Abstract
Background:
Prostate cancer is one of the most common cancer types both in western and eastern countries, involving mostly elder men. The mechanisms underlying the prostate cancer development remain unclear. Prostate-specific antigen (PSA) is the only well accepted marker for prostate cancer diagnosis and prognosis. The diagnosis and treatment of prostate cancer are facing big challenges. Here, we evaluated the expression of Dipeptidyl peptidase IV (CD26/DPPIV) and C-X-C chemokine receptor type 4 (CXCR4), two known cancer-related molecules but without clear data on prostate cancer population, and their correlation with clinical parameters in prostate cancer tissue array. To explore the correlation of CD26 and CXCR4 expression in prostate carcinoma and their relationship with clinical parameters.
Materials and Methods:
We immunohistochemically stained the tissue array containing samples from 36 cases with prostate cancer with CD26 and CXCR4 antibodies. Then we analyzed the expression of CD26 and CXCR4 and its relationship with clinical parameters. We used immunohistochemical staining to evaluate the expression of CD26 and CXCR4 in a set of tissue array containing 36 cases of prostate cancers and eight peritumoral normal prostatic tissues. The data were statistically analyzed with Statistical Package for Social Sciences (SPSS) 16.0 software. The difference between parameters was compared with nonparametric test and correlation analysis was performed with Spearman test. P < 0.05 was considered as significant.
Results:
We found both CD26 and CXCR4 expression were higher in cancer tissue than in normal tissues. CD26 and CXCR4 levels were correlated with each other. Moreover, CD26 was correlated with PSA level, tumor residue, cancer stage, and tumor size in the studied samples.
Conclusion:
Our data indicate that CD26 may be a good indicator for cancer behaviors of prostate cancer in clinic.
Keywords: Biomarker, CD26, CXCR4, immunohistochemistry, prostate cancer
INTRODUCTION
Prostate cancer is a growing public health problem worldwide; it is the fifth most common incident cancer in the world. One in six American men will be diagnosed in their lifetime with prostate cancer (PCa), which is the second leading cause of male cancer death in United States.[1] The incidence of prostate cancer is also rising each year and in China. More than 30,000 men are diagnosed with prostate cancer every year.[2] Treatment options by hormone ablation therapy, radiation, and surgery for advanced PCa do not offer cure but delay the inevitable recurrence of the lethal hormone-refractory disease.
CD26/Dipeptidylpeptidase IV is expressed on the surface of various cell types. The major physiological function of CD26 is to cleave the N-terminal dipeptide from diverse molecules, such as cytokines and hormones.[1] It also binds to the extracellular matrix (ECM), which bear a great part of responsibility for cancer invasion. CD26 has been reported to be useful as a marker in some cancers. However, CD26 levels in are not in a consistent trend for various cancers. In some cancer types, CD26 was upregulated, while in others, it was downregulated.[2] No study on CD26 level has been performed in prostate cancer. Here, we evaluated the CD26 level and its significance in prostate cancer.
The chemokine receptor CXCR4 is a member of the large superfamily of G protein-coupled receptors. It has been revealed to play an important role in a number of biological and pathological processes, including the trafficking and homeostasis of T-lymphocytes and cancer development.[3] CXCR4 has also been identified as a prognostic marker in a number of cancer types, including leukemia and breast cancer, and recent evidence has highlighted the role of CXCR4 in the prostate cancer that CXCR4 was associated with the survival of prostate cancer patients and activated serine—threonine kinase B (Akt) and Matrix metallopeptidase 9 (MMP 9) expressions.[4,5]
To provide clues of CD26 and CXCR4 expression in prostate cancer, and possible relationship of these two molecules with clinical parameters, we here detected the expression levels of CD26 and CXCR4, as well as their relationship with the clinical parameters of prostate cancer in a group of prostate cancer patients. The data from this study will benefit further research and understanding of prostate cancer.
MATERIALS AND METHODS
The tissue array containing prostate cancer tissue spots (Lot. PR955) was prepared by Xi’an Alenobio Company (Xi’an, China). All the prostate cancer patients underwent prostatectomy and the tumor samples were pathologically examined. The set of tissue array includes duplicate prostate cancer tissue spots from 36 cancer patients and eight peritumoral normal prostate tissues. The clinical and pathological characteristics of the patients were summarized in Table 1.
Table 1.
Demographic characteristics of the prostate cancer patients in this study
The general two-steps immunohistochemistry staining was adopted. Phosphate buffered saline (PBS) was used in negative control instead of the primary antibodies. The goat Immunoglobulin G (IgG) was used as an isotype control at the same concentration as primary antibodies. Briefly, the tissue array was hydrated in gradient alcohol and antigen-retrieval was performed in Ethylenediaminetetraacetic acid (EDTA)-containing antigen retrieval buffer (pH = 8.0) in 95°C followed by 3% H2O2 incubation for 30 minutes. After blocking by goat serum for 10 minutes, various primary antibodies (Rabbit anti-human CD26 polyclonal antibody (Bioss, bs-2570R) and rabbit anti-human CXCR4 polyclonal antibody (Bioss, bs-1011R)) were incubated overnight at 4°C. The corresponding Horseradish peroxidase (HRP)-conjugated secondary antibodies (Zhong Shan Jin Qiao Bio Tech., PV900) were incubated for 25 minutes at room temperature before visualization by diaminobenzidine (DAB) reagent. The tissue array slides then were stained with hematoxylin and mounted with slide cover for microscopic evaluation.
The interpretation of the tissue slide staining was independently performed by two experienced pathologists. The diagnosis was conformed and the final score for each sample was calculated by averaging the two scores from the two pathologists. The criteria for staining evaluation were as follows:[6] 0, <5%; 1, 5-25%; 2, 26-50%; 3, 51-75%; 4, >75%. The intensity of the staining was also graded: 0, negative; 1, weak; 2, moderate; 3, strong. For each tissue spot, a score between 0-12 was achieved by multiplying the extent of positivity and intensity. The final score was the average of parallel tissue spots. Scores were classified as strong (8-12), moderate (4-8), and weak (0-4).
The data were statistically analyzed with Statistical Package for Social Sciences (SPSS) 16.0 software. The difference between parameters was compared with nonparametric test and correlation analysis was performed with Spearman test. P < 0.05 was considered as significant.
RESULTS
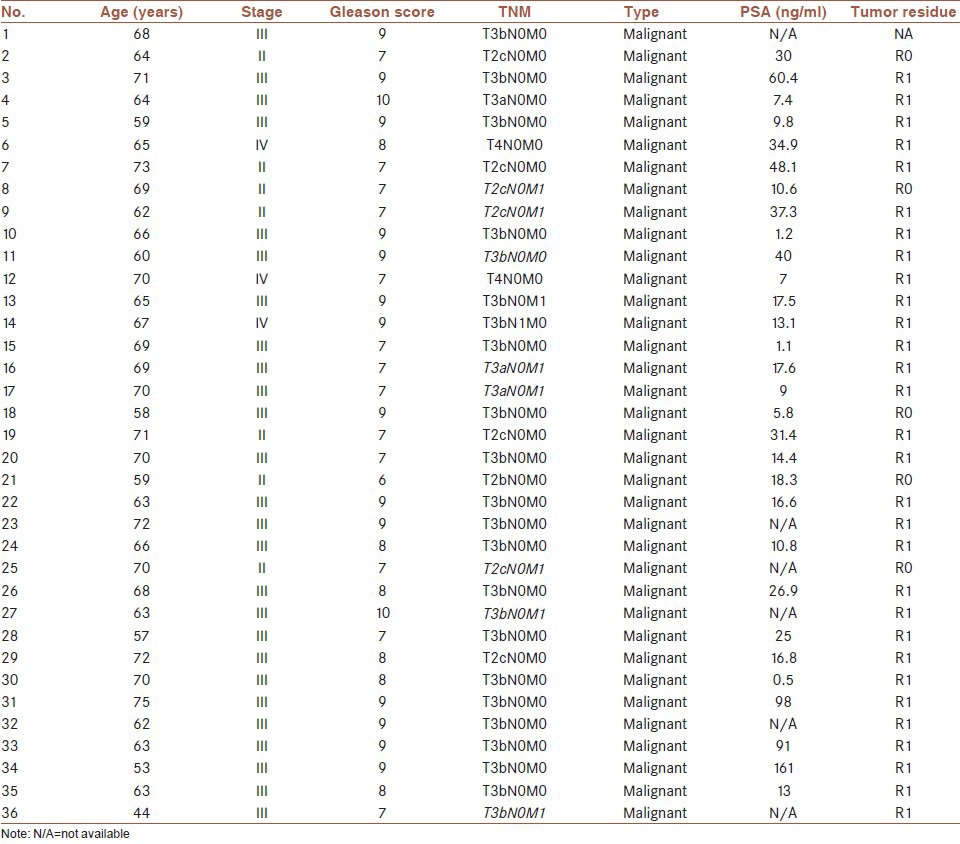
The pattern of expression for CD26 and CXCR4 was predominantly cytoplasm and membrane staining in normal and cancer tissues. The expression levels of CD26 and CXCR4 in cancer tissues were both significantly higher than that of normal tissues [Figure 1].
Figure 1.
Representative expression of CD26 and CXCR4 (a) brown colored cytoplasm and membrane staining with CD26 and CXCR4 is found in most of the tumor cells with higher positivity and intensity than normal tissue. CD26 expression overlaps with CXCR4 expression in consecutive sections of same tumor tissue, suggesting a possible cooperating mechanism for the two factors in prostate cancer (b) comparison of CD26 and CXCR4 between tumor and normal tissues
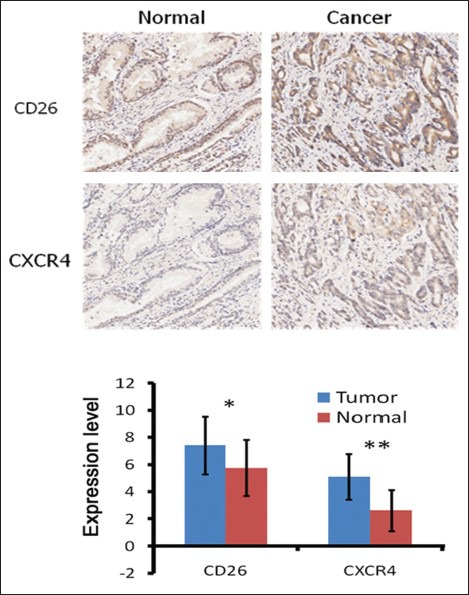
High levels of CD26 and CXCR4 were significantly correlated with each other (r = 0.500, P < 0.001) [Figure 2]. From the consecutive serial sections, we can see the expression of CD26 and CXCR4 was basically overlapped, suggesting potential regulation between these two pathways in prostate cancer [Figure 1]. The CD26-positive tumors were significantly with higher CXCR4 expression.
Figure 2.
Correlations between CD26 and CXCR4 expression. The strong correlation between CD26 and CXCR4 is found (r=0.500, P <0.001)
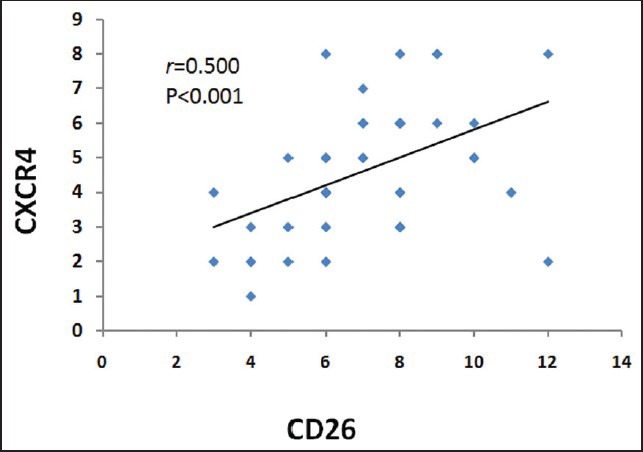
Among the 36 cases, we analyzed the correlation of CD26 and CXCR4 with other pathological and clinical parameters. CD26 was highly recommended as a marker for prostate cancer marker since it was significantly correlated with PSA level (r = 0.334, P = 0.043), tumor residue (r = 0.471, P = 0.022), cancer stage (0.572, P = 0.000), and tumor size (r = 0.616, P = 0.000). We did not find the significant correlation of CD26 with other clinical parameters, such as cancer metastasis. For CXCR4, we only found that it was correlated with tumor residue (r = 0.456, P = 0.011) but not other parameters [Table 2]. CD26 expression was increased with cancer stage advancement [Figure 3].
Table 2.
CD26 was correlated with PSA level, tumor residue, stage, and tumor size, while CXCR4 was correlated with tumor residue
Figure 3.
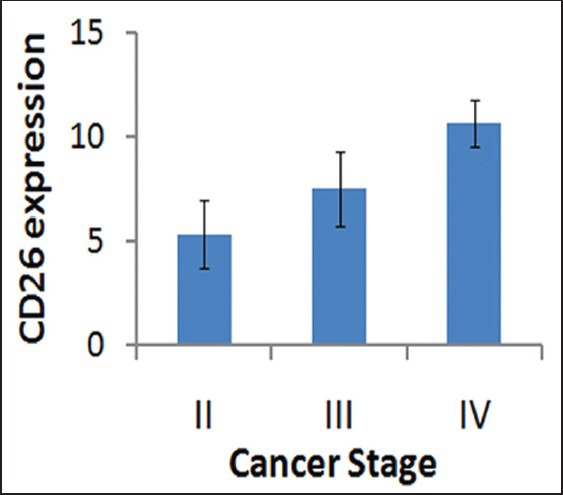
CD26 expression was increased with the advancement of cancer stage. CD26 expression reached a very high level at the stage IV (P <0.01)
Moreover, we saw certain percentage of ectopic expression of CD26 and CXCR4. In normal tissues and in most of the cancer tissues, the expression of CD26 and CXCR4 were in cytoplasm and on membrane. But in some cancer cases, the nuclear expression of these two markers was observed, which was statistically higher than that of normal tissues (P < 0.05) [Figure 4]. By correlation assay, we found that the nuclear expression of CD26 was correlated with the age of cancer patients but not other parameters.
Figure 4.
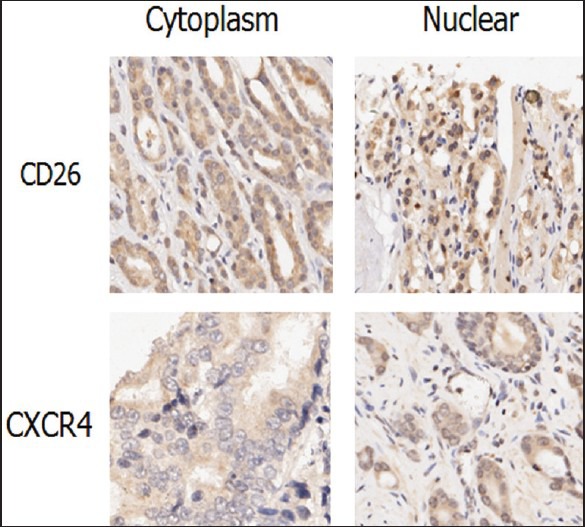
Ectopic expression of CD26 and CXCR4. The localization of CD26 and CXCR4 was expected to be in the cytoplasm. In the prostate cancer tissues, CD26 and CXCR4 were expressed in cytoplasm and nuclear at various extend
DISCUSSION
Prostate cancer is a prominent problem in aged men in Western countries and also has been drawing increasing attention with the progression of China into an aged society. Currently, surgical procedure in the early stage is expected to result in best prognosis. There are no satisfying strategies when the disease develops into an advanced stage except for palliative androgen deprivation therapy. It is an urgent task to explore the mechanisms of prostate cancer to hatch novel therapeutic strategies.
CD26 has been recognized as a marker of T-lymphocytes activation. Recently, it has been reported to be associated with malignant behaviors in various cancer types.[2,7,8] CD26 plays important roles in tumor behaviors with its levels on the cell surface increased in some cancer types and decreased in others.[2] Silence of CD26 by specific Small interfering Ribonucleic Acid (siRNA) inhibited cell growth, enhanced sensitivity to selected chemotherapeutic agents, and enhanced survival of tumor-bearing mouse model.[2] Pang R et al., identified that a subpopulation of CD26+ human colorectal cancer stem cells were enriched for metastatic capacity.[9]
Key points in this paper: 1) CD26 and CXCR4 was up-regulated in prostate cancer comparing with their normal counterparts; 2) CD26 was correlated with CXCR4 expression in the prostate cancer tissue samples, indicating potential interacting networks between the two pathways; 3) CD26 was correlated with PSA level, tumor residue, cancer stage, and tumor size highlighting its significance in diagnosis and prognosis of prostate cancer patients. All these data suggest an active role of CD26 during prostate cancer development and advancement.
However, as the only previously reported data on CD26 in prostate cancer we know, Sun YX et al., found that CD26 regulated the metastasis capacity of prostate cancer cell line.[10] They suggested that inhibition of CD26 may be a trigger for prostate cancer metastasis. This is not consistent with our results that CD26 expression is correlated with the stages but not metastasis of prostate cancer. The reason for this discrepancy remains to be investigated. But they did not evaluate CD26 expression in prostate cancer tissues. They drew the conclusion from cell line research and they used a co-culture system with endothelial cells. The system and methods used in the study may, in part, explain the difference in the data between the two studies. We suggested that CD26 has a positive correlation with prostate cancer malignancy by showing the relationship of CD26 with PSA, tumor residue, cancer stage, and tumor size. PSA is a well-accepted clinical marker for prostate cancer recurrence and prognosis. The correlation between CD26 and PSA indicates a potential clinical application of CD26 as an indicator of prostate cancer status. The trend of CD26 in prostate cancer reported in this paper is consistent with that in leukemia and hepatocellular carcinoma.[11,12] Varona A suggested that CD26 expression is tumor-type-dependent.[13] CD26 was considered as a novel therapeutic target for cancer and immune disorders.[14] Further study will define the expression pattern of CD26 in various cancer types which will provide detailed clues for CD26-targeted cancer therapy strategies.
CXCR4 is the receptor for stromal cell-derived factor-1 (SDF-1/CXCL12). The SDF-1/CXCR4 axis plays an important role in prostate cancer metastasis,[15] especially metastasis to bone.[5,16] However, in our study, we did not find a significant correlation of CXCR4 with other parameters except for the correlation of CXCR4 with CD26. This may be caused by the relatively small sample size. Before this study, no relationship between CD26 and CXCR4 in prostate cancer has been reported. But several papers had indicated a CXCR4/CD26 interacting network in other biological systems. CD26 may directly modulate SDF-1α/CXCR4 axis to activate human lymphocytes.[17] Altered CXCR4/CD26 balance will impair the recruitment of Hereditary Hemorrhagic Telangiectasia Type 1 (HHT-1) mononuclear cells in the ischemic heart. Here, we did not find the correlation of CXCR4 with most of the studied parameters.[18] We need to testify the result in a larger sample group in the future to avoid the false negativity resulted from a limited sample size.
The ectopic expression of some protein molecules in cancer tissues is not a rare phenomenon. A typical example is the localization of beta-catenin in cancer samples. Normally, beta-catenin is expressed in cytoplasm. During carcinogenesis, it is translocated into nuclear to activate a series of cancer-related genes.[19,20] Metastasis-associated protein 1 (MTA 1) is another example for ectopic expression during carcinogenesis. MTA 1 is normally expressed in nuclear to regulate transcriptional status of chromatin. However, it is translocated into cytoplasm increasingly with the advancement of cancer stage in esophageal squamous cell carcinoma.[6] Though, we don’t know the mechanisms of CD26 and CXCR4 nuclear localization yet, this result provides new clue for their roles in prostate cancer development.
Among the 36 prostate cancer cases included in this tissue array, eight have bone metastatic tumor samples in the array. But the decalcification process of bone samples made the immunohistochemical staining unreadable with background brown color. Also, for a relatively short period of follow-up, only three patients were dead of cancer at the time of analysis, which made the survival assay impossible. These are the points to be resolved in further studies.
In brief, we found the significance of CD26 as a marker for prostate cancer malignancy and the correlation of CD26 with CXCR4. Though there are limitations in drawing more conclusions from the study because of the limited sample size and integrity of data of the cases, this study adds new insights into the prostate cancer profiles which had not been reported before and guarantees further exploration of CD26 as a therapeutic target in prostate cancer patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Matteucci E, Giampietro O. Dipeptidyl peptidase-4 (CD26): Knowing the function before inhibiting the enzyme. Curr Med Chem. 2009;16:2943–51. doi: 10.2174/092986709788803114. [DOI] [PubMed] [Google Scholar]
- 2.Havre PA, Abe M, Urasaki Y, Ohnuma K, Morimoto C, Dang NH. The role of CD26/dipeptidyl peptidase IV in cancer. Front Biosci. 2008;13:1634–45. doi: 10.2741/2787. [DOI] [PubMed] [Google Scholar]
- 3.Furusato B, Mohamed A, Uhlen M, Rhim JS. CXCR4 and cancer. Pathol Int. 2010;60:497–505. doi: 10.1111/j.1440-1827.2010.02548.x. [DOI] [PubMed] [Google Scholar]
- 4.Akashi T, Koizumi K, Tsuneyama K, Saiki I, Takano Y, Fuse H. Chemokine receptor CXCR4 expression and prognosis in patients with metastatic prostate cancer. Cancer Sci. 2008;99:539–42. doi: 10.1111/j.1349-7006.2007.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chinni SR, Sivalogan S, Dong Z, Filho JC, Deng X, Bonfil RD, et al. CXCL12/CXCR4 signaling activates Akt-1 and MMP-9 expression in prostate cancer cells: The role of bone microenvironment-associated CXCL12. Prostate. 2006;66:32–48. doi: 10.1002/pros.20318. [DOI] [PubMed] [Google Scholar]
- 6.Qian H, Lu N, Xue L, Liang X, Zhang X, Fu M, et al. Reduced MTA1 expression by RNAi inhibits in vitro invasion and migration of esophageal squamous cell carcinoma cell line. Clin Exp Metastasis. 2005;22:653–62. doi: 10.1007/s10585-006-9005-2. [DOI] [PubMed] [Google Scholar]
- 7.De Chiara L, Rodriguez-Pineiro AM, Rodriguez-Berrocal FJ, Cordero OJ, Martinez-Ares D, Paez de la Cadena M. Serum CD26 is related to histopathological polyp traits and behaves as a marker for colorectal cancer and advanced adenomas. BMC Cancer. 2010;10:333. doi: 10.1186/1471-2407-10-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abe M, Havre PA, Urasaki Y, Ohnuma K, Morimoto C, Dang LH, et al. Mechanisms of confluence-dependent expression of CD26 in colon cancer cell lines. BMC Cancer. 2011;11:51. doi: 10.1186/1471-2407-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pang R, Law WL, Chu AC, Poon JT, Lam CS, Chow AK, et al. A subpopulation of CD26+ cancer stem cells with metastatic capacity in human colorectal cancer. Cell Stem Cell. 2010;6:603–15. doi: 10.1016/j.stem.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Sun YX, Pedersen EA, Shiozawa Y, Havens AM, Jung Y, Wang J, et al. CD26/dipeptidyl peptidase IV regulates prostate cancer metastasis by degrading SDF-1/CXCL12. Clin Exp Metastasis. 2008;25:765–76. doi: 10.1007/s10585-008-9188-9. [DOI] [PubMed] [Google Scholar]
- 11.Aldinucci D, Poletto D, Lorenzon D, Nanni P, Degan M, Olivo K, et al. CD26 expression correlates with a reduced sensitivity to 2’-deoxycoformycin-induced growth inhibition and apoptosis in T-cell leukemia/lymphomas. Clin Cancer Res. 2004;10:508–20. doi: 10.1158/1078-0432.ccr-0755-03. [DOI] [PubMed] [Google Scholar]
- 12.Stecca BA, Nardo B, Chieco P, Mazziotti A, Bolondi L, Cavallari A. Aberrant dipeptidyl peptidase IV (DPP IV/CD26) expression in human hepatocellular carcinoma. J Hepatol. 1997;27:337–45. doi: 10.1016/s0168-8278(97)80180-8. [DOI] [PubMed] [Google Scholar]
- 13.Varona A, Blanco L, Perez I, Gil J, Irazusta J, Lopez JI, et al. Expression and activity profiles of DPP IV/CD26 and NEP/CD10 glycoproteins in the human renal cancer are tumor-type dependent. BMC Cancer. 2010;10:193. doi: 10.1186/1471-2407-10-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson MA, Ohnuma K, Abe M, Morimoto C, Dang NH. CD26/dipeptidyl peptidase IV as a novel therapeutic target for cancer and immune disorders. Mini Rev Med Chem. 2007;7:253–73. doi: 10.2174/138955707780059853. [DOI] [PubMed] [Google Scholar]
- 15.Gladson CL, Welch DR. New insights into the role of CXCR4 in prostate cancer metastasis. Cancer Biol Ther. 2008;7:1849–51. doi: 10.4161/cbt.7.11.7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62:1832–7. [PubMed] [Google Scholar]
- 17.Herrera C, Morimoto C, Blanco J, Mallol J, Arenzana F, Lluis C, et al. Comodulation of CXCR4 and CD26 in human lymphocytes. J Biol Chem. 2001;276:19532–9. doi: 10.1074/jbc.M004586200. [DOI] [PubMed] [Google Scholar]
- 18.Post S, Smits AM, van den Broek AJ, Sluijter JP, Hoefer IE, Janssen BJ, et al. Impaired recruitment of HHT-1 mononuclear cells to the ischaemic heart is due to an altered CXCR4/CD26 balance. Cardiovasc Res. 2010;85:494–502. doi: 10.1093/cvr/cvp313. [DOI] [PubMed] [Google Scholar]
- 19.Stenner M, Yosef B, Huebbers CU, Preuss SF, Dienes HP, Speel EJ, et al. Nuclear translocation of beta-catenin and decreased expression of epithelial cadherin in human papillomavirus-positive tonsillar cancer: An early event in human papillomavirus-related tumour progression? Histopathology. 2011;58:1117–26. doi: 10.1111/j.1365-2559.2011.03805.x. [DOI] [PubMed] [Google Scholar]
- 20.Li A, Zhou T, Guo L, Si J. Collagen type I regulates beta-catenin tyrosine phosphorylation and nuclear translocation to promote migration and proliferation of gastric carcinoma cells. Oncol Rep. 2010;23:1247–55. doi: 10.3892/or_00000757. [DOI] [PubMed] [Google Scholar]


