Abstract
Background:
Hepatitis C virus (HCV) is the main causative agent of post-transfusion hepatitis. The virus is distributed worldwide with varying prevalence in different countries, which could easily lead to chronic infections, cirrhosis, and even hepatocellular carcinoma. The aim of this study was to investigate prevalence of HCV infection and its trend in Iranian blood donors.
Materials and Methods:
Literatures on the HCV prevalence among blood donors in Iran were acquired through searching PubMed, Magiran, IranMedex, Scientific Information Databank, and Google databases. All the potentially relevant papers were reviewed independently by two investigators by assessing the eligibility of each paper and abstracting data. Prevalence was calculated using random effects model for meta-analysis.
Results:
Forty-eight studies with total samples of 10,739,221 persons from 1996 to 2011 were combined and meta-analyzed, the pooled prevalence of HCV infection among blood donors in Iran provinces and cities was 0.5% (95% CI: 0.4-0.6%). Trend of HCV infection was decreasing in recent years.
Conclusion:
This study provides a comprehensive and reliable data on the prevalence and trend of HCV infection among blood donors and may be helpful in providing insight into disease burden and opportunities for prevention. In comparison with countries in this geographic region, Iran has the lowest rate of HCV infection.
Keywords: Blood donors, hepatitis C virus, Iran, prevalence, seroepidemiology, transfusion
INTRODUCTION
Hepatitis C virus (HCV) is the main causative agent of post-transfusion hepatitis.[1] The virus is distributed worldwide with prevalence varying from 0.2% up to 40% in different countries,[2] which could easily lead to chronic infections, cirrhosis, and even hepatocellular carcinoma.[1,3] Estimated that, about 170 million people are infected by HCV worldwide,[2,4] about 3.5 million new cases occur annually,[3] and there are about 21.3 million HCV carriers in the Eastern Mediterranean countries.[5] The virus is transmitted through blood transfusion, intravenous injection, and exposures to contaminated medical practices, and sexual intercourses.[6]
The prevention and control of HCV infection, describing its geographic distribution, determining its risk factors, and evaluating co-factors that accelerate infection progression is somehow complicated.[5] HCV and other transfusion-associated infections have been significantly decreased in the countries where routine serologic screening of blood donation has been implemented.[2,6] Prevalence of HCV infection in blood donors is low in North European (0.01% - 0.02%) and in South European (1-1.5%) countries. In contrast, HCV is higher among developing countries, because there are problems such as low quality in blood screening tests, unsafe medical practices, and intravenous drug abuse with shared needle. For example, higher HCV prevalence have been reported in Southeast Asian countries, including India (1.5%), Malaysia (2.3%), Philippines (2.3%), Pakistan (8.1%), and in equatorial Africa (6.5%), as high as 20% in Egypt.[7,8]
“The first study on HCV prevalence in Iran was on healthy blood donors in 1994 and showed a seroprevalence of 0.25%. In that year, blood donors were not selected and anyone allowed donating blood. A recent study, on blood donors, indicated an almost stable seroprevalence of 0.13% during the period of 2004-2007. Reports on the prevalence of HCV infection in special populations in Iran are as high as 11-25% for patients on hemodialysis, 11-52% for intravenous drug abusers, and 15-76% for hemophilia and thalassemia patients”.[9] For example, in a study, seroprevalence of HCV infection in persons with high-risk behaviors who referred to the behavioral counseling center in Hamadan, west of Iran, was 35.6%.[10]
Blood transfusions contribute to the expanding transmission pool of viral infections, wherein even an asymptomatic person can transmit the infection. Screening and assessment of donors not only lessens the risk of transmission through infected blood products, but also gives an idea about the prevalence rates of the infections in the community. Evaluation and monitoring the prevalence and trend of HCV in blood donors is important for assessing quality and effectiveness of donor screening, public education, blood screening tests, and potential risk of transfusion-transmitted HCV infection.[6,11] In Iran Blood Transfusion Organization (IBTO), screening of blood donors became mandatory for HCV from 1996.[6] All donations of blood and blood derivatives are screened by serologic tests for HCV antibody by employing ELISA and confirmed by Recombinant Immunoblot Assay (RIBA) in IBTO centers. However, Nucleic Acid Testing (NAT) has not been implemented in all IBTO centers.[12]
No vaccine exists to prevent HCV infection, and treatment for HCV infection is costly. Thus, the prevention of primary HCV infection is very important. Any strategy to prevent HCV infection must be based on accurate data, including information about its prevalence. A few amounts of studies have been done on the prevalence of HCV infections in past years among blood donors in some provinces of Iran. However, they are little and incomplete. The aim of this study was to investigate prevalence of HCV infection and its yearly trends in Iranian blood donors up to the date of writing this paper. In this paper, we review the available national studies so as to provide comprehensive and reliable prevalence and trends of HCV infection among Iranian blood donors, which is speculated to help prevention of this infection and guide further research.
MATERIALS AND METHODS
Literature search
Literatures on the HCV prevalence among blood donors in Iran were acquired through searching PubMed, Magiran, IranMedex, Scientific Information Databank, and Google scholar Databases from 1996 to 2011. To search and include related studies as many as possible, we used including words: “Hepatitis C Virus,” “prevalence,” “blood donor,” “seroepidemiology,” “transfusion,” “Iran.”
Data selection and abstraction
All the potentially relevant papers were reviewed independently by two investigators through assessing the eligibility of each article and abstracting data in Excel data sheet. Non-relevant papers were put away and not analyzed. The following information were extracted from the literatures: First author's name, publication date, published journal name, study period, province or city of sampling, blood donor recruitment methods (first time, continuous, repeat or volunteer donors), sampling size, the number of subjects that had positive anti-HCV antibody (HCV Prevalence), type of HCV detection tests, gender, age, marriage status, risk factors etc., although some papers did not contain all of them. Study period were considered the end date of study mentioned in papers’ methods and used for analysis of trend.
The inclusion criteria were: 1 studies with full text or abstract, despite the language of original text; 2 studies reporting anti-HCV positive prevalence among blood donors in Iran; 3 studies using anti-HCV as a detection index of HCV and confirmed by RIBA. The exclusion criteria were: 1 studies without known sample origins; 2 studies with overlapping time or place of sample collection; 3 studies that failed to present data clearly.
Statistical analysis
We considered 48 studies from Iran; prevalence and 95% confidence interval were calculated using random effects model for meta-analysis. Considering the possibility of significant heterogeneity between studies was tested with the Q test (P < 0.10 was considered indicative of statistically significant heterogeneity) and the I2 statistic. Freeman-Tukey double arcsin transformation was used to stabilize variances, and also Forest plot was performed by study locations and year. Data manipulation and statistical analyzes were undertaken using the statistical software package Meta R Version 2.13.
RESULT
According to the literature search strategies, 53 relevant studies were identified, 19 studies in English language journals, 33 studies in Persian language journals, and 1 IBTO bulletin found in databases such as PubMed, Google Scholar, Magiran, IranMedex, and Scientific Information Databank. Some studies were excluded based on the inclusion and exclusion criteria, and finally, 48 studies were included in meta-analysis.
General information of samples
Our review involved 48 studies done in 26 provinces or cities as their names appeared in paper's titles or methods, the names are shown in Table 1. A total of 10,739,221 blood donors were included. The donor samples mainly came from Tehran (1,656,978) and then from Shiraz (1,172,837). Studies had duration variation from 2 days to as longer as 6 years period in original researches. The most of research methods were cross-sectional or retrospective. All blood donors were voluntary, and most of blood donors were men. Marriage status of blood donors was not identified in the most of the papers. Major risk factors for HCV infection were age, marriage status, transfusion, surgery, medical procedures, lifestyle, prison history, tattooing, and intravenous injections.
Table 1.
Data derived from published documents about prevalence of HCV in Iranian blood donors and its lower and upper limits were calculated (95% confidence)
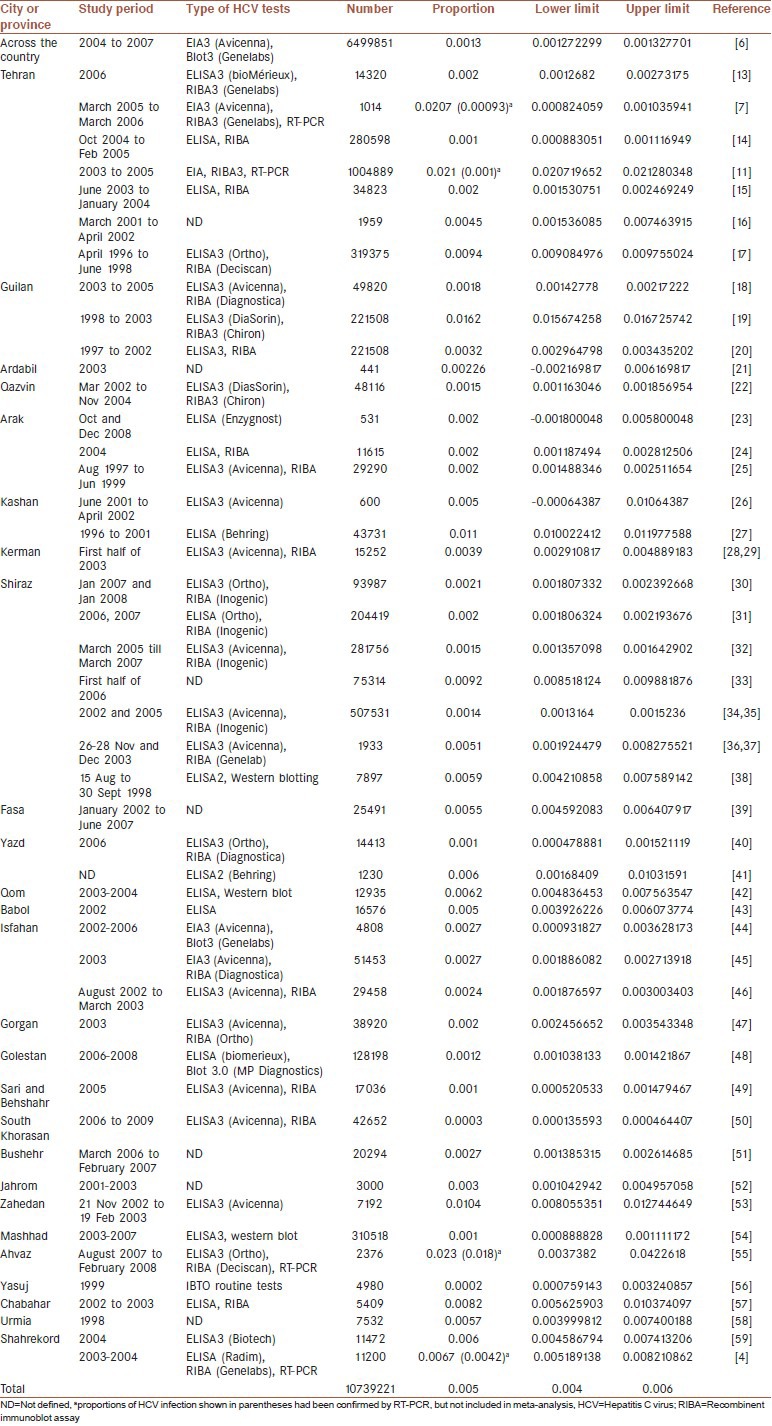
Screening tests of HCV infection in studies were based on detection of anti-HCV antibodies using ELISA and confirmed by RIBA; kit generation number or manufacturer's names are shown in Table 1. In some studies, results of mentioned tests were verified by molecular method RT-PCR. Proportion of HCV positive results using NAT are shown in parentheses and specified by letter a Table 1, but these results were not included in meta-analysis.
Prevalence of HCV infection among blood donors in Iran provinces and cities
Results of 48 studies with total samples of 10,739,221 persons from 1996 to 2011 were combined and meta-analyzed, the pooled prevalence rate of HCV infection among blood donors in Iran provinces and cities was estimated 0.5% (95% CI: 0.4% - 0.6%), using random effect model, Heterogeneity, I-squared = 99.9, Heterogeneity Chi-squared = 31478.61 (d.f = 47) P < 0.001, Estimate of between-study variance Tau-squared = 0.000.
Based on statistical analysis, no dramatic geographic difference in prevalence rates of HCV infection among blood donors from different provinces or cities of Iran was observed. The highest rate of pooled prevalence of HCV infection was in Kashan a city in Isfahan province, 1.09% (95% CI: 1.0% - 1.19%), and the lowest prevalence was in South Khorasan province with the rate of 0.03% (95% CI: 0.02% - 0.05%). Seropositivity of HCV and distribution by region is depicted in Graph 1.
Graph 1.
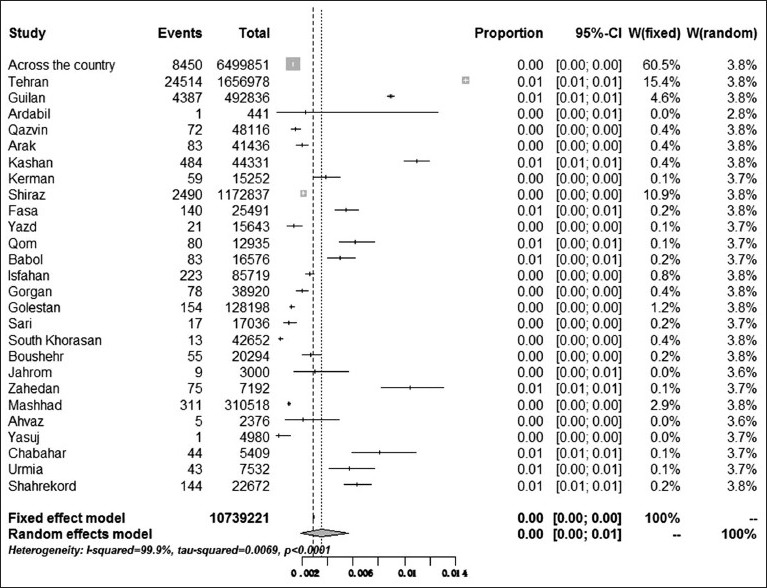
Prevalence of HCV infection among blood donors in Iran provinces and cities. The software package was limited in reporting the number of decimals more than two digits
Time
In data analyzing the study periods that prolonged more than one year, the final year of study was considered as study period. All studies were divided into 11 groups according to the year of study. Graphs 2 and 3 shows the pooled prevalence of HCV in each year group. The annual HCV prevalence rates and their trends over 11 years are shown in Figure 1. During 1998, the HCV prevalence rate was 0.93% (95% CI: 0.89% - 0.96%) in Iranian blood donors. Then, prevalence of HCV infection had fluctuation till 2006. The highest prevalence rate was 1.39% in 2005 (95% CI: 1.37% - 1.41%) then, the annual HCV prevalence rates showed decreasing linear trend to 0.13% in 2007 (95% CI: 0.13% - 0.14%) and to its lowest rate 0.03% in 2009, (95% CI: 0.02% -0.05%) (See Graph 3).
Graph 2.
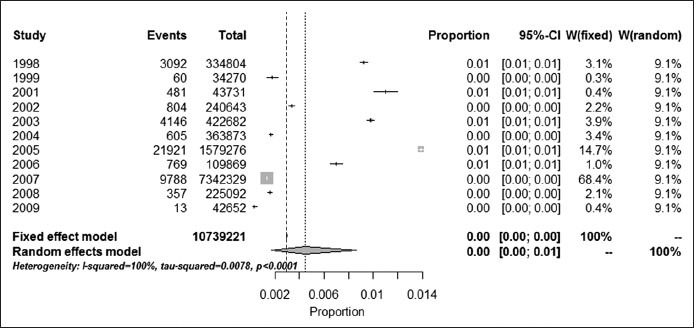
Prevalence of HCV infection among blood donors study periods in Iran
Graph 3.
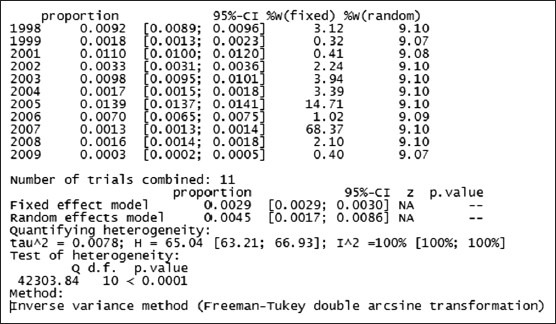
Statistical analysis of HCV infection among blood donors in Iran based on study periods
Figure 1.
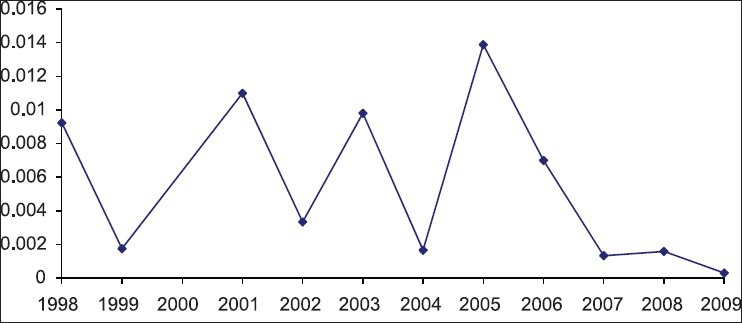
Prevalence (proportion) of HCV infection among blood donors at different study periods
DISCUSSION
The purpose of this study was to assess the comprehensive prevalence and trends of HCV infection in Iranian blood donors by a meta-analysis using published papers. We included 48 papers from the Iranian authors investigating for HCV. In this systematic review, we calculated that 0.5% (95% CI: 0.4% - 0.6%) of the blood donors were positive for HCV antibodies in 10,739,221 persons from 1996 to 2011. Information about HCV prevalence provides insight into disease burden and opportunities for prevention.
Prevention of transfusion-transmitted infections in Iran has been achieved by reducing unnecessary transfusions, using voluntary donors, excluding donors with specific risk factors, and systematic screening of all donated blood for infection. Although these interventions in Iran are applied, the risk of HCV infections remains,[4,7,60] because the problem of chronic infection with HCV may be greater than generally recognized. By considering the vast population of the country, a prevalence of 0.5% leads to thousands of seropositive patients, if we assume that seronegative donors were not viremic. Therefore, every blood transfusion carries a potential risk for transmission.
The global seroprevalence of HCV among blood donors varies from 0.4% to 19.2%.[61] Our study showed that the overall 11-year HCV seropositivity was 0.5%. Early studies in 1994 on blood donors point to a seroprevalence of approximately 0.25% for HCV infection in Iran.[62] In another study, the prevalence of HCV among Iranian blood donors was 0.13%, in which the data were collected from all the blood transfusion centers across the country in a total of 6,499,851 donations during the four year period from 2004 through 2007.[6] In comparison with countries in this geographic region, we have the lowest rate of HCV infection.[7,63]
The prevalence of infections among blood donors has been used as a surrogate marker for the prevalence of infections in the general population, although there are certain pitfalls like the exclusion of people below 18 years and over 60 years and low number of female donors. The prevalence of viral markers in the donor population is lower than general population, because donors are a selected population at low risk of infectious diseases due to public education and donor health assessment.[64] The frequency of HCV exposure in the general population in Iran had been increased from 0.3% in 1997 to approximately 1% in 2006.[6] In a recent study, the seroprevalence of HCV in the general population had been 0.5%.[9] Authors of studies suggest that the prevalence of HCV infection in Iran is rising, and in the future, hepatitis C will replace hepatitis B as the most common cause of chronic viral liver disease in Iran because HBV has vaccine, but no vaccine exist for HCV.[2,9]
Most routine screening tests for HCV infection in IBTO centers were third generation ELISA and RIBA. However, the actual prevalence of HCV is difficult to assess because serological tests do not discriminate among acute, chronic, or resolved infection. In four papers, in addition to routine methods, the blood donors were tested for HCV RNA by reverse transcriptase polymerase chain reaction (RT-PCR). This test is designed based on reverse transcription of viral RNA; by using this technique, the prevalence of HCV was lower than routine methods [Table 1]. One explanation to these lower results is that antibody-positive participants did not have positive RT-PCR results because they had cleared HCV viremia. Other probability is that false-positive ELISA and RIBA results for HCV are frequent.[1] Thus, researchers were attempted to design more useful techniques to identify viral genomes, such as RT-PCR.
Use of RT-PCR in healthy volunteer blood donors has also contributed to the decrease in rates of HCV infections. For example, HCV prevalence decreased significantly since NAT introduction in USA.[65] Although the NAT are recommended for screening blood donations, they are not widely available in developing countries. Currently, IBTO is evaluating implementation of the NAT techniques for improving the blood safety.[12] Detecting of HCV RNA also differentiates between active and resolved infection. Other advantage of NAT assay is capability of detecting HCV genotypes specific to the provinces and cities.
Because of incompleteness of data in selected papers, we were unable to analysis the risk factors of HCV infection. The association between HCV positivity and risk factors mentioned in some papers were similar to previous knowledge in this area.[17,54] Risk factors for HCV infection were age, marriage status, transfusion, surgery, medical procedures, lifestyle, prison history, tattooing, and intravenous injections. The main risk factor for acquiring HCV infection before the routine anti-HCV screening of blood donors was blood transfusion. Now the relatively high proportion of non-transfused hepatitis C cases suggests that transfusion is not the predominant route of transmission of HCV. Nowadays, intravenous drug abuse is the major risk factor for HCV infection.[66]
There were no data about HCV prevalence in some provinces of Iran. However, our study showed that prevalence of HCV is variable in different cities and provinces. Other seroepidemiological studies have suggested different rates of HCV infection in healthy blood donors in different provinces of Iran.[2,4,9] In other parts of the world, the prevalence of the HCV infection differs in blood donor and general population, which reflects the health, social status in those regions. For example, in the Middle East countries, prevalence of HCV infection have been as: 7.1% in Iraq, 6.3% in Qatar, 0.65-6.25% in Jordan, 0.8% in Kuwait; among general population, 0.46% in Cyprus among soldiers from Turkey, blood donors, and soldiers from Northern Cyprus; 0.9% in Oman, 0.95% in Syria; 0.6% in Lebanon; 2.7% in Yemen, 14.5% in Egypt among blood donors; 1-2% in Turkey among healthy individuals.[5] Control strategies should take these differences into account.
The prevalence and trend of HCV infection fluctuated in the past donors compared to recent donors [Figure 1]. Reasons may be that prevalence was variable in past, and numbers of studies were fewer than recent years. However, study periods, sample sizes, geographic regions, and type of kits used in detecting antibodies associated with the prevalence of the infection in gathered documents were different. These reasons would be the cause of zigzag appearance of trend in past years. Reasons about increase in HCV prevalence around 2005 in our study in consistent with result of other study was that total number of donations in that year was higher may be due to an increase in number of first-time donors and change in confirmation kits used during that period leaded to increase in number of positives results.[6]
As the sensitivity of HCV screening tests had increased, viral infection transmission through blood products has been eliminated in developed countries and has decreased in developing countries. The significant decreasing trend of HCV infection among blood donors in the recent five years from 0.14% in 2006 to 0.03% in 2009 is consistent with other study that the prevalence of HBV and HCV is decreased in 2005 compared to 2003.[11] In another study also HCV prevalence showed a slight decline in blood donations from 0.14% in 2005 to 0.12% in 2007.[6] This decrease can be attributable to increase of public knowledge on transfusion-transmitted infections and improving of the safety measures employed in recent years[5,6] such as: The IBTO centers screen all blood donors for HCV prior to donation, using of uniform standards, physical examination, application of strict questionnaire to potential donors, standard operating procedures, instruments, validation of procedures and training across the country, implementing of voluntary blood donation to 100% since 2007, removing replacement donation, increasing number of regular donations, effective donor selection program such as confidential unit exclusion, improvement in automation, data registry of blood donors with history of positive screening tests, using of high sensitive screening test kits.[12]
CONCLUSIONS
Control and prevention of HCV infection is an important public health problem by screening of blood donors because the most of infections are asymptomatic and do not resolve but lead to chronic infection. Therefore, control of HCV transmission requires continuous monitoring and surveillance for prevention. This meta-analysis provides a comprehensive and reliable data on the prevalence and trend of HCV infection among Iranian blood donors and may be helpful in prevention. Trend of HCV infection was decreasing in recent years, and these trends of HCV prevalence suggest that the safety measures implemented in recent years in Iran have been useful. In comparison with countries in geographic region, Iran has lowest rate of HCV infection.[7,63]
ACKNOWLEDGEMENT
We would like to thank Kurdistan University of Medical Sciences for their supports.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Hassanshahi G, Arababadi MK, Assar S, Hakimi H, Karimabad MN, Abedinzadeh M, et al. Post-transfusion-transmitted hepatitis C virus infection: A study on thalassemia and hemodialysis patients in southeastern Iran. Arch Virol. 2011;156:1111–5. doi: 10.1007/s00705-011-0950-y. [DOI] [PubMed] [Google Scholar]
- 2.Alavian SM. Hepatitis C infection in Iran; A review article. Iranian Journal of Clinical Infectious Diseases (IJCID) 2009;4:47–59. [Google Scholar]
- 3.Gao X, Cui Q, Shi X, Su J, Peng Z, Chen X, et al. Prevalence and trend of hepatitis C virus infection among blood donors in Chinese mainland: A systematic review and meta-analysis. BMC Infect Dis. 2011;11:88. doi: 10.1186/1471-2334-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doosti A, Amini-Bavil-Olyaee S, Tajbakhsh E, Adeli A, Mahboudi F. Prevalence of viral hepatitis and molecular analysis of HBV among voluntary blood donors in west Iran. New Microbiol. 2009;32:193–8. [PubMed] [Google Scholar]
- 5.Fallahian F, Najafi A. Epidemiology of hepatitis C in the middle East. Saudi J Kidney Dis Transpl. 2011;22:1–9. [PubMed] [Google Scholar]
- 6.Amini Kafi-abad S, Rezvan H, Abolghasemi H, Talebian A. Prevalence and trends of human immunodeficiency virus, hepatitis B virus, and hepatitis C virus among blood donors in Iran, 2004 through 2007. Transfusion. 2009;49:2214–20. doi: 10.1111/j.1537-2995.2009.02245.x. [DOI] [PubMed] [Google Scholar]
- 7.Khedmat H, Fallahian F, Abolghasemi H, Alavian SM, Hajibeigi B, Miri SM, et al. Seroepidemiologic study of hepatitis B virus, hepatitis C virus, human immunodeficiency virus and syphilis infections in Iranian blood donors. Pak J Biol Sci. 2007;10:4461–6. doi: 10.3923/pjbs.2007.4461.4466. [DOI] [PubMed] [Google Scholar]
- 8.Sharifi Z, Mahmoudian Shooshtari M. Hepatitis C virus infection and genotypes in blood donors. Iran J Virol. 2008;2:17–22. [Google Scholar]
- 9.Merat S, Rezvan H, Nouraie M, Jafari E, Abolghasemi H, Radmard AR, et al. Seroprevalence of hepatitis C virus: The first population-based study from Iran. Int J Infect Dis. 2010;14(Suppl 3):e113–6. doi: 10.1016/j.ijid.2009.11.032. [DOI] [PubMed] [Google Scholar]
- 10.Keramat F, Eini P, Majzoobi M. Seroprevalence of HIV, HBV and HCV in persons referred to hamadan behavioral counseling center, West of Iran. Iran Red Crescent Med J. 2011;13:42–6. [PMC free article] [PubMed] [Google Scholar]
- 11.Khedmat H, Alavian SM, Miri SM, Amini M, Abolghasemi H, Hajibeigi B, et al. Trends in seroprevalence of hepatitis B, hepatitis C, HIV and Syphilis infections in Iranian blood donors from 2003 to 2005. Hepat Mon. 2009;9:24–8. [Google Scholar]
- 12.Abolghasemi H, Maghsudlu M, Amini Kafi-Abad S, Cheraghali A. Introduction to Iranian blood transfusion organization and blood safety in Iran. Iran J Public Health. 2009;38:82–7. [Google Scholar]
- 13.Omid Khoda A, Gharehbaghian A, Jamali M, Ahmad Beigi N, Hashemi S, Rahimi A, et al. Comparison of the prevalence of major transfusion-transmitted infections among Iranian blood donors using confidential unit exclusion in an Iranian population. Hepat Mon. 2011;1:11–3. [PMC free article] [PubMed] [Google Scholar]
- 14.Sharifi S, Attarchi Z. Comparison of first time and repeated donors blood test results. Northern center region blood transfusion Bultin (in Persian) 4:1–6. [Google Scholar]
- 15.Attarchi Z, Ghafouri M, Hajibaygi B, Assari S, Alavian SM. Donor deferral and blood-borne infections in blood donors of Tehran (in Persian) Sci J Iranian Blood Transfus Organ. 2006;2:353–64. [Google Scholar]
- 16.Pourshams A, Malekzadeh R, Monavvari A, Akbari MR, Mohamadkhani A, Yarahmadi S, et al. Prevalence and etiology of persistently elevated alanine amino transferase levels in healthy Iranian blood donors. J Gastroenterol Hepatol. 2005;20:229–33. doi: 10.1111/j.1440-1746.2004.03511.x. [DOI] [PubMed] [Google Scholar]
- 17.Alavian SM, Gholami B, Masarrat S. Hepatitis C risk factors in Iranian volunteer blood donors: A case-control study. J Gastroenterol Hepatol. 2002;17:1092–7. doi: 10.1046/j.1440-1746.2002.02843.x. [DOI] [PubMed] [Google Scholar]
- 18.Taheri Azbarmi Z, Nouri S, Joukar F, Jafarshad R, Haajikarimian K, Alinejad S, et al. Transfusion transmitted diseases in Rasht blood donors (in Persian) Sci J Iranian Blood Transfus Organ. 2008;4:337–43. [Google Scholar]
- 19.Mansour-Ghanaei F, Fallah M, Jafarshad R, Joukar F, Salari A, Tavafzadeh R. Prevalence of hepatitis B surface antigen and hepatitis C virus antibody and their risk factors among Guilan's volunteer blood donors (1998-2003) Hepat Mon. 2007;7:239–41. [Google Scholar]
- 20.Mansour Ghanaei HR, Fallah MS, Jafarshad R, Joukar F, Salari A, Tavafzadeh R, et al. Prevalence of hepatitis B and hepatitis C, and their risk factors among Guilan blood donors (in Persian) Sci J Iranian Blood Transfus Organ. 2008;4(5):331–6. [Google Scholar]
- 21.Habibzadeh S, Davarnia B, Bazazataei A, Bagherzadeh S, Hamid Kholgh GR. Epidemiological evaluation of transfusion transmitted diseases in Ardabil in Tasoua and Ashoura 1381(2003) (in Persian) Sci J Iranian Blood Transfus Organ. 2003;1:55–60. [Google Scholar]
- 22.Bozorgi S, Ahmadzad Asl M, Ramezani H, Kargarfard H, Alavian S. Study of viral infections prevalence in blood donors of qazvin province in different time intervals and during bam earthquake (in Persian) Govaresh. 2006;11:242–8. [Google Scholar]
- 23.Sofian M, Aghakhani A, Izadi N, Banifazl M, Kalantar E, Eslamifar A, et al. Lack of occult hepatitis B virus infection among blood donors with isolated hepatitis B core antibody living in an HBV low prevalence region of Iran. Int J Infect Dis. 2010;14:e308–10. doi: 10.1016/j.ijid.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 24.Mahdaviani F, Saremi S, Maghsoudlu M, Pourfathollah AA. Prevalence of blood transmitted viral infections in regular and non-regular donors of Arak Blood Center (in Persian) Sci J Iranian Blood Transfus Organ. 2006;2:343–51. [Google Scholar]
- 25.Tavallaei SG. The prevalence of hepatitis C virus among blood donors in Markazi province (in Persian) Journal of Arak University of Medical Sciences. 2000;3:11–5. [Google Scholar]
- 26.Moniri R, Mosayebii Z, Mossavi G. Seroprevalence of Cytomegalovirus, Hepatitis B, Hepatitis C and human immunodeficiency virus antibodies among volunteer blood donors. Iran J Public Health. 2004;33:38–42. [Google Scholar]
- 27.Afzali H, Taghavi Ardakani A, Vali GR. Seroepidemiology of Hepatitis B and C in blood donors in Kashan, 1996-2001 (in Persian). Feyz. Kashan University of Medical Sciences and Health Services. 2002;6:43–50. [Google Scholar]
- 28.Delavari M, Tabatabaie SM, Sheikh Bardsiri H, Maarefdust Z, Zandieh T. The prevalence of Hepatitis C and its related factors among blood donors of Kerman Blood Center (in Persian) Sci J Iranian Blood Transfus Organ. 2006;2:269–71. [Google Scholar]
- 29.Delavari M, Tabatabaei S. Frequency of Hepatitis C and its related factors in blood donors in Kerman in 2003 (in Persian) Journal of Army University of Medical Sciences (JAUMS) 2004;2:353–7. [Google Scholar]
- 30.Kasraian L, Tavassoli AR. Prevalence of hepatitis C and its risk factors in blood donors at Shiraz transfusion center (in Persian) Koomesh, Journal of Semnan University of Medical Sciences. 2008;10:7–12. [Google Scholar]
- 31.Kasraian L, Tavasoli A. Positivity of HIV, hepatitis B and hepatitis C in patients enrolled in a confidential self-exclusion system of blood donation: A cross-sectional analytical study. Sao Paulo Med J. 2010;128:320–3. doi: 10.1590/S1516-31802010000600002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kasraian L. Survay the rate of blood donors loss due to unconfirmed ELISA test in blood transfusion organization in Fars province (in Persian) Journal of Guilan University of Medical Science. 2008;67:80–7. [Google Scholar]
- 33.Kasraian L, Torab Jahromi SA. Study of confidential self-exclusion cases in Shiraz regional blood transfusion center (in Persian) Daneshvar. 2008;15:71–8. [Google Scholar]
- 34.Kasraian L, Torab Jahromi SA. Prevalence of major Transfusion-transmissible viral infections in blood donors attending fars blood transfusion center, Shiraz, Southern Iran: 2002-05. Iran J Med Sci. 2007;32:114–7. [Google Scholar]
- 35.Kasraian L, Torab Jahromi SA. Prevalence of major transfusion transmitted viral infections (HCV, HBV, HIV) in Shiraz blood donors from 2000 to 2005 (in Persian) Sci J Iranian Blood Transfus Organ. 2007;3:373–8. [Google Scholar]
- 36.Kasraian L, Torab-Jahromi S. The effect of Bam earthquake on blood supply and safety in Shiraz blood transfusion organization, winter 1382 (in Persian) Journal of Shahrekord University of Medical Sciences Journal (JSKUMS) 2008;1:9–13. [Google Scholar]
- 37.Kasraian L. National disasters in Iran and blood donation: Bam earthquake experience. Iran Red Crescent Med J. 2010;12:316–8. [Google Scholar]
- 38.Ghavanini A, Sabri MR. Hepatitis B surface antigen and anti-hepatitis C antibodies among blood donors in the Islamic Republic of Iran. East Mediterr Health J. 2000;6:1114–6. [PubMed] [Google Scholar]
- 39.Yavari Barhaghtalab MJ, Saboori S, Damiri M, Ekrahi M. Prevalence of viral markers for hepatitis B and C in healthy volunteer blood donors in fasa region, South Iran. 13th International Congress on Infectious Diseases Abstracts, Poster Presentations. International Journal of Infectious Diseases. 2008;12(1):e87–e. [Google Scholar]
- 40.Javadzadeh Shahshahani H. Comparison of the positive predicative value of two enzyme immunoassay screening kits for hepatitis C in blood donors (in Persian) Sci J Iranian Blood Transfus Organ. 2007;4:51–7. [Google Scholar]
- 41.Salman Roghani H. Prevalence of Hepatitis B Ab with or without anti-HBSAb in HBSAg seronegative blood donors (in Persian) Journal of Shaheed Sadoughi University of Medical Sciences and Health services. 2005;12:10–6. [Google Scholar]
- 42.Mardani A, Hosseini S, Kheirkhahi N. Study of confidential self-exclusion cases in Qom Regional Blood Transfusion Center (in Persian) Sci J Iranian Blood Transfus Organ. 2006;3:183–9. [Google Scholar]
- 43.Agha Jani Poor K, Zandieh T. Seroepidemiological investigation of hepatitis B, C and HIV virus in safe blood donors of Babol Blood Transfusion Center (in Persian) Sci J Iranian Blood Transfus Organ. 2006;2:339–41. [Google Scholar]
- 44.Salehi H, Salehi M, Khish Ardestani M, Khorvash F, Mz K. Comparison of the blood safety from Viral makers of B and C hepatitis, AIDS and Addiction, on the blood donors within the religious ceremonies and routine conditions (in Persian) Journal of Isfahan Medical School. 2011;28:56–62. [Google Scholar]
- 45.Pourazar A, Akbari N, Hariri M, Yavari F, Akbari S. Evaluation of demographic profiles and prevalence of major viral markers in first time vs repeat blood donors in Esfahan (in Persian) Sci J Iranian Blood Transfus Organ. 2006;2:323–9. [Google Scholar]
- 46.Masaeli Z, Jaberi MR, Magsudlu M. A comparison of seroprevalence of blood-borne infections among regular, sporadic, and first-time blood donors in Isfahan (in Persian) Sci J Iranian Blood Transfus Organ. 2006;2:301–7. [Google Scholar]
- 47.Kazemi Nejad V, Azar Housh R, Molana A, Dehbashi GR. Frequency of Hepatitis B virus, Hepatitis C virus and human immunodeficiency virus in blood donors and patients in Gorgan Blood Transfusion Organization in 2003 (in Persian) Journal of Gorgan University of Medical Sciences. 2005;15:84–6. [Google Scholar]
- 48.Bani Aghil S, Abbasi S, Arab M, Seyedein MS. The Prevalence of HCV, HBV, HIV in Blood Donors of Golestan (in Persian) Medical Laboratory Journal. 2009;2:1–5. [Google Scholar]
- 49.Nour Kojory S, Alaoddowleie H, Seddighian F. Efficacy of confidential self-exclusion and failed systems on blood donation safety in Sari and Behshahr blood donors (in Persian) Sci J Iranian Blood Transfus Organ. 2007;4:153–8. [Google Scholar]
- 50.Ghafouri M, Ameli MR. Comparing prevalence of transfusion transmitted viral infections in various population groups of South Khorasan (in Persian) Blood SJIBTO. 2011;7:242–8. [Google Scholar]
- 51.Esmaieli H. Seroepidemiological survey of hepatitis B, C, HIV and syphilis among blood donors in Bushehr-Iran (in Persian) Iranian South Medical Journal. 2009;2:183–90. [Google Scholar]
- 52.Emamghorashi F, Fathi GH, Mohtashami A. Evaluation of demographic characteristics and hepatitis B, C and HIV prevalence among blood donors in Jahrom (in Persian) Sci J Iranian Blood Transfus Organ. 2006;2:373–8. [Google Scholar]
- 53.Sanei Moghaddam E, Khosravi S, Gharibi T. Prevalence of HBsAg and Anti-HCV reactivity in donors embarking on direct blood donation and among first-time blood donors in Zahedan Blood Transfusion Center. Blood SJIBTO. 2005;1:19–25. [Google Scholar]
- 54.Vossoughinia H, Taghi Shakeri M, Mokhtari Amirmajdi E, Ravan bakhsh F, Abedini5 S. Risk factors for hepatitis B and C in 400 blood donor volunteers in mashhad during 2003-2007: A case-control study (in Persian) Ofogh-e-Danesh (Ufuq-i dāish) 2010;16:68–76. [Google Scholar]
- 55.Farshadpour F, Makvandi M, Samarbafzadeh AR, Jalalifar MA. Determination of hepatitis C virus genotypes among blood donors in Ahvaz, Iran. Indian J Med Microbiol. 2010;28:54–6. doi: 10.4103/0255-0857.58731. [DOI] [PubMed] [Google Scholar]
- 56.Nabavizadeh SH, Haghbeen S. Prevalence of blood transmitted infection in donors of Yasuj blood transfusion organization (in Persian) Journal of Guilan University of Medical Sciences. 2000;35:64–7. [Google Scholar]
- 57.Jabbari H, Karami S, Fattahi F, Jam S, Mohraz M. “Seroprevalence of Human Immunodeficiency Virus, Hepatitis B and C viruses among blood donors in Chabahar Iran”. International Journal of Infectious Diseases. 2008;12(Supplement 1(0)):e424. [Google Scholar]
- 58.Sadeghi A, Shariat Zadeh M, Lamei A. Evaluation of the prevalence of hepatitis C virus (HCV) among blood donors in Urmia transfusion center (in Persian) Urmia Medical Journal. 1999;9:242–8. [Google Scholar]
- 59.Tajbakhsh E, Yaghobi R, Vahedi A. A serological survey on hepatitis C virus Antibody in blood donors with an ELISA method (in persian) Tehran University Medical Journal. 2007;65:69–73. [Google Scholar]
- 60.Alavian SM, Miri SM, Keshvari M, Elizee PK, Behnava B, Tabatabaei SV, et al. Distribution of hepatitis C virus genotype in Iranian multiply transfused patients with thalassemia. Transfusion. 2009;49:2195–9. doi: 10.1111/j.1537-2995.2009.02252.x. [DOI] [PubMed] [Google Scholar]
- 61.Meena M, Jindal T, Hazarika A. Prevalence of hepatitis B virus and hepatitis C virus among blood donors at a tertiary care hospital in India: A five-year study. Transfusion. 2011;51:198–202. doi: 10.1111/j.1537-2995.2010.02801.x. [DOI] [PubMed] [Google Scholar]
- 62.Rezvan H, Ahmadi J, Farhadi M, Tardyan S. A preliminary study of prevalence of HCV infection in healthy Iranian blood donors. Vox Sang. 1994;67:149. [Google Scholar]
- 63.Sultan F, Mehmood T, Mahmood MT. Infectious pathogens in volunteer and replacement blood donors in Pakistan: A ten-year experience. Int J Infect Dis. 2007;11:407–12. doi: 10.1016/j.ijid.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 64.Mahmoodian-Shooshtari M, Pourfathollah A. An overview analysis of blood donation in the Islamic Republic of Iran. Arch Iran Med. 2006;9:200–3. [PubMed] [Google Scholar]
- 65.Zou S, Dorsey KA, Notari EP, Foster GA, Krysztof DE, Musavi F, et al. Prevalence, incidence, and residual risk of human immunodeficiency virus and hepatitis C virus infections among United States blood donors since the introduction of nucleic acid testing. Transfusion. 2010;50:1495–504. doi: 10.1111/j.1537-2995.2010.02622.x. [DOI] [PubMed] [Google Scholar]
- 66.Fallahian F, Najafi A, Alavian S. Intravenous drug use: The predominant risk factors for hepatitis C Virus Infection. Shiraz E-Medical Journal (SEMJ) 2010;11(4):209–218. [Google Scholar]


