Abstract
Background:
The objective of this study is to optimize the effective dose of heparin and ligustrazine hydrochloride injection (LHI) for drug combination.
Materials and Methods:
The animal clinical study of LHI was performed by the rat's model of induced arteriovenous shunt thrombosis. Experimental animals were grouped into several groups and separately treated with both LHI (20, 40, 80 mg/kg, i.p.) and heparin (60, 55, and 50 U/kg; 5000 U/ml; Sigma, i.v). The study had used thrombus weight, protein concentration in thrombus homogenate, inhibition rate of thrombosis, and plasma anti-thrombin activity as indications.
Results:
The group combination (50, 80) got the result of 100% antithrombotic activity with 0 ± 0 mg of thrombus weight, 14 ± 3 μg/ml of protein concentration in thrombus homogenate and 1.5 ± 0.04 U/ml of plasma anti-thrombin activity. Its anti-thrombotic effect was much better than individual groups treated with LHI in a dose of 0 mg/kg and group of combination (0, 80) (P < 0.05) while antithrombotic effect of 55 and 60 U/kg heparin alone was only 37-58%. Therefore, the group of combination (50, 80) was optimal for 100% antithrombotic activity.
Conclusion:
Optimal combined doses of LHI and heparin preventing blood coagulation were determined and the results were available. It may give some hint for the further clinical application on human.
Keywords: Anti-thrombotic, heparin, ligustrazine hydrochloride injection
INTRODUCTION
Ligustrazine is the main component of Chinese medicine Rhizoma Chuanxiong and play an important role in the treatment of angiocardiopathy. It inhibits platelet aggregation,[1,2,3] improves atherosclerosis situation,[4] protects endothelium injury,[5] and has antithrombotic effects.[6] In the research of pharmacokinetics by rats, it could be learned that the dose of injection of ligustrazine used was 40 mg/kg by i.p.[7] While in the study of pharmacology by rats, the dose of ligustrazine hydrochloride injection (LHI) applied was 35 mg/kg.[8] So that in our animal clinical study, the doses of 20, 40, 80 mg/kg were selected not only via investigating previous studies,[7,8,9] but also based on our previous studies of LHI for antithrombotic effect in clinical. In the clinical studies of LHI many years ago,[10,11] clinicians utilized single LHI to patients for antiplatelet aggregation or cerebral embolism with different doses such as 80, 120, 160, 240, 320 and 480 mg/50 kg. Referring the dose conversion rate between rats and human in the related paper and guidance,[12,13] the dose of rats should be within 16~96 mg/kg substantially. So, it could also be learned that the doses of LHI adopted in our study were reasonable. In our research, LHI was studied for antithrombotic effects with several doses on rats for the first time. There is no doubt that based on ligustrazine concentrate LHI had further therapy effects on various thrombosis and cardiovascular diseases as well.
LHI therapy associated with heparin for acute phase arterial and venous thrombosis was very popular in China.[14,15] However, the optimized dose of combination therapy was unknown. Here, we studied antithrombotic activity of a Chinese drug product LHI combined with heparin in rats with various combinations of doses via experimental arteriovenous shunt thrombosis for the first time. The combination therapy will be much more helpful in the treatment of post-operative thrombosis, pulmonary embolism, heart failure, respiratory failure of pulmonary heart disease and so on.
MATERIALS AND METHODS
Experiments were performed on male Wistar rats (n = 140, weight between 180 g and 250 g) with an average body weight of 200 g at the approximate age of 9 months. The temperature in an animal room was (25 ± 2)°C with 12 h artificial light. It's airing all the time. Experimental animals were randomly divided into eight groups according to the body weight, and separately treated with both of LHI (0, 20, 40 or 80 mg/kg, i.p.) and heparin (60, 55, 50 or 0 U/kg; 5000 U/ml; Sigma, i.v). The eight groups could be named as combination (0, 0) combination (0, 80), combination (50, 0), combination (50, 80), combination (55, 0), combination (55, 40), combination (60, 0), combination (60, 20), separately, according to its own dose combination condition. The rats of control groups 1 (combination [0, 0]) and 2 (combination [0, 80]) only received 0.9% NaCl and 80 mg/kg LHI, respectively. LHI is administered via combining with heparin for the treatment of some thrombotic disorders because heparin catalyzes inhibitory activity of serpin relative to serine proteinases.
LHI is from Shandong Weifang Pharmaceutical Factory CO., Ltd. Preclinical studies of LHI preparation were performed via the rat's model of induced arteriovenous shunt thrombosis. The animals received an intra-peritoneal injection of Nembutal in a dose of 60 mg/kg (1 ml/200 g body weight). A skin flap was prepared on the neck and cranial part of the ventral chest area, the left carotid artery, left and right jugular vein was separated (1.0-1.5 cm). A cannula for repeated administration of substances was inserted into the left jugular vein. Atropine sulfate in a dose of 5 mg/kg was used to prevent a protective response of the anticoagulant system. The arteriovenous shunt thrombosis was induced as described elsewhere.[16] The only different content was polyethylene tubes only filled with saline solution. The anticoagulant system in rat blood was activated with silk thread 15 min after administration of antithrombotic substances.
Antithrombotic activity was scored by thrombus weight,[16] inhibition rate of thrombosis and protein concentration (Lowry method),[17] in thrombus homogenate (in 0.2 ml normal sodium). Protein concentration was evaluated by the calibration cure constructed using fibrinogen (Sigma) in concentrations of 10-400 μg/ml. Inhibition rate of thrombosis could be calculated as follows:

Anti-thrombin activity of blood plasma was determined by the ability of heparins to activate LHI and inhibit amidolytic activity of thrombin.[18] Anti-thrombin (250 μl, Sigma; 1 U/ml 0.05 M Tris-HCl buffer with 0.0075 M Na2-EDTA and 0.175 M NaCl, pH 7.4) was incubated with platelet-depleted plasma (100 μl) at 37°C for 3 min, then bovine thrombin (Sigma, 2 NIH U/ml Tris-EDTA buffer) was added. Thrombin-specific chromogenic substrate (Sigma, 200 μl solution, 2 mM Tris-EDTA buffer) was added after 30 s. Optical density of the solution was recorded on an Shimadzu spectrophotometer at 405 nm over 1 min. The calibration curve for plasma anti-thrombin activity was constructed using international standard unfractionated heparin (World Health Organization) obtained from the National Institute for Biological Standards and Control (United Kingdom).
All the data were analyzed by using the SPSS 18.0. Results were expressed as the mean ± SEM (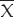 ± s). Data were analyzed by one-way ANOVA, followed by the Student's two-tailed t-test for comparison between two groups. P < 0.05 was considered to be significant.
± s). Data were analyzed by one-way ANOVA, followed by the Student's two-tailed t-test for comparison between two groups. P < 0.05 was considered to be significant.
RESULTS
Table 1 showed antithrombotic activity of LHI and heparin. Combination (50, 80) group got the result of 100% antithrombotic activity. The antithrombotic effect was most pronounced after injection of 50 U/kg heparin and 80 mg/kg LHI. Antithrombotic effect of 55 and 60 U/kg heparin alone was 37-58%. Therefore, group of combination (50, 80) was optimal for 100% antithrombotic activity.
Table 1.
Effect of combined injection treatment with LHI and heparin on the course of experimental arteriovenous shunt thrombosis in rats 15 min after administration of anticoagulants (Mean +/-SD)
Optimal dosages of LHI and heparin were effective over 60 min [Table 2]. Anti-thrombin activity of the plasma remained high even 90 min after treatment. We conclude that optimal dosages of Chinese drug products LHI and heparin completely prevent thrombus growth in rats with experimental arteriovenous shunt thrombosis.
Table 2.
Duration of antithrombotic effect after combined injection treatment with 80 mg/kg LHI and 50 U/kg heparin (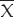 ± s)
± s)
DISCUSSION
From this animal clinical study, it indicated that there was a kind of synergy effect in the combination treatment of heparin and LHI for thrombosis disorders in this induced model of arteriovenous shunt thrombosis. It would be very hard to do the combination therapy research in real clinical. So, it is an important way to select optimized combination proposal of heparin and LHI for cardiovascular diseases. The study was focused on their natural synergy effects on antithrombotic activity instead of animal models itself. Furthermore, the optimized doses could be calculated to human doses via the dose conversion rate between rats and human.
About limitations of our study, there was still a lot of work need to be performed on other animal thrombotic models. As the study was only based on arteriovenous shunt thrombosis model, the data from many other models such as carotid artery thrombosis model, blood clot dissolution experiments or even platelet aggregation experiment in vitro were also very important and kind of crucial. Otherwise, the doses could be further subdivided. It also would be guidance for the further study in clinical.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Sheu JR, Kan YC, Hung WC, Lin CH, Yen MH. The antiplatelet activity of tetramethylpyrazine is mediated through activation of NO synthase. Life Sci. 2000;67:937–47. doi: 10.1016/s0024-3205(00)00686-x. [DOI] [PubMed] [Google Scholar]
- 2.Cheng XC, Liu XY, Xu WF, Guo XL, Zhang N, Song YN. Ligustrazine derivatives. Part 3: Design, synthesis and evaluation of novel acylpiperazinyl derivatives as potential cerebrocardiac vascular agents. Bioorg Med Chem. 2009;17:3018–24. doi: 10.1016/j.bmc.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Sheu JR, Kan YC, Hung WC, Ko WC, Yen MH. Mechanisms involved in the antiplatelet activity of tetramethylpyrazine in human platelets. Thromb Res. 1997;88:259–70. doi: 10.1016/s0049-3848(97)00253-3. [DOI] [PubMed] [Google Scholar]
- 4.Jiang F, Qian J, Chen S, Zhang W, Liu C. Ligustrazine improves atherosclerosis in rat via attenuation of oxidative stress. Pharm Biol. 2011;49:856–63. doi: 10.3109/13880209.2010.551776. [DOI] [PubMed] [Google Scholar]
- 5.Li WM, Liu HT, Li XY, Wu JY, Xu G, Teng YZ, et al. The effect of tetramethylpyrazine on hydrogen peroxide-induced oxidative damage in human umbilical vein endothelial cells. Basic Clin Pharmacol Toxicol. 2010;106:45–52. doi: 10.1111/j.1742-7843.2009.00470.x. [DOI] [PubMed] [Google Scholar]
- 6.Sheu JR, Hsiao G, Lee YM, Yen MH. Antithrombotic effects of tetramethylpyrazine in in vivo experiments. Int J Hematol. 2001;73:393–8. doi: 10.1007/BF02981969. [DOI] [PubMed] [Google Scholar]
- 7.Guo XL, Huang XZ, Shi D. Cardiac hemodynamics after suprarenal ligation of the inferior vena cava and the resection of the right kidney or injection of ligustrazine in rats. Ai Zheng. 2004;23:481–6. [PubMed] [Google Scholar]
- 8.Ma M, Zhang GJ, Ma Y, Li DH, Zhai HN. Effect of salviae miltiorrhizae and ligustrazine hydrochloride injection on axonal regeneration and nerve growth factor expression in a rat model of sciatic nerve injury. Neural Regen Res. 2009;4:1002–6. [Google Scholar]
- 9.Mu YM, Dai Y, Zhang B. Protective effect of tetramethylpyrazine on acute lung injury induced by LPS in mice (in Chinese) China Med. 2004;2:43–5. [Google Scholar]
- 10.Cai YL, Ren MS, Yang RM, Wang GQ. Observation on Curative Effect of Acute Ischemic Cerebrovascular Disease Treated with Different Dosage of Ligustrazine (in Chinese) Chin J Integr Med. 2000;10:747–9. [PubMed] [Google Scholar]
- 11.Yu SZ, Li CY. The effects of different doses of tetramethylpyrazine on platelet aggregation of cerebral thrombosis patients (in Chinese) J Apoplexy Nerv Dis. 1986;3:148–9. [Google Scholar]
- 12.Tibbitts J, Cavagnaro JA, Haller CA, Marafino B, Andrews PA, Sullivan JT. Practical approaches to dose selection for first-in-human clinical trials with novel biopharmaceuticals. Regul Toxicol Pharmacol. 2010;58:243–51. doi: 10.1016/j.yrtph.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Sargent EV, Faria E, Pfister T, Sussman RG. Guidance on the establishment of acceptable daily exposure limits (ADE) to support risk-based manufacture of pharmaceutical products. Regul Toxicol Pharmacol. 2013;65:242–50. doi: 10.1016/j.yrtph.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Zhang DH, Tian ZF, Zhao SZ, Jin XS, Cheng SJ. Clinical observation of prophylaxis of venous thrombosis after the great saphenous vein operation with low molecular heparin and ligustrazine injection. Med Res Educ. 2011;2:43–5. [Google Scholar]
- 15.Chen Z, Li XP. Impaction on blood rheology of chronic pulmonary heart disease in acute phase after treated by tetramethylpyrazine and heparin (in Chinese) China J Chin Med. 2010;2:276–8. [Google Scholar]
- 16.Kou J, Tian Y, Tang Y, Yan J, Yu B. Antithrombotic activities of aqueous extract from Radix Ophiopogon japonicus and its two constituents. Biol Pharm Bull. 2006;29:1267–70. doi: 10.1248/bpb.29.1267. [DOI] [PubMed] [Google Scholar]
- 17.Yang H, Wang Y, Xiao Y, Wang Y, Wu J, Liu C, et al. A bi-functional anti-thrombosis protein containing both direct-acting fibrin(ogen)olytic and plasminogen-activating activities. PLoS One. 2011;6:e17519. doi: 10.1371/journal.pone.0017519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pletcher CH, Cunningham M, Nelsestuen GL. Kinetic analysis of various heparin fractions and heparin substitutes in the thrombin inhibition reaction. Biochim Biophys Acta. 1985;838:106–13. doi: 10.1016/0304-4165(85)90256-9. [DOI] [PubMed] [Google Scholar]


