Abstract
Background:
Regular exercise has been associated with improved quality of life (QoL) in patients with heart failure (HF). However, less is known on the theoretical framework, depicting how educational intervention on psychological, social, and cognitive variables affects physical activity (PA). The purpose of this study is to assess the effectiveness of a social cognitive theory-based (SCT-based) exercise intervention in patients with HF.
Materials and Methods:
This is a randomized controlled trial, with measurements at baseline, immediately following the intervention, and at 1, 3, and 6 months follow-up. Sixty patients who are referred to the cardiac rehabilitation (CR) unit and meet the inclusion criteria will be randomly allocated to either an intervention group or a usual-care control group. Data will be collected using various methods (i.e., questionnaires, physical tests, paraclinical tests, patients’ interviews, and focus groups). The patients in the intervention group will receive eight face-to-face counseling sessions, two focus groups, and six educational sessions over a 2-month period. The intervention will include watching videos, using book and pamphlets, and sending short massage services to the participants. The primary outcome measures are PA and QoL. The secondary outcome measures will be the components of SCT, heart rate and blood pressure at rest, body mass index, left ventricular ejection fraction, exercise capacity, and maximum heart rate.
Conclusion:
The findings of this trial may assist with the development of a theoretical model for exercise intervention in CR. The intervention seems to be promising and has the potential to bridge the gap of the usually limited and incoherent provision of educational care in the CR setting.
Keywords: Cardiac rehabilitation, exercise, heart failure, quality of life, randomized controlled trial, social cognitive theory
INTRODUCTION
Chronic heart failure (CHF) is one of the most common and debilitating conditions in older patients,[1] frequently resulting from coronary artery disease or chronic hypertension. Patients commonly experience a decline in their health-related quality of life (QoL),[2] due to severe impairments in physical and social functioning, as well as associated psychological disorder[3] such as depression.[4] Major symptoms associated with heart failure (HF) are fatigue, shortness of breath at rest or during exercise, dyspenia, tachypnea, cough, diminished exercise capacity, orthopnea, paroxysmal noctural dyspnea, nocturia, weight gain, and edema.[5] These disabilities correlate well with physical, sociological, environmental, and social limitations.[6]
Cardiac rehabilitation (CR) program is a part of outpatient secondary prevention in patients with HF[7] and is aimed at promoting QoL, functional capacity, exercise tolerance,[8] reducing mortality,[9] and the need for frequent hospitalizations.[10] Moreover, CR has beneficial effects on systolic blood pressure and serum total cholesterol level,[11] symptoms of angina and dyspnea, weight loss, smoking, anxiety, and psychosocial well-being.[8] Major components of CR include patient education on blood lipids, hypertension, smoking cessation, diabetes, physical training, counseling on psychological and dietary aspect of life style.[7,12]
Researchers have investigated the positive effects of exercise-training in patients with HF.[12] However, we are noticing an increasing epidemic of physical inactivity among HF patients.[13] Exercise interventions can lead to increase the levels of physical activity (PA) in daily activity.[14] In this study, CR will be exploited as an exercise model.
PA is affected by factors including demographic variables and potentially malleable psychosocial variables such as self-efficacy, outcome expectations, and self-regulation.[15] Although some studies have examined exercise training in patients with HF in the CR context, they often lack a theoretical framework depicting how educational emphasis on psychological, social, and cognitive variables can affect PA.
Social cognitive theory (SCT) provides a framework that has been successfully guided behavioral intervention, especially for organizing, understanding, and increasing PA.[16] In general, SCT puts forward that personal, environmental, and behavioral factors are reciprocally prominent in determining behavior. Self-efficacy is defined as “people's judgments of their capabilities to execute given level of performance”.[17] It is concerned not with the skills individual has but with the judgments of what one can do with whatever skills one possesses.[18] Self-efficacy is believed to be reinforced in individuals by the following four means: performance accomplishment, vicarious experience, verbal persuasion, and physiological arousal.[19] Outcome expectation is derived from SCT and is defined as a person's belief that a specific behavior will lead to desired outcomes, which is also related to PA. Outcome expectations from exercise are both positive (e.g., improved cardiovascular condition) and negative (e.g., fear of falls), and depend on the maintenance of the exercise habit.[20,21] Strategies to change behavior include goal setting, problem solving, self-monitoring, being receptive to feedback, and supervised exercise have been more successful than giving or receiving advice only.[22] These components are termed self-regulation. Self-regulation may be defined as “a sequence of actions and/or steering processes intended to attain a personal goal”.[23] Self-regulation is thought to play a key role in initiating and maintaining the behavioral change such as PA.[24] Both the acceptance and maintenance of exercise habit depend on the individual's perceived support from the family, friends, and other participants in the cardiac rehab program.[25] Additionally, social support is negatively correlated with mortality due to cardiac events.[26]
OBJECTIVES AND HYPOTHESES
Our primary objective is to determine the effectiveness of the SCT-based exercise intervention in patients with HF and whether this model would lead to improved levels. We hypothesize that the theoretically based educational intervention will significantly increase the level of PA.
Our secondary objectives are as follows:
To examine the change in score of SCT components including exercise self-efficacy, self-regulation, outcome expectation, and perceived social support.
To investigate the change in QoL, depressive symptoms, and anxiety indicators, and to evaluate the change in ejection fraction, exercise capacity and PA, BMI, heart rate, and blood pressure.
To explore the predicting role of demographic determinants and comorbidity conditions for a successful PA
MATERIALS AND METHODS
Design
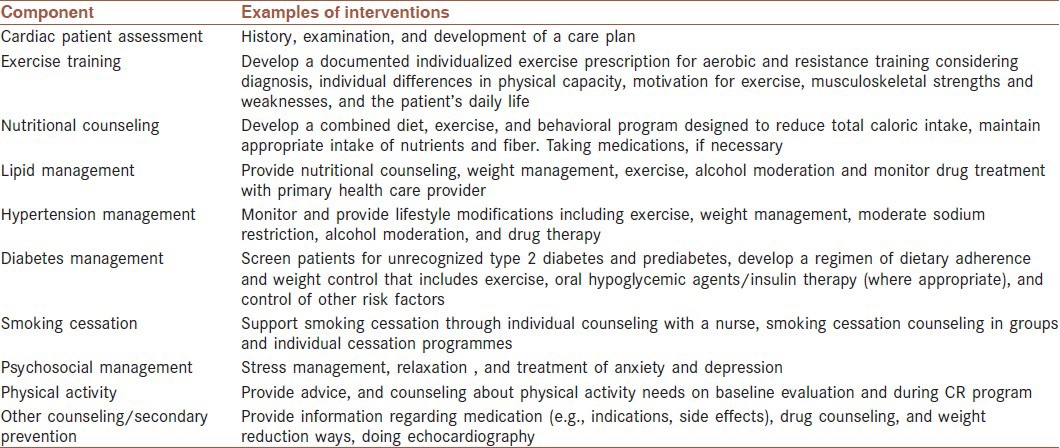
This single-blind randomized control trial (RCT) will be conducted with the participants attending a specific CR program. The participants, who will be recruited from among patients with HF, are assigned to the two groups: SCT-based exercise intervention group (trial) and usual care group (control). The components of a typical usual care in the CR program are shown in Table 1.
Table 1.
The components of a cardiac rehabilitation program
Setting
All patients are recruited those diagnosed with cardiovascular disease and who are referred to the CR in the Isfahan Cardiovascular Research Center by their cardiologists between May 2013 and August 2013. This study was approved by Isfahan University of Medical Science Ethics Committee (Number 391016). The cost of the 20 session CR will be covered by the University's Medical Research Assistant.
Patient eligibility
Patients will be accepted into the study if they are diagnosed with HF. Inclusion criteria are: (a) aged 30 years or over, (b) having been treated medically, (c) being able to speak and read Persian, (d) having a resting left ventricular ejection fraction (LVEF) of ≤40, (e) being assessed as (NYHA) II and III by the cardiac function classification method of New York Heart Association, and (f) living with at least one family member who is willing to accompany the patient throughout the study.
Participants are excluded from the study if they meet the following criteria, as determined by the study's cardiologist: (a) presence of unstable angina pectoris, (b) participation in another aerobic exercise program more than three times a week within the past 12 months, (d) having symptoms of acute decompensated CHF, (e) having suffered from severe mental illnesses, (g) having prior orthopedic or neuromuscular conditions, preventing participation in exercise and strength/resistance training, and (f) currently being a participant in a similar study.
Before being recruited into the study, all participants are screened by a sports medicine professional to assess whether they are medically cleared for the CR.
Sample size
The effect size for this study has obtained from a study performed by Pozehl et al. (2010) based on 80% power with a two-tailed significance level of 0.05 and for detecting a standardized effect size (Δ = 0.47)[27] in outcome for the two groups. Thus, 30 patients per group have been estimated. Considering a dropout rate of 10% at the follow-up, we aim to recruit 60 patients for the study.
Measurements and procedures
All participants will undergo four measurements: on entry to the study (pretest), and after having gone through the 2-month intervention (posttest), and 1, 3, and 6 months after rehabilitation will be repeated for following up.
Randomization and blinding
All eligible patients referred to the Cardiac Rehabilitation Research Center will be requested to complete the informed consent form. Patients who consent to take part in the study are randomly allocated to either the intervention group or control group after the baseline measurements. Participants are randomized to either the intervention or control group, using permuted block randomization after completing the baseline questionnaires and the pretest assessments.
Assessor and sports medicine specialist will be blind to the interventions. Blinding the patients to the allocation is not possible due to the nature of the intervention.
Outcome measures
The outcomes will be assessed at baseline (prior to randomization), at the end of the intervention period, and after 1-, 3-, and 6-months follow-up [Figure 1]. Baseline assessments involve demographics, general information (including age, gender, medical history, and use of medications), examination, SCT construct questionnaires, and anxiety and depression questionnaires will be collected. Echocardiography will be performed by one experienced cardiologist for estimating the ejection fraction using Simpson's method, and modified Bruce protocol is used for the exercise test by a nurse practitioner. All blood pressures will also be assessed by that nurse practitioner.
Figure 1.
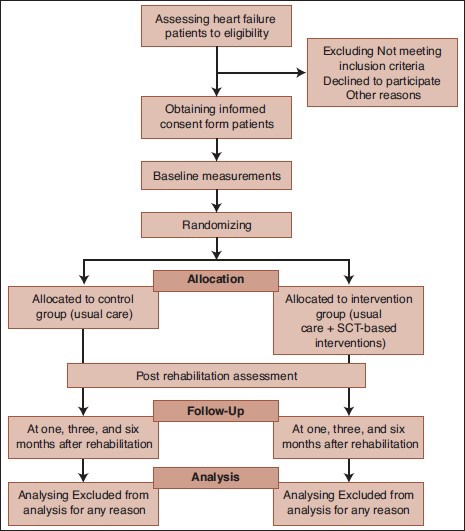
study flow
Primary outcome measures
Physical activity
Physical activity will be assessed by a translated version of validated International Physical Activity Questionnaire (IPAQ).[28] The long form of IPAQ assesses PA over the past 7 days across four domains: occupation, transportation, house/yard work, and recreation/leisure. Both continuous and categorical indicators will be assessed from IPAQ. The continuous indicator will be expressed as metabolic equivalent of the task (MET) minutes per week derived from walking and vigorous- or moderate-intensity activities in IPAQ. The categorical indicator groups the individual in the low, moderate, and high PA levels. Transportation- and recreation-based physical activities are the main outcomes of interest. Transportation activity consists of weekly minutes of walking and bicycling for transportation. Recreational activity consists of weekly minutes of walking for leisure and moderate and vigorous leisure-time activity.[29] For the IPAQ long form, most of the test — retest reliability coefficients are around 0.80 across 12 countries.[30]
Health-related quality of life
Health-related quality of life will be evaluated by the Minnesota Living with Heart Failure questionnaire (MLHF)[31,32,33] and the Persian version of the generic SF-36 Health Survey Questionnaire.[34] The MLHF tool is a 21-item structured questionnaire that assesses patient's perceptions about the effects of a range of physical, emotional, and related impairments that relate to HF. We chose this instrument because it is a commonly used disease-specific HQOL questionnaire used for patients with HF. Respondents are asked to rate the degree to which each HF-related impairment prevent them from living as they desired during the previous month. HQOL impairment is evaluated using a 6-point scale ranging from 0 (no impact/not applicable [best score]) to 5 (severe impact [worst score]). The MLHFQ produces a total score (21 items, range 0-105) and physical (PDS) and emotional dimension (EDS) Lower scores indicate better HQOL; a change >5 points in the total score is considered clinically meaningful.[35] Because of the popular use of the 36-Item Short Form Health Survey (SF-36) in CR setting, we use the Persian version of this form. The instrument comprises of 36 questions, measuring eight multiitem components (subscales [2-10 items each]), including physical function, physical role, social function, social role, mental health, bodily pain, vitality, and general health perception. Responses subscale items will be transformed to a scale ranging from 0 to 100. Furthermore, physical (PCS) and mental (MCS) component summary scores (both standardized) can be calculated using a PCS and MCS scoring algorithm. In contrast to the MLHF, high scores on the SF-36 indicate better QoL.[36]
Secondary outcome measures
Cardiac exercise self-efficacy
Cardiac Exercise Self-efficacy Scale (CESE) will be used for measuring patients’ exercise self-efficacy. This instrument devised by Hickey et al., (1992) measures patients’ opinion of their confidence to tolerate exercise after their cardiac event. The overall CESE scores will be calculated by mean scores of the actual values of the 16 items of each of the scales. This instrument has been found to have considerable internal consistency with an alpha coefficient of 0.9.[37]
Outcome expectations for exercise
Use of exercise outcome strategies will assess by two scales: (a) the Multidimensional Outcome Expectations for Exercise Scale (MOEES)[38] and (b) the Outcome Expectations for Exercise-2 Scale (OEE-2).[39] The MOEES is composed of 15 items (i.e., 6 physical, 4 social, and 5 self-evaluative outcome expectation). Some of these questions are “Exercise will improve my ability to perform daily activities”, and “Exercise will provide companionship”, and “Exercise will improve my psychological state”. Patients will be asked to rate how strongly they agree with each of the 15 items on a 5-point scale (1 = strongly disagree, 2 = disagree, 3 = neutral, 4 = agree, and 5 = strongly agree). A total scale score will be driven by summing the score of the individual item. Possible scores for each participant will be ranged from 15 to 75. The lowest scores indicate that patients have low outcome expectancy of doing exercise and vice versa.
Another questionnaire, called outcome expectations for exercise-2 Scale (OEE-2) will be used. This is a 13-item tool with two subscales: (a) positive outcome expectation for exercise (POEE) and, (b) negative outcome expectations for exercise (NOEE). The nine items are included in the OEE are all positive benefits associated with exercise such as “gives me a sense of personal accomplishment”, and “makes me more alert mentally”, etc. In addition, the four items included are POEE such as “exercise places too much stress on my heart so I avoid it”. To complete the OEE scale, the participant will be asked to listen to each statement and answer to any question if they strongly agree (1), agree (2), neither agree nor disagree (3), disagree (4), or strongly disagree with the stated outcomes of exercise. The scale is scored by summing the numerical ratings for each response and dividing by the number of responses. The subscales are scored separately.
Exercise self-regulation
Self-regulation for exercise will be assessed with a 43-item questionnaire developed by Petosa[40] to measure the level to which self-regulation strategies are applied to lead exercise behavior adoption and adherence. Patients will rate how often they used each strategy for exercising in the past month on a scale of 1 to 5, (1 = never and 5 = very often). Participants’ total self-regulation scores will be calculated by summing the scores of the individual items; the total score could range from 43 to 215, where higher scores indicate greater use of self-regulation strategies. These measurements are administered at baseline, 2-, 3-, and 6-month follow-up. The Cronbach's α is 0.96.
Social support for exercise
Sallis's Social Support Scale for Exercise Behavior will be used in this study. This scale consisting of 18 items is used to assess the social support (friend and family) in exercise behavior. The instrument has a 5-point Likert-type scale ranging from (1 = none to 5 = very often). The scale has two subscales: family support with 12 items and friend support with 5 items. The Cronbach's coefficient α for the friend and family support scale is 0.86. For the purpose of this study, the perceived social support will be emotional (steam support and confidence support) and informational support[41] received from staff and the patients’ family.
Anxiety
Anxiety will be measured by means of Spielberger Anxiety Inventory, which includes the 4-point scale items using a score ranging from 0 (absence) to 3 (severe). The cutoff scores for anxiety are as follows: <20, no anxiety; 20-39, mild; 40-59, moderate; and >60, severe. The questionnaire's validity and reliability have been reported by Hazavehei.[42] Reliability and validity of the Persian version of the questionnaire was reported by Kalkhoran et al. (r = 0.97).[43]
Depression
Beck's Depression Inventory (BDI) will be used to assess depression.[44] It is a structured self-report, 21-item scale used to evaluate depression more extensively in psychiatric patients and to identify depression in other patients. Each item consists of four self-evaluative items of increasing severity. The total score of BDI can range from 0 to 63, indicating no depression to a severe state of clinical depression, respectively. The following categorization is used to guide decision making in clinical and research settings: scores 0-9, normal; 10-16 mild depression; 17-20 moderate depression; 21-30 severe depression; and scores >30 indicate very severe depression. Validity and reliability study of BDI for the Persian language was done by Ghassemzadeh et al. (Cronbach's α = 0.87).[45]
Heart rate and blood pressure at rest
The average heart rate for 1 min will be assessed. The baseline heart rate mean will be calculated as beat/mean. In addition, all patients will complete after rehabilitation at which time resting heart rate will be reevaluated. Blood pressure (BP) measurements are performed by a standard mercury sphygmomanometer. BP is measured twice from the right arm with an appropriate-sized cuff in the sitting position after a 5-min rest by a nurse. The average of the two BP values is calculated as the baseline blood pressure. BP will be measured before and after the intervention for all participating patients.
Exercise capacity, maximum heart rate
Submaximal treadmill exercise testing will be carried out under the supervision of a physician, with continuous electrocardiographic monitoring, using the Bruce protocols, according to the standard guidelines and depending on individual fitness for each patient.[46] The test will be terminated at 85% of age-appropriate target heart rate or at the request of the patient because of his/her symptoms. Blood pressure will be monitored every 3 min during the treadmill walk and at peak exertion. Heart rate will be recorded at 30, 60, 90, and 120 s into recovery. METs will be recorded for each patient as the exercise capacity.
Left ventricular ejection fraction (LVEF)
Each participant will undergo an initial resting three-dimensional (3-D) echocardiogram in multiviews before the exercise program will be initiated. Another echocardiogram will be taken upon completion of the 2-month CR program. LVEF is then computed by dividing the stroke volume by the end-diastolic volume. A normal ejection fraction is considered to be 50%.
Body mass index
Body mass index or BMI is a measurement of the body composition based on an individual's weight in kilograms (kg) divided by height in meters (m2).
Intervention
Theoretical framework
SCT principles are the guidelines for the educational intervention design used in this study. In the social—cognitive model of PA, self-efficacy, outcome expectation, self-regulation, social support are the determinant components. We will mainly establish the intervention model based on the principal SCT components. Patients randomized to the intervention group will receive 8 weekly face-to-face counseling (lasting, 30-45 min) over a 2-month period. Moreover, on the basis of assessment of patients’ needs emerged from the two focus groups, six educational intervention sessions will be held for the intervention group, one topic per week for the first 6 weeks. Two focus groups will include patients, patients’ family, and two moderator and facilitator. In general, the intervention will include following educational methods and materials: face-to-face and group sessions, focus group, video shows, book and pamphlets review, and short massage services.
In the face-to-face sessions, we will advise patients on doing exercise 3 days a week for at least 30 min, except for attendance the CR program.[47] The best and often easiest type of exercise is walking. Exercise levels will be tailored to the patient's baseline fitness, performance status, and exercise preferences, and we take into account personal facilitators and barriers to exercise for each patient. The aerobic walking has two aims in mind: (i) to increase the total level of PA and (ii) to improve patient's symptoms of HF. It is expected that most participants will engage in walking as their preferred form of exercise; however, other forms of aerobic exercise such as swimming, cycling, or running are acceptable in this study.
Participants will be given a DVD, containing a video about an energetic patient that, despite suffering from several comorbidities, continues with the aerobic exercise. This video has been awarded the best documentary at the Third Heath Media Festival.[48] The patients will also be given pamphlet on factual exercise benefits in patients with HF.
We will apply six self-regulation strategies, eliciting from a 43-item physical activity questionnaire, containing items on self-monitoring, goal setting, eliciting social support, reinforcement, time management, and relapse prevention. In the subsequent face-to-face visits, the first author will encourage participants to establish individual goals for performing regular exercise, implement self-monitoring, enlist self-incentive, and identify personal barriers, and explain social support to sustain exercise. We will ask patients to complete daily behavior checklists that address competence in self-regulation toward exercise, for example “I often had my exercise intention in mind,” and “I really tried hard to exercise regularly”. Furthermore, selected goals will be evaluated and reassessed. Barriers to exercise such as fear of falling will be explored during both face-to-face counseling and CR. If a patient expresses fear of exercise, the educator (first author) will explain to patients how this fear may be relieved so as not to negatively affect their exercise habit. Tailor-made feedback will be provided to each patient based on how close the patient came to the optimal level of exercise. Patients who will report doing three or more days of exercise will receive complementary feedback about health benefits and will be encouraged to maintain their level of PA. Participants who will report doing one or two days of exercise per week will be given positive feedback but will be encouraged to increase their effort to the recommended level. Participants who will report doing less than one day of exercise per week will be encouraged to increase exercise. All these strategies will be applied during the CR sessions. Completion of behavior checklists will been encouraged with raffle. In addition, patients will be given pamphlets containing written and visual information about the type of aerobic exercise they should do, how much and how hard should they push themselves. Participants will be reminded by a phone call reminder to ensure all of them will complete the program.
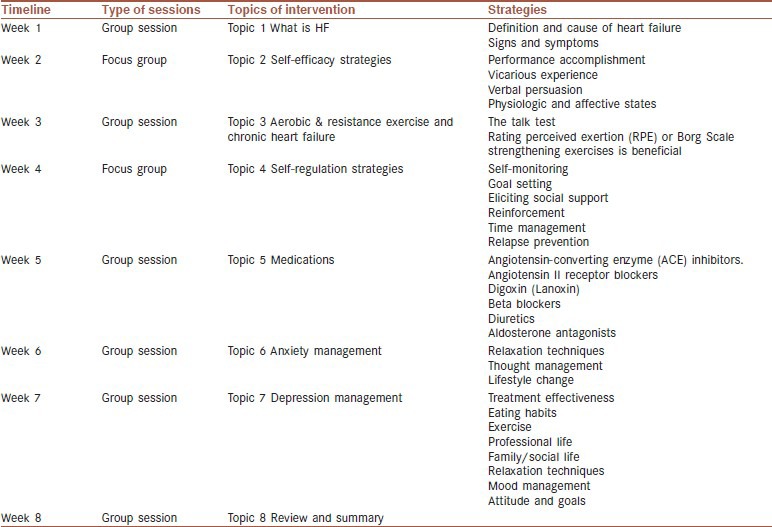
Intervention topics of group sessions will be included the exercise and HF topics. Table 2 reveals the topic of sessions and timeline of intervention. Educational information will be taken from the guidelines of Heart Failure Society of America and European Association for Cardiovascular Prevention and Rehabilitation (EACPR).[49,50] A textbook of cardiovascular medicine will be used to standardize the CR program.[5]
Table 2.
Topical listing for timeline of group sessions and focus group
Self-efficacy influence by four main sources of information including experience, vicarious experience, verbal persuasion or similar sources of social influences and physiological and affective states.[19] These strategies can be integrated either singly or in combination, into a rehabilitation program.[50]
We will ask the patients to set their own goals to perform a specific exercise, making sure their goals are attainable and safe. If patients have had success at a particular exercise skill in the past, they will probably believe that they will be successful at skills in the future.[50] We will also recommend to patients to do exercise together with a friend or a family member. The CR setting provides this situation spontaneously as delivering in a group format. In addition, we will ask patients to watch the movie at home. Patients will also be recommended to list their ability for exercising after watching the movie. We propose that the first movie character play the role of model in a participant's exercise learning acquisition. The movie is about a patient that suffers from cardiovascular disease and several cancers. The observing success of others, or vicarious experiences, can raise the patient's self-efficacy.[50] The patient continues to exercise and lives with cancers by high levels of exercise self-efficacy despite disabilities. The movie shows the patient can overcome any difficulties. Presents will be discussed about their ability according to the movies during two group discussions. Furthermore, to enhance the effect of vicarious experience, a variety of successful patients observed who participated in previous CR programs will be attended in some sessions.
Verbal persuasion will be used in two ways: congratulatory verbal encouragement by the health educator during rehabilitation, and sending short message service (SMS) throughout the CR. This part of the intervention will be continued weekly for 3 months after rehabilitation. To increase interaction with patients, they will be asked to respond to the SMS messages if they wish. The information is likely to be regarded positively by patients and may help increase their self-efficacy to achieve the rehabilitation goals. The wording of some of the SMS texts may be as follows:
“Do you know that occurrence of major cardiovascular events during supervised aerobic exercise training in cardiac rehabilitation programs is very rare?”. Patients at low clinical risk with CHF can be assigned an aerobic exercise training of moderate to vigorous intensity of 3-5 sessions per week, 30 min per session” and
“Don’t forget to note the successful exercise sessions! It will improve your adherence”.
After the intervention and during the follow-up, patients will receive one text message per week to motivate them about the topics learned in the CR.
Statistical analysis
The clinical outcome variables will be analyzed by the intention-to-treat principle. Survey data will be coded and analyzed using the Statistical Package for the Social Sciences. A normal distribution will be checked using the Kolmogorov—Smirnove test. Differences based on continuous data with normal distribution will be analysed using an independent t-test and Mann — Whitney U-test for nonnormally distributed data. A paired t-test (or Wilcoxon signed-rank test) and Repeated Measure ANOVA (or ANCOVA as appropriate) (or mixed model ANOVA (or ANCOVA) will be used for before and after intervention data analysis and Bonferroni post-hoc test will be used for pairwise comparisons. Association between categorical data will be analyzed using Chi-square test. A P-value of <0.05 will be considered statistically significant and all tests will be two tailed.
DISCUSSION
There is an urgent need to identify interventions for increasing and adhering to PA in patients with HF. This article presents a study protocol with an innovative approach for educational intervention to improve patients PA and subsequently their QoL. This protocol has the potential to improve the standard care of patients with HF with a simple, accessible, and relatively inexpensive therapy that could improve signs and symptoms and help them to remain physically active for as long as possible. This new SCT-based intervention in the CR setting could lead to significant and generalized benefits, reducing sedentary lifestyle and several consequences of HF such as depression and anxiety by improving physical, emotional, and behavioral functioning and perceived social support. It is hoped that such a socio-cognitive shift in educational intervention will produce more favorable outcomes with respect to mood status, PA levels, and perception of health-related QoL.
Overall, multiple interventions, such as watching movie, learning by reviewing informational documents, using notebooks, etc., can provide exceptionally promising and efficient tools to facilitate behavior changes in patients. Mobile phone text massage-based interventions have been confirmed effective to broadly reach specific populations and deliver specific messages.[50]
To strengthen the quality of our data collection, we plan to extend our resources by using a mixture of measuring methods. Besides the quantitative methods for the exploration of self-efficacy and other components of SCT, the study design integrates qualitative methods for the exploration of PA barriers to make them adhere to cognitive behavioral strategies by organizing focus groups.
If the intervention is found to improve PA behaviors either during the rehabilitation program or follow-up periods, future experimental work may be required to identify the most important component of SCT-based intervention in the rehabilitation setting. The result of this study will provide useful insight into the role that a SCT-based intervention program could play in improving QoL in HF patients. If successful, the intervention may be adopted in other patient population and RCT study designs.
The proposed study has a number of potential limitations. First, there is only a limited time period in which the participants can be recruited. Second, convening patients in a given day for watching movies will be difficult. Third, patients’ adhering to a CR program regularly may be difficult, so a high attrition rate may be expected. Fourth, it should also be noted that a cost-effective analysis is likely to be more difficult in the CR setting where the cost per hospitalization is not available. Fifth, it is essential to tailor the prescribed exercise regimen. Although we will tailor the type of exercise intervention, dose of exercise, and length of exercise according to baseline assessment for any patient, all educational materials will be similar. Future research should be performed with respect on the cause of heart failure.
Footnotes
Source of Support: This article is a protocol study of Ph.D thesis of educational exercise intervention based on social cognitive theory in patients empowerment diagnosed with heart failure; hence, we appreciate all the professors supporting this research
Conflict of Interest: None declared.
REFERENCES
- 1.Du H, Newton P, Zecchin R, Denniss R, Salamonson Y, Everett B, et al. An intervention to promote physical activity and self-management in people with stable chronic heart failure The Home-Heart-Walk study: Study protocol for a randomized controlled trial. Trials. 2011;12:63. doi: 10.1186/1745-6215-12-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saxton JM, Zwierska I, Mathur A, Channer KS. Study protocol to investigate the effects of testosterone therapy as an adjunct to exercise rehabilitation in hypogonadal males with chronic heart failure. BMC Cardiovasc Disord. 2006;6:46. doi: 10.1186/1471-2261-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carels RA, Musher-Eizenman D, Cacciapaglia H, Pérez-Benıtez CI, Christie S, O’Brien W. Psychosocial functioning and physical symptoms in heart failure patients: A within-individual approach. J Psychosom Res. 2004;56:95–101. doi: 10.1016/S0022-3999(03)00041-2. [DOI] [PubMed] [Google Scholar]
- 4.Koenig H. Depression in hospitalized older patients with congestive heart failure. Gen Hosp Psychiatry. 1998;20:29–43. doi: 10.1016/s0163-8343(98)80001-7. [DOI] [PubMed] [Google Scholar]
- 5.Bonow RO, Mann DL, Zipes DP, Libby P. 2 Set. Philadelphia: Saunders; 2011. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. [Google Scholar]
- 6.Ola BA, Adewuya AO, Ajayi OE, Akintomide AO, Oginni OO, Ologun YA. Relationship between depression and quality of life in Nigerian outpatients with heart failure. J Psychosom Res. 2006;61:797–800. doi: 10.1016/j.jpsychores.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 7.Wise FM. Exercise based cardiac rehabilitation in chronic heart failure. Aust Fam Physician. 2007;36:1019–24. [PubMed] [Google Scholar]
- 8.Grace SL, Abbey SE, Shnek ZM, Irvine J, Franche RL, Stewart DE. Cardiac rehabilitation I: Review of psychosocial factors. Gen Hosp Psychiatry. 2002;24:121–6. doi: 10.1016/s0163-8343(02)00178-0. [DOI] [PubMed] [Google Scholar]
- 9.Suaya JA, Stason WB, Ades PA, Normand SL, Shepard DS. Cardiac rehabilitation and survival in older coronary patients. J Am Coll Cardiol. 2009;54:25–33. doi: 10.1016/j.jacc.2009.01.078. [DOI] [PubMed] [Google Scholar]
- 10.Grace SL, Racco C, Chessex C, Rivera T, Oh P. A narrative review on women and cardiac rehabilitation: Program adherence and preferences for alternative models of care. Maturitas. 2010;67:203–8. doi: 10.1016/j.maturitas.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Taylor RS, Brown A, Ebrahim S, Jolliffe J, Noorani H, Rees K, et al. Exercise-based rehabilitation for patients with coronary heart disease: Systematic review and meta-analysis of randomized controlled trials. Am J Med. 2004;116:682–92. doi: 10.1016/j.amjmed.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 12.West R. Cardiac rehabilitation of older patients. Rev Clin Gerontol. 2003;13:241–56. [Google Scholar]
- 13.Gordon NF, Gulanick M, Costa F, Fletcher G, Franklin BA, Roth EJ, et al. Physical Activity and Exercise Recommendations for Stroke Survivors. Circulation. 2004;109:2031–41. doi: 10.1161/01.CIR.0000126280.65777.A4. [DOI] [PubMed] [Google Scholar]
- 14.Marcus BH, Williams DM, Dubbert PM, Sallis JF, King AC, Yancey AK, et al. Physical activity intervention studies what we know and what we need to know: A scientific statement from the American Heart Association Council on Nutrition, physical activity, and metabolism (Subcommittee on Physical Activity); Council on cardiovascular disease in the Young; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research. Circulation. 2006;114:2739–52. doi: 10.1161/CIRCULATIONAHA.106.179683. [DOI] [PubMed] [Google Scholar]
- 15.Bandura A. New York: W.H. Freeman; 1997. Self-efficacy: The exercise of control. [Google Scholar]
- 16.Meland E, Mæland JG, Lærum E. The importance of self-efficacy in cardiovascular risk factor change. Scand J Public Health. 1999;27:11–7. doi: 10.1177/14034948990270011001. [DOI] [PubMed] [Google Scholar]
- 17.Bandura A. Recycling misconceptions of perceived self-efficacy. Cognit Ther Res. 1984;8:231–55. [Google Scholar]
- 18.Bandura A. United States: Prentice-Hall, Inc; 1986. Social foundations of thought and action: A social cognitive theory. [Google Scholar]
- 19.Bandura A. Self-efficacy: Toward a unifying theory of behavioral change. Psychol Rev. 1977;84:191. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 20.Resnick B, Jenkins LS. Testing the reliability and validity of the self-efficacy for exercise scale. Nurs Res. 2000;49:154–9. doi: 10.1097/00006199-200005000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Melillo KD, Futrell M, Williamson E, Chamberlain C, Bourque AM, MacDonnell M, et al. Perceptions of physical fitness and exercise activity among older adults. J Adv Nurs. 2006;23:542–7. doi: 10.1111/j.1365-2648.1996.tb00017.x. [DOI] [PubMed] [Google Scholar]
- 22.Marcus B, Williams D, Dubbert P, Sallis J, King A, Yancey A, et al. Physical activity intervention studies: What we know and what we need to know: A scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity); Council on Cardiovascular Disease in the Young; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research. Circulation. 2006;114:2739–52. doi: 10.1161/CIRCULATIONAHA.106.179683. [DOI] [PubMed] [Google Scholar]
- 23.Huisman S, de Gucht V, Maes S, Schroevers M, Chatrou M, Haak H. Self-regulation and weight reduction in patients with type 2 diabetes: A pilot intervention study. Patient Educ Couns. 2009;75:84–90. doi: 10.1016/j.pec.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 24.Stadler G, Oettingen G, Gollwitzer PM. Physical activity in women: Effects of a self-regulation intervention. Am J Prev Med. 2009;36:29–34. doi: 10.1016/j.amepre.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 25.Courneya KS, McAuley E. Cognitive mediators of the social influence-exercise adherence relationship: A test of the theory of planned behavior. J Behav Med. 1995;18:499–515. doi: 10.1007/BF01904776. [DOI] [PubMed] [Google Scholar]
- 26.Frasure-Smith N, Lespérance F, Gravel G, Masson A, Juneau M, Talajic M, et al. Social support, depression, and mortality during the first year after myocardial infarction. Circulation. 2000;101:1919–24. doi: 10.1161/01.cir.101.16.1919. [DOI] [PubMed] [Google Scholar]
- 27.Pozehl B, Duncan K, Hertzog M, Norman JF. Heart Failure Exercise And Training Camp: Effects of a multicomponent exercise training intervention in patients with heart failure. Heart Lung. 2010;39(6 Suppl):S1–13. doi: 10.1016/j.hrtlng.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dekker J, van Dijk G, Veenhof C. Risk factors for functional decline in osteoarthritis of the hip or knee. Curr Opin Rheumatol. 2009;21:520–4. doi: 10.1097/BOR.0b013e32832e6eaa. [DOI] [PubMed] [Google Scholar]
- 29.Katz S. Assessing Self-Maintenance - Activities of Daily Living, Mobility, and Instrumental Activities of Daily Living. J Am Geriatr Soc. 1983;31:721–7. doi: 10.1111/j.1532-5415.1983.tb03391.x. [DOI] [PubMed] [Google Scholar]
- 30.VanderZee K, Sanderman R, Heyink J. A comparison of two multidimensional measures of health status: The Nottingham Health Profile and the RAND 36-item health survey 1.0. Qual Life Res. 1996;5:165–74. doi: 10.1007/BF00435982. [DOI] [PubMed] [Google Scholar]
- 31.Bennett SJ, Oldridge NB, Eckert GJ, Embree JL, Browning S, Hou N, et al. Discriminant properties of commonly used quality of life measures in heart failure. Quality of Life Research. 2002;11:349–59. doi: 10.1023/a:1015547713061. [DOI] [PubMed] [Google Scholar]
- 32.Rector TS, Kubo SH, Cohn JN. Validity of the Minnesota Living with Heart Failure questionnaire as a measure of therapeutic response to enalapril or placebo. Am J Cardiol. 1993;71:1106–7. doi: 10.1016/0002-9149(93)90582-w. [DOI] [PubMed] [Google Scholar]
- 33.Rector TS, Cohn JN. Assessment of patient outcome with the Minnesota Living with Heart Failure questionnaire: Reliability and validity during a randomized, double-blind, placebo-controlled trial of pimobendan. American Heart Journal. 1992;124:1017–25. doi: 10.1016/0002-8703(92)90986-6. [DOI] [PubMed] [Google Scholar]
- 34.Victor CR, Ross F, Axford J. Capturing lay perspectives in a randomized control trial of a health promotion intervention for people with osteoarthritis of the knee. J Eval Clin Pract. 2004;10:63–70. doi: 10.1111/j.1365-2753.2003.00395.x. [DOI] [PubMed] [Google Scholar]
- 35.Supino PG, Borer JS, Franciosa JA, Preibisz JJ, Hochreiter C, Isom OW, et al. Acceptability and psychometric properties of the Minnesota Living With Heart Failure Questionnaire among patients undergoing heart valve surgery: Validation and comparison with SF-36. J Card Fail. 2009;15:267–77. doi: 10.1016/j.cardfail.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Holla J, Steultjens MP, Roorda LD, Heymans MW, Ten Wolde S, Dekker J. Prognostic factors for the two-year course of activity limitations in early osteoarthritis of the hip and/or knee. Arthritis Care Res. 2010;62:1415–25. doi: 10.1002/acr.20263. [DOI] [PubMed] [Google Scholar]
- 37.Lau-Walker M. Importance of illness beliefs and self-efficacy for patients with coronary heart disease. J Adv Nurs. 2007;60:187–98. doi: 10.1111/j.1365-2648.2007.04398.x. [DOI] [PubMed] [Google Scholar]
- 38.Sharma L, Cahue S, Song J, Hayes K, Pai Y, Dunlop D. Physical functioning over three years in knee osteoarthritis - Role of psychosocial, local mechanical, and neuromuscular factors. Arthritis Rheum. 2003;48:3359–70. doi: 10.1002/art.11420. [DOI] [PubMed] [Google Scholar]
- 39.Resnick B. Reliability and validity of the Outcome Expectations for Exercise Scale-2. J Aging Phys Act. 2005;13:382–94. doi: 10.1123/japa.13.4.382. [DOI] [PubMed] [Google Scholar]
- 40.Petosa PS. Ohio, USA: Ohio State University; 1993. Use of social cognitive theory to explain exercise behavior among adults. [Google Scholar]
- 41.Wills T.A, Shinar O. Measuring perceived and received social support. In: Cohen S, Underwood LG, Gottleib BH, editors. Social support measurement and intervention. Oxford, UK: Oxford University Press; 2000. pp. 86–135. [Google Scholar]
- 42.Hazavehei S, Sabzmakan L, Hassanzadeh A, Rabiei K. The effect of PRECEDE Model-based educational program on depression level in patients with coronary artery bypass grafting. J Qazvin Univ Med Sci. 2008;12:32–40. [Google Scholar]
- 43.Kalkhoran MA, Karimollahi M. Religiousness and preoperative anxiety: A correlational study. Ann Gen Psychiatry. 2007;6:17. doi: 10.1186/1744-859X-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beck AT, Steer RA. New York: Psychological Corporation; 1987. BDI, Beck depression inventory: manual. [Google Scholar]
- 45.Ghassemzadeh H, Mojtabai R, Karamghadiri N, Ebrahimkhani N. Psychometric properties of a Persian-language version of the Beck Depression Inventory-Second edition: BDI-II-PERSIAN. Depress Anxiety. 2005;21:185–92. doi: 10.1002/da.20070. [DOI] [PubMed] [Google Scholar]
- 46.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: Implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–63. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 47.Piña IL, Apstein CS, Balady GJ, Belardinelli R, Chaitman BR, Duscha BD, et al. Exercise and heart failure: A statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation. 2003;107:1210–25. doi: 10.1161/01.cir.0000055013.92097.40. [DOI] [PubMed] [Google Scholar]
- 48.Health Media Festival. (HMF). Ministry of Health and Medical Education. 2012. [Last updated on 2013 Mar 2; cited on 2013 May 4]. Available from: http://www.healthfestival.ir/fr .
- 49.Everett B, Salamonson Y, Davidson PM. Bandura's exercise self-efficacy scale: Validation in an Australian cardiac rehabilitation setting. Int J Nurs Stud. 2009;46:824–9. doi: 10.1016/j.ijnurstu.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 50.Goode AD, Reeves MM, Eakin EG. Telephone-delivered interventions for physical activity and dietary behavior change: An updated systematic review. Am J Prev Med. 2012;42:81–8. doi: 10.1016/j.amepre.2011.08.025. [DOI] [PubMed] [Google Scholar]


