Abstract
This report describes a 66-year-old male who had a long history of hepatitis B virus (HBV) infection. He was found hepatocellular carcinoma (HCC) 5 months after lamivudine resistance mutation and then received a successful hepatectomy. Three years later, hepatitis B envelope antigen seroconversion was achieved and nucleoside analogs were discontinued. After the withdrawn of antiviral treatment, HBV reactivated and acute-on-chronic liver failure (ACLF) occurred. Anti-HBV treatment improved the patient clinical condition. Three months after the remission of ACLF, the patient was diagnosed as HCC recurrence and received another hepatectomy. This case illustrates indefinite duration antiviral therapy and tight viral control should be performed in patients with HBV-related HCC.
Keywords: Carcinoma, hepatitis B virus, hepatocellular, recurrence, therapy
INTRODUCTION
China has the greatest economic and social burdens of hepatitis B and hepatocellular carcinoma (HCC) in the world. An estimated 1/3 of global individuals infected with the hepatitis B virus (HBV) reside in China, with 130 million carriers, 30 million chronically infected and 300 thousand HBV-related deaths each year.[1] HBV-related HCC is a leading cause of cancer death in China.[1] A high serum HBV deoxyribonucleic acid (DNA) level has been found to be a key risk factor for HCC development in HBV carriers.[2] Therefore, the effective antiviral therapy suppressing HBV virus load could reduce HCC risk and also the risk of HCC recurrence after hepatic resection for HBV-related HCC.[3] However, in patients with HCC complicating chronic hepatitis B, the evidence is still insufficient whether antiviral treatment is protective against new HCC development (level III).[4] For patients undergoing surgical treatment for HBV-related HCC, a number of important questions remain undefined, including for when antiviral therapy should be initiated and what are the endpoints of therapy. In this report, we described the unusual case of a patient who suffered post-operative HBV reactivation closely followed by HCC recurrence due to the withdrawal of nucleoside analogs after sustained viral clearance.
CASE REPORT
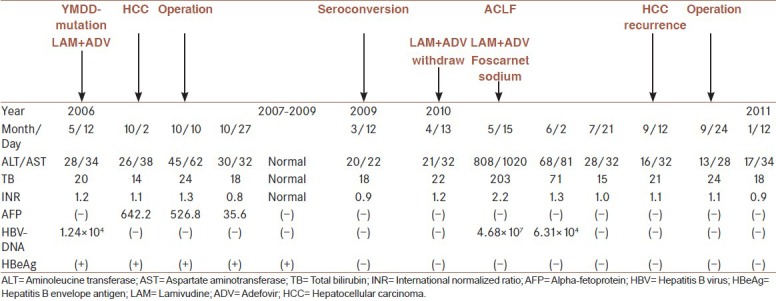
A 66-year-old male patient was admitted to First Affiliated Hospital, Zhejiang University School of Medicine, China in January 2006. The patient who had a 15-year history of HBV infection and treated with lamivudine (LAM) (100 mg once daily, take orally, GlaxoSmithKline, UK), was found LAM-resistant mutation in the YMDD motif of the polymerase gene (rtM204I) in 2006; thus an additional adefovir (ADV) (10 mg once daily, take orally, GlaxoSmithKline, UK) was given. Five months later, the patient was admitted to our hospital for a high alpha-fetoprotein (AFP) level (642.2 ng/ml). HCC was highly suspected by the computed tomography (CT) [Figure 1]. Therefore, a local tumor resection was performed and the pathology confirmed the mass nature of HCC with high-moderate differentiation [Figure 2]. Serum AFP level decreased sharply after the operation and viral load was stable with persistent undetectable HBV DNA. During the following long-term follow-up, the patient was in a satisfactory status [Table 1].
Figure 1.
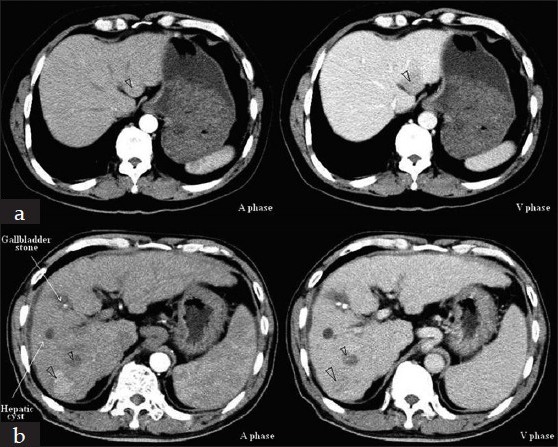
Computed tomography (CT) scans of the primary and recurrent hepatocellular carcinoma. The liver CT scan showed a 2.6 cm × 2.4 cm mass in the segment III in 2006 (a) and two occupancies (3.0 cm × 2.2 cm and 1.5 cm × 1.3 cm) located in the right lobe of cirrhotic liver in 2010 (b)
Figure 2.
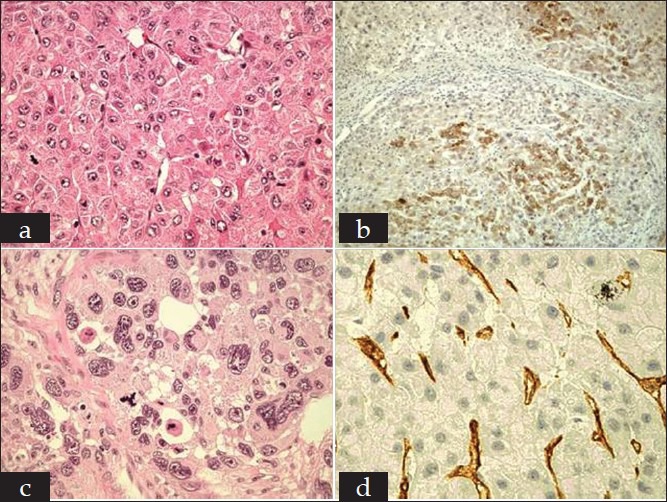
Histopathological findings of the primary and recurrent hepatocellular carcinoma (HCC). In 2006, the patient was found a focal lesion in the segment III of liver with cirrhosis. Histopathological examination of liver tumor showed HCC with high-moderate differentiation (a, H and E, original magnification ×400). Immunohistochemical study showed alpha-fetoprotein (AFP) (+) (b, H and E, original magnification ×100), CD34 (−) and cytokeratin (CK) (−). In 2010, the patient was found two occupancies located in the right lobe of cirrhotic liver. Both tumors were confirmed by pathology as poorly differentiated HCC (c, H and E, original magnification ×400) and the immunohistochemisty showed CD34 (+) (d, H and E, original magnification ×400), AFP (−) and CK (−)
Table 1.
Patient hepatic status and viral loads during eight years follow up

In March 2009, the patient was found hepatitis B envelope antigen (HBeAg) seroconversion. He discontinued antiviral treatment after a 13-month consolidation therapy. One month later, the patient suffered fatigue, nausea, jaundice, abdominal distension and then hepatic encephalopathy. The initial serology showed high serum levels of total bilirubin (TB) (203 umol/L) (normal range: 3.4-17.1 umol/L), aminoleucine transferase (ALT) (808 IU/L) (normal range: 0-40 U/L), aspartate aminotransferase (AST) (1020 U/L) (normal range: 4-40 U/L) and international normalized ratio (INR) (2.2) (normal range: 2.0-2.5). Viral markers showed HBV reactivation (HBsAg 421.91 ng/ml (normal range: 0-0.18 ng/ml), HBeAg 11.7 ng/ml, antibody to hepatitis B core antigen 120.63 ng/ml and HBV DNA 4.68 × 107 copies/ml (normal range: <103 copies/ml). Both the AFP level and radiological assessment of liver were negative for HCC recurrence. The patient was diagnosed as HBV-related acute-on-chronic liver failure (ACLF) and treated with combined nucleoside analogs (LAM [100 mg once daily, take orally, GlaxoSmithKline, UK] and ADV [10 mg once daily, take orally, GlaxoSmithKline, UK]) and foscarnet sodium injection (3 g once daily, Chia-tai Tianqing Pharmaceutical Co., Ltd., China) besides other conservative therapies. Two weeks later, the patient was noted an obviously improved liver function (ALT/AST 68/81 IU/L, TB 71 umol/L, INR 1.3) and a decreased viral load (HBV DNA 6.31 × 104 copies/ml). A gradually resolution of hepatic encephalopathy and ascites was also achieved. After a 2-month conservative treatment, the patient gained a sustained clinical remission of ACLF with normal liver function and undetectable HBV DNA.
In September 2010, the patient was found two occupancies located in the right lobe of cirrhotic liver [Figure 1] with normal serum AFP level. HCC recurrence was highly suspected and curative hepatic segmentectomy was performed. Both tumors were confirmed by pathology as poorly differentiated HCC and the immunohistochemistry showed different results from the initial one's [Figure 2]. In addition, real-time polymerase chain reaction revealed positive results of covalently closed circular DNA (cccDNA) and HBV-x gene levels in the recurrent HCC and primary one, although serum HBV DNA levels were undetectable [Figure 3]. The patient was subsequently discharged after 2 weeks of operation. CT scan performed post-operatively showed no evidence of disease recurrence up to now.
Figure 3.
Polymerase chain reaction (PCR) results of the primary and recurrent hepatocellular carcinoma (HCC). Real-time PCR showed positive expression of covalently closed circular deoxyribonucleic acid (a) and hepatitis B virus-x genes (b) in the primary and recurrent HCC; although, the serum viral load was undetectable
DISCUSSION
The prevention and treatment of hepatitis B and HCC is a long-term program and challenging task. Although several programs such as universal HBV vaccination in infants, HBV guidelines and HCC screening have been initiated by the Chinese government, it is a long way to win the war against HBV infection in China. This case presented nature history of chronic HBV infection and typical progression from HBV infection to cirrhosis and finally HCC. In the management of this patient, several questions had been addressed: What is the optimal duration of antiviral therapy in patients with HBV-related HCC? What is the relationship between HBV reactivation and HCC recurrence after surgery? How important is the serum AFP level as the predictor of recurrence?
Antiviral therapy for HBV-related HCC
The development of nucleoside analogs has made great strides in the treatment of HBV infections. It is well-known now that treatment with LAM not only delays the disease progression, but also reduces the incidence of HCC. The addition of ADV to ongoing LAM therapy could achieve the excellent virological response and biochemical response in LAM-resistant patients; however, it cannot suppress hepatocarcinogenesis.[5] This case proved again that HCC surveillance is important for patients with LAM-resistant mutation.
For patients with HBV infection, serum HBV DNA levels is not only the key risk factor for development of HCC, but also a strong predictor of post-operative HCC recurrence.[3] Consequently, LAM based antiviral therapy was applied to patients after hepatic resection for HBV-related HCC recently and it was found improving liver function, decreasing the risk of liver failure and HCC recurrence.[3] In this case, nucleoside analogs treatment showed potent effect in stabilizing inflammatory activity and inhibiting HBV DNA replication even during the perioperative period, when the patient had marked decreased immunologic function. Interferon was not applied in this case and other cirrhotic patients with HBV-related HCC in our center because it may lead to hepatitis flare. The well control of viral load before and after operation led to a satisfactory tumor-free survival.
Until date, current guidelines have not differentiated the management of patients with HBV-related HCC differently from those without HCC. The optimal antiviral treatment protocol for patients with HCC complicating chronic hepatitis B, especially in those who underwent curative resection is still unknown. A recent meta-analysis reviewed 9 cohort studies including 551 patients showed that antiviral therapy could significantly reduce the risk of tumor recurrence after curative treatment of HBV-related HCC.[6] There is an increasing consensus in support of antiviral therapy after curative treatment of HCC. However, an important question that addressed by this case is to be defined, how long is the antiviral treatment duration.
Until date, only a few publications have mentioned the duration of antiviral treatment after surgery.[7,8] Study from Kuzuya et al. showed a mean treatment period of 22.7 ± 14.2 months during a 38.0 ± 21.6 months follow-up among 16 patients who received LAM therapy after treatment for HCC. They found 2/4 patients achieved seroconversion while 5/16 exhibited the emergence of YMDD mutants.[7] However, they did not show the reason why discontinued of antiviral therapy and the consequence. They did not analyze the relationship between the withdrawal of treatment and tumor recurrence either.
HBeAg seroconversion is a critical milestone in the clinical course of chronic hepatitis B and an allowance for the withdrawal of antiviral treatment with sustained low viral load. A suggestion from Asian-Pacific guidelines showed that the nucleoside analogs treatment can be discontinued if HBeAg seroconversion with undetectable HBV DNA on 2 separate occasions at least 6 months apart in HBeAg-positive patients.[9] However, this case demonstrated that even 13 months of consolidation therapy was not enough for patients who underwent surgical resection for HBV-related HCC. High levels of HBV cccDNA which serves as an indicator of HBV replication in the tumor tissues were found; although, the serum viral load was well-controlled. As we all know, HBV is associated with liver carcinogenesis through integration of the HBV genome into the host chromosome and expression of trans-activating factors. Because HBV-induced inflammatory damage and gene mutations have been proved to be associated to HCC, The thresholds of viremia to initiate therapy and long-term antiviral therapy applied in patients with HBV-related HCC should be lower than those without HCC. After HCC surgery, indefinite duration antiviral treatment is essential to tight control the viral load and to suppress the hepatocarcinogenesis, regardless of HBeAg seroconversion.
Intensive researches are imperative to deeply understand the management of patients with HBV-related HCC; although, people start to realize the management of HBV is as important as treating HCC in the past decade.
HBV reactivation-induced ACLF after curative resection
So far limited data have focused on the HBV reactivation-induced ACLF after curative resection for HCC. According to the data from Singapore, the incidences of post-operative ACLF were 8.5% and most of ACLF developed in the early post-operative period due to the declined immune function.[10] Different form the reported cases, this case suffered ACLF in the long-term post-operative follow-up and due to the HBV reactivation caused by the withdrawal of antiviral treatment. Sharply accumulation of endotoxins in circulation leaded to the severe impairment of organs including brain, liver, kidney and gastrointestinal tract. To rapidly reduce the viral load and improve patient condition, the patient was given combined nucleoside analogs and foscarnet sodium injection. Foscarnet is a viral DNA polymerase inhibitor and has been found valid in quickly reducing HBV DNA levels in chronic HBV infected patients.[11] Foscarnet sodium injection is of great important for ACLF patients with vomit or variceal bleeding or hepatic coma or other conditions with invalid gut absorption. Moreover, it is cheap and can be widely accepted by most of patients in mainland China. Further large-sample and well-designed studies are needed to evaluate the efficacy of foscarnet on patients with HBV reactivation induced ACLF.
HBV reactivation-associated late HCC recurrence after curative resection
The association between HBV reactivation and HCC recurrence after HCC surgery is remain unclear. In general, there are two types of HCC recurrence after curative resection. Early HCC recurrence were found associated with tumor factors and surgical strategy and may due to intra-hepatic metastasis.[12] While late HCC recurrence were related to high viral loads and hepatic inflammatory activity.[12] Our results showed different pathologies between the recurrent HCC and the initial one, which indicated that the late HCC recurrence possibly due to de novo hepatocarcinogenesis of non-cancerous liver tissues rather than intra-hepatic metastasis. This case was also consisted with the previous studies that post-operative viral load were highly associated with late recurrence of HCC.[13] Moreover, the time interval from the occurrence of HBV reactivation-induced ACLF to HCC recurrence was so short and we found high levels of cccDNA and HBV-x gene in the tumor tissues. Up to now, in the absence of a dominant oncogene encoded by the HBV genome, only indirect roles of HBV on hepatocarcinogenesis have been proposed, including HBV DNA integration into the host genome, induction of genetic instability by viral integration or by the regulatory protein HBx.[14] Our findings suggested that reactivation of HBV replication seemed to play a direct role in the late recurrence of HCC after surgical resection.
AFP and surveillance of tumor recurrence
AFP has long been used as a standard serum tumor marker for the screening and diagnosis of HCC since the 1970s. It is also widely used for the evaluation of treatment response and detection of recurrence after curative resection in AFP-producing HCC. However, some patients with high AFP level at initial HCC diagnosis presented normal AFP levels at HCC recurrence, which was consistent with our findings.[15] The monitor of serum AFP level is not deemed sufficient for surveillance of tumor recurrence in such patients. Besides the measurement of AFP levels and other so called tumor markers, the imaging tests should be performed regularly and frequently to monitor the tumor recurrence directly.
In conclusion, our findings suggest that HBV reactivation after HCC surgery may induce hepatitis flare and hepatocarcinogenesis, thus indefinite duration antiviral therapy and tight viral control should be performed in patients with chronic hepatitis B and HBV-related HCC.
ACKNOWLEDGEMENTS
This work was supported by the Program of Zhejiang Medical and Health Platform (2011ZDA007), the project of Zhejiang medical science and technology plan (2013RCA013) and Zhejiang Provincial Program for the Cultivation of High-level Innovative Health talents.
Footnotes
Source of Support: This work was supported by the Program of Zhejiang Medical and Health Platform (2011ZDA007), the project of Zhejiang medical science and technology plan (2013RCA013) and Zhejiang Provincial Program for the Cultivation of High-level Innovative Health talents
Conflict of Interest: None declared.
REFERENCES
- 1.Liu J, Fan D. Hepatitis B in China. Lancet. 2007;369:1582–3. doi: 10.1016/S0140-6736(07)60723-5. [DOI] [PubMed] [Google Scholar]
- 2.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 3.Chuma M, Hige S, Kamiyama T, Meguro T, Nagasaka A, Nakanishi K, et al. The influence of hepatitis B DNA level and antiviral therapy on recurrence after initial curative treatment in patients with hepatocellular carcinoma. J Gastroenterol. 2009;44:991–9. doi: 10.1007/s00535-009-0093-z. [DOI] [PubMed] [Google Scholar]
- 4.Asia-Pacific Working Party on Prevention of Hepatocellular Carcinoma. Prevention of hepatocellular carcinoma in the Asia-Pacific region: Consensus statements. J Gastroenterol Hepatol. 2010;25:657–63. doi: 10.1111/j.1440-1746.2009.06167.x. [DOI] [PubMed] [Google Scholar]
- 5.Elefsiniotis I, Buti M, Jardi R, Vezali E, Esteban R. Clinical outcome of lamivudine-resistant chronic hepatitis B patients with compensated cirrhosis under adefovir salvage treatment. Importance of HCC surveillance. Eur J Intern Med. 2009;20:478–81. doi: 10.1016/j.ejim.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Wong JS, Wong GL, Tsoi KK, Wong VW, Cheung SY, Chong CN, et al. Meta-analysis: The efficacy of anti-viral therapy in prevention of recurrence after curative treatment of chronic hepatitis B-related hepatocellular carcinoma. Aliment Pharmacol Ther. 2011;33:1104–12. doi: 10.1111/j.1365-2036.2011.04634.x. [DOI] [PubMed] [Google Scholar]
- 7.Kuzuya T, Katano Y, Kumada T, Toyoda H, Nakano I, Hirooka Y, et al. Efficacy of antiviral therapy with lamivudine after initial treatment for hepatitis B virus-related hepatocellular carcinoma. J Gastroenterol Hepatol. 2007;22:1929–35. doi: 10.1111/j.1440-1746.2006.04707.x. [DOI] [PubMed] [Google Scholar]
- 8.Koda M, Nagahara T, Matono T, Sugihara T, Mandai M, Ueki M, et al. Nucleotide analogs for patients with HBV-related hepatocellular carcinoma increase the survival rate through improved liver function. Intern Med. 2009;48:11–7. doi: 10.2169/internalmedicine.48.1534. [DOI] [PubMed] [Google Scholar]
- 9.Liaw YF, Leung N, Kao JH, Piratvisuth T, Gane E, Han KH, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: A 2008 update. Hepatol Int. 2008;2:263–83. doi: 10.1007/s12072-008-9080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thia TJ, Lui HF, Ooi LL, Chung YF, Chow PK, Cheow PC, et al. A study into the risk of exacerbation of chronic hepatitis B after liver resection for hepatocellular carcinoma. J Gastrointest Surg. 2007;11:612–8. doi: 10.1007/s11605-007-0121-3. [DOI] [PubMed] [Google Scholar]
- 11.Han YX, Xue R, Zhao W, Zhou ZX, Li JN, Chen HS, et al. Antiviral therapeutic efficacy of foscarnet in hepatitis B virus infection. Antiviral Res. 2005;68:147–53. doi: 10.1016/j.antiviral.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Wu JC, Huang YH, Chau GY, Su CW, Lai CR, Lee PC, et al. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J Hepatol. 2009;51:890–7. doi: 10.1016/j.jhep.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Qu LS, Jin F, Huang XW, Shen XZ. High hepatitis B viral load predicts recurrence of small hepatocellular carcinoma after curative resection. J Gastrointest Surg. 2010;14:1111–20. doi: 10.1007/s11605-010-1211-1. [DOI] [PubMed] [Google Scholar]
- 14.De Mitri MS, Cassini R, Bernardi M. Hepatitis B virus-related hepatocarcinogenesis: Molecular oncogenic potential of clear or occult infections. Eur J Cancer. 2010;46:2178–86. doi: 10.1016/j.ejca.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 15.Hsieh CB, Chen TW, Chu CM, Chu HC, Yu CP, Chung KP. Is inconsistency of alpha-fetoprotein level a good prognosticator for hepatocellular carcinoma recurrence? World J Gastroenterol. 2010;16:3049–55. doi: 10.3748/wjg.v16.i24.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]


