8 nmol/l vs. 63.5 ± 22.7 nmol/l, P < 0.001). The area under the ROC was 77.0% (95% confidence interval [CI] 71.3-78.8). The optimal cut-off value for detecting GDM was a SHBG ≥62.5 nmol/l. For every 1 nmol/l increase in SHBG value, there was a 7% reduction in the risk for development of GDM (Odds ratio 0.93 [95% CI 0.90-0.96], P < 0.001).
Conclusion:
In women with PCOS preconception, SHBG levels are strongly associated with development of GDM.
Keywords: Gestational diabetes, polycystic ovary syndrome, Rotterdam criteria, SHBG
INTRODUCTION
Polycystic ovarian syndrome (PCOS) is the most common endocrinopathy, present in 15.2% of the Iranian women of reproductive age, based on the Rotterdam criteria.[1] Insulin may act directly and/or indirectly through the pituitary to stimulate ovarian androgen production.[2,3]
The most commonly used definition of PCOS include at least two of the three following criteria: Clinical and/or biochemical signs of hyperandrogenism, oligo—and/or anovulation and polycystic ovary on ultrasonography excluding other related diseases such as adrenal congenital hyperplasia, Cushing's syndrome, and androgen secreting tumors.[4] During pregnancy, increased peripheral tissue resistance to insulin has been demonstrated, which is primary caused by placental hormone and a preexisting state of insulin resistance frequently associated with PCOS.[5] In nonpregnant women, low level of sex hormone binding globulin (SHBG) have been associated with insulin resistance and the subsequent development of type 2 diabetes mellitus.[6,7,8,9] Among pregnant women, low level of SHBG have been found at the time of the diagnosis of gestational diabetes mellitus (GDM).[10] A recent study of different SHBG polymorphism showed evidence of a genetic correlation between SHBG levels and type 2 diabetes independent of body mass index (BMI).[11] This could indicate a possible causal role of SHBG in the glucose regulation pathway.
The purpose of this study was to investigate whether the SHBG levels before conception are predictive of GDM in women with PCOS.
MATERIALS AND METHODS
This prospective cohort study was conducted from January 2009 to March 2013 in Isfahan, Iran. Our study sample comprised 180 infertile women diagnosed with PCOS that were conceived. All the patients attended the clinics in Shahid Beheshti Hospital, which is affiliated to Isfahan University of Medical Sciences, Iran (research project Number 390038). We only included the subjects who were aged between 18 and 42 years. They were diagnosed retrospectively according to the European Society for Human Reproduction and Embryology and the American Society for Reproductive Medicine (ESHRE/ASRM) criteria, that is, presence of at least two of the following conditions: chronic anovulation, hyperandrogenism, and polycystic ovaries.[4]
Written informed consent was obtained from all participants and the study was approved by the ethics committee at Isfahan University of Medical Sciences. All women underwent standardized screening at the study inclusion, including a physical examination, transvaginal ultrasound scan of ovaries and metabolic and endocrine laboratory measurements, as described previously.[12]
Waist and hip circumference was measured to the nearest 0.5 cm with a measuring tape. Waist was measured midway between the lower rib margin and the iliac-crest at the end of a gentle expiration. Hip circumference was measured over the greater trochanters directly over the underwear. Laboratory measurements were repeated annually until pregnancy was achieved. We excluded women with fasting plasma glucose levels >92 mg/dl at first prenatal care and women with any type of pre-GDM and/or concomitant disease such as heart disease, kidney disease, chronic hypertension, hypothyroidism, hyperthyroidism or autoimmune disease, and asthma. Screening for GDM was performed in 24-28 gestational weeks using a oral glucose tolerance test, 3 hours after 100 mg of glucose (OGTT-3h-100 g). The diagnosis of GDM was established with two or more abnormal values in accordance with the American Diabetes Association criteria[13] (Fasting ≥ 92 mg/dl, 1h ≥ 180 mg/dl, 2h ≥ 153 mg/dl). SHBG was quantified using the modular E 170 (Roche diagnostic, Mannheim, Germany).
Statistical analysis
Quantitative variables were expressed as mean ± SD and were compared between groups using student t-test. Quantitative variables were expressed as frequencies in percents and were analyzed by chi-square or Fisher exact test. Multivariate binary logistic regressions were fitted to identify predictors of new-onset GDM using the SPSS version 15 for Windows (SPSS Inc., Chicago, IL, USA). We considered the following covariates in the multivariate-adjusted analyses: Age, waist circumference, BMI, HDL, triglyceride, SHBG, insulin, and HOMA-IR. The ability of SHBG value to predict incidence of GDM was examined by receiver operating characteristic (ROC) curve and their respective areas under the curve, in which sensitivity is plotted as a function of 1-specificity. The area under the ROC curve is a global summary statistic of the discriminative value of a model, describing the probability that the score is higher in an individual developing than in an individual not developing GDM. All tests for statistical significance were two-tailed, and performed assuming a type I error probability of <0.05.
RESULTS
Of the 189 pregnant women with PCOS, 9 women were excluded because of incomplete data. Of the remaining 180 women, 50 (27.8%) had GDM. The preconceptional characteristics of the 130 (72.2%) PCOS women without GDM and 50 (27.8%) with GDM are shown in Table 1. Those who developed GDM had higher mean waist circumference, weight, triglyceride, insulin and HOMA-IR, and have lower preconception SHBG and HDL level. The mean (SD) SHBG was 44.4 (14.9) nmol/l for those with GDM and 63.5 (32.7) nmol/l for those without GDM (P < 0.001).
Table 1.
Preconceptional characteristic of PCOS women with and without GDM
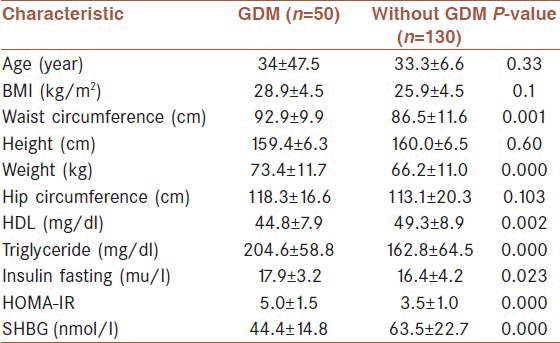
The overall incidence of subsequent GDM was 27.8% (95% CI: 21.2-34.3). To determine the independent predictors of factors associated with GDM, stepwise binary logistic regression was used to test seven predictor variables (age, waist circumference, BMI, HDL, triglyceride, insulin, and HOM-IR). The risk of GDM increased with decreasing SHBG levels. In adjusted analyses, there was a significant negative association between SHBG levels and GDM (Odds ratio [OR] [95% CI] = 0.93 [0.90-0.96], P < 0.001).
The ROC curve for the incidence of GDM for SHBG is shown in Figure 1. The areas under the ROC curves were 0.769 (95% CI 0.692-0.845) for SHBG. SHBG was a significant predictor for future risk of GDM (P < 0.001). The optimal cut-off value for detecting GDM was a SHBG level of greater or equal to 62.5, at which the sensitivity was 92% and specificity was 50.8. The positive and negative predictive value of the SHBG for prediction of GDM development were 41.8% and 94.3%, respectively.
Figure 1.
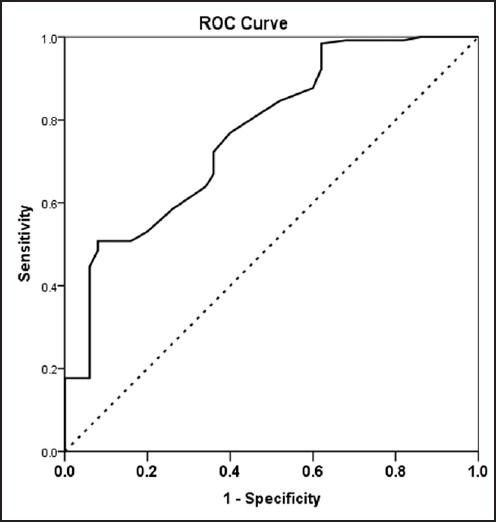
Receiver operating characteristic (ROC) curve for SHBG for prediction of gestational diabetes in PCOS women. The estimates of the area under the ROC curves and their 95% confidence intervals are shown
DISCUSSION
In this study, the SHBG has a good ability to predict GDM in a cohort of 180 PCOS pregnant women, with an area under the ROC of 77%, and comparable to its ability to predict GDM. The worldwide prevalence of GDM varies from 1% to 14% depending on the population studied and the diagnostic criteria used.[14] Women with PCOS have a 3-fold increased risk of developing GDM compared with women without PCOS.[15] We found that the lower preconceptional SHBG values were associated with an increased risk of the development of GDM, which was diagnosed at 24-28 weeks of gestation. Women with PCOS display decreased level of SHBG. This glycoprotein, produced in the liver, binds most sex steroids. The synthesis of SHBG is suppressed by insulin. SHBG levels have also been linked to impaired glucose control and a risk for developing type 2 diabetes mellitus (DM).[11] The mechanism of this association is not fully understood and may reflect a role for SHBG in glucose homeostasis. Moreover, in several studies, a relationship has been found between the lower plasma SHBG levels in first trimester pregnancies and subsequent GDM.[16,17]
Importantly, unlike other markers of insulin resistance, SHBG is reliable in the nonfasting state and exhibits no diurnal variation,[18] which renders SHBG as a unique marker of insulin resistance that is useful especially in clinical situations when fasting blood samples are not collected routinely such as during prenatal care. Women in the two groups (GDM and non-GDM) were not significantly different in terms of BMI, but the mean of the waist circumference was significantly higher in GDM women. The distribution of body fat has a strong influence on SHBG levels. Android or central fat is located in the abdominal wall and visceral mesenteric locations. This fat distribution is associated with hyperinsulinemia and decreased level of SHBG.
This study may draw criticism because of the use of a relatively small sample size of PCOS women. Large prospective studies of PCOS need to be performed to determine the actual risk of developing GDM and, in addition, to search for differing biological mechanisms that may explain why some PCOS women appear to remain clinically silent for life or develop GDM very late. Despite the above limitations, the findings here add to our understanding of the developing rate to GDM in women with PCOS in Iran. Furthermore, this study provides new data from Iran, a developing country that has been underrepresented in past studies.
Reliable prediction of GDM would need interventions aimed at reducing the incidence of GDM in women with a history of PCOS such as life style changing and medical treatment with the use of pre- and transgestational metformin.
CONCLUSION
These data provide further evidence that the preconception SHBG is an effective tool for identification of PCOS women at increased risk of developing GDM in Iran.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Mehrabian F, Khani B, Kelishadi R, Ghanbari E. The prevalence of polycystic ovary syndrome in Iranian women based on different diagnostic criteria. Endokrynol Pol. 2011;62:238–42. [PubMed] [Google Scholar]
- 2.Cara JF, Rosenfield RL. Insulin-like growth factor I and insulin potentiate luteinizing hormone-induced androgen synthesis by rat ovarian thecal-interstitial cells. Endocrinology. 1988;123:733–9. doi: 10.1210/endo-123-2-733. [DOI] [PubMed] [Google Scholar]
- 3.Nestler JE, Jakubowicz DJ. Decreases in ovarian cytochrome P450c17 alpha activity and serum free testosterone after reduction of insulin secretion in polycystic ovary syndrome. N Engl J Med. 1996;335:617–23. doi: 10.1056/NEJM199608293350902. [DOI] [PubMed] [Google Scholar]
- 4.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Dunaif A. Insulin resistance and the polycystic ovary syndrome: Mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 6.Haffner SM, Katz MS, Stern MP, Dunn JF. The relationship of sex hormones to hyperinsulinemia and hyperglycemia. Metabolism. 1988;37:683–8. doi: 10.1016/0026-0495(88)90091-1. [DOI] [PubMed] [Google Scholar]
- 7.Haffner SM, Valdez RA, Morales PA, Hazuda HP, Stern MP. Decreased sex hormone-binding globulin predicts noninsulin-dependent diabetes mellitus in women but not in men. J Clin Endocrinol Metab. 1993;77:56–60. doi: 10.1210/jcem.77.1.8325960. [DOI] [PubMed] [Google Scholar]
- 8.Lindstedt G, Lundberg PA, Lapidus L, Lundgren H, Bengtsson C, Björntorp P. Low sex-hormone-binding globulin concentration as independent risk factor for development of NIDDM. 12-yr follow-up of population study of women in Gothenburg, Sweden. Diabetes. 1991;40:123–8. doi: 10.2337/diab.40.1.123. [DOI] [PubMed] [Google Scholar]
- 9.Sherif K, Kushner H, Falkner BE. Sex hormone-binding globulin and insulin resistance in African-American women. Metabolism. 1998;47:70–4. doi: 10.1016/s0026-0495(98)90195-0. [DOI] [PubMed] [Google Scholar]
- 10.Bartha JL, Comino-Delgado R, Romero-Carmona R, Gomez-Jaen MC. Sex hormone-binding globulin in gestational diabetes. Acta Obstet Gynecol Scand. 2000;79:839–45. [PubMed] [Google Scholar]
- 11.Ding EL, Song Y, Manson JE, Hunter DJ, Lee CC, Rifai N, et al. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med. 2009;361:1152–63. doi: 10.1056/NEJMoa0804381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehrabian F, Khani B, Kelishadi R, Kermani N. The prevalence of metabolic syndrome and insulin resistance according to the phenotypic subgroups of polycystic ovary syndrome in a representative sample of Iranian females. J Res Med Sci. 2011;16:763–9. [PMC free article] [PubMed] [Google Scholar]
- 13.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34(Suppl):562. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27(Suppl 1):S5–10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- 15.Boomsma CM, Eijkemans MJ, Hughes EG, Visser GH, Fauser BC, Macklon NS. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update. 2006;12:673–83. doi: 10.1093/humupd/dml036. [DOI] [PubMed] [Google Scholar]
- 16.Smirnakis KV, Plati A, Wolf M, Thadhani R, Ecker JL. Predicting gestational diabetes: choosing the optimal early serum marker. Am J Obstet Gynecol. 2007;196:410.e1–7. doi: 10.1016/j.ajog.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 17.Thadhani R, Wolf M, Hsu-Blatman K, Sandler L, Nathan D, Ecker JL. First-trimester sex hormone binding globulin and subsequent gestational diabetes mellitus. Am J Obstet Gynecol. 2003;189:171–6. doi: 10.1067/mob.2003.343. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton-Fairley D, White D, Griffiths M, Anyaoku V, Koistinen R, Seppälä M, et al. Diurnal variation of sex hormone binding globulin and insulin-like growth factor binding protein-1 in women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 1995;43:159–65. doi: 10.1111/j.1365-2265.1995.tb01910.x. [DOI] [PubMed] [Google Scholar]


