Abstract
Background:
We analyzed factors associated with worsened paresis in a large series of patients with brain lesions located within or near the primary motor area (M1) to establish protocols for safe, awake craniotomy of eloquent lesions.
Methods:
We studied patients with brain lesions involving M1, the premotor area (PMA) and the primary sensory area (S1), who underwent awake craniotomy (n = 102). In addition to evaluating paresis before, during, and one month after surgery, the following parameters were analyzed: Intraoperative complications; success or failure of awake surgery; tumor type (A or B), tumor location, tumor histology, tumor size, and completeness of resection.
Results:
Worsened paresis at one month of follow-up was significantly associated with failure of awake surgery, intraoperative complications and worsened paresis immediately after surgery, which in turn was significantly associated with intraoperative worsening of paresis. Intraoperative worsening of paresis was significantly related to preoperative paresis, type A tumor (motor tract running in close proximity to and compressed by the tumor), tumor location within or including M1 and partial removal (PR) of the tumor.
Conclusions:
Successful awake surgery and prevention of deterioration of paresis immediately after surgery without intraoperative complications may help prevent worsening of paresis at one month. Factors associated with intraoperative worsening of paresis were preoperative motor deficit, type A and tumor location in M1, possibly leading to PR of the tumor.
Keywords: Awake surgery, brain tumor, complication, failure, neurological deficit, primary motor area
INTRODUCTION
Awake craniotomy has been reported to preserve neurological function in eloquent areas with maximal removal of brain tumors compared with surgery under general anesthesia.[6,14,18,19,23,32] One of the reasons for the reduced incidence of neurological deficit using awake surgery is the detection of safe margins for surgical resection of tumors by brain mapping, but identification of eloquent areas increases the risk of postoperative deficit. This presumably occurs because positive mapping indicates close proximity of functional areas to the brain tumor, resulting in deterioration of neurological outcome.[14] Thus, for the preservation of neurological function, brain mapping as well as continuous monitoring of neurological function during awake surgery is important.[26] In addition, preoperative functional magnetic resonance imaging (fMRI) and diffuse tensor imaging (DTI) can suggest safe approaches to brain tumors in eloquent areas.[26,31]
Tumors in eloquent areas include mainly language- and motor-related lesions. Many investigators have reported the removal of brain tumors in language-related areas by awake surgery, but relatively few reports have analyzed awake surgery for brain tumors located in motor-related areas.[3,12,13,20,30] The present study performed preoperative fMRI and DTI with continuous monitoring of motor function during awake surgery for brain tumors in motor-related areas, namely the primary motor area (M1), premotor area (PMA), and primary sensory area (S1). Our goal was to analyze factors associated with the deterioration of motor function in a large series of patients to establish safe, awake craniotomy for these lesions.
MATERIALS AND METHODS
Patients
A total of 102 patients with brain lesions within or near M1, who underwent awake surgery between 2003 and 2013 in Komagome Metropolitan Hospital, were analyzed in the present study. Informed consent to perform awake surgery was obtained from all patients. Lesions were in regions including M1, PMA, S1, and combinations of these areas. The patients included 55 men and 47 women, with a median age of 61 years (range, 34-80 years). Forty-nine patients had brain lesions on the left side, whereas the remaining 53 patients had brain lesions on the right side. The histology of brain lesions was astrocytoma grade IV in 17 patients, astrocytoma grade III in 6, astrocytoma grade II in 3, metastasis in 57, meningioma in 16, primary central nervous system lymphoma in 1, cavernous angioma in 1, and hematoma in 1. Tumor location was M1 in 24 tumors, PMA in 25, S1 in 17, M1 and PMA in 17, M1 and S1 in 11, PMA and S1 in 1, and M1, PMA and S1 in 7.
Although many surgical teams use awake cortical mapping for low grade gliomas, epileptic heterotopia, or nonlesional cases where the lesion borders are very indistinct, it is important to note that there were few of these types of cases in this series.
Preoperative evaluation of location of M1 by fMRI
All imaging studies were performed using a 1.5-T Signa Horizon Lx imager (General Electric, Tokyo, Japan). The fMRI and image analysis were performed as described previously.[25] Briefly, the fMRI acquisition sequence consisted of a gradient echo-Echo Planar Imaging (EPI) (echo time (TE), 82.5 ms; repetition time (TR), 3000 ms; matrix, 128 × 128; field of view, 24 × 24 cm; block design, 30-s intervals). The reconstructed voxel size was 1.88 × 1.88 × 2.3 mm3. Slices of 5-mm thickness were obtained with 70 repetitions. The patient was asked to make a fist repetitively (hand clenching), or alternating backward and forward swings of the arm around the shoulder (shoulder swing) during imaging approximately 7 days before surgery. The fMRI acquisition time was approximately 3.5 min. Anatomical images were acquired using a 3D-fast spoiled gradient echo (FSPGR) sequence (TE, 2.4 ms; TR, 26.0 ms; flip angle, 30°; bandwidth, 31.25 kHz; image matrix, 256 × 256; slice thickness, 2.3 mm). After reconstruction, EPI images were aligned to correct for head motion and coregistered with anatomic images. EPI images were smoothed using isotropic Gaussian kernels of 4 mm and statistically analyzed using SPM software (free software written by the Wellcome Department of Imaging Neuroscience at University College London).[11] Activations showing values of P < 0.05 were considered significant.
Quantitative histological examination of specimens from autopsy cases have demonstrated that localization of M1 in both cerebral hemispheres is symmetrical.[33] Thus, using the principles of symmetry, fMRI characterization of M1 on the contralateral normal side can yield valuable information regarding the localization of M1 on the affected side, even in the face of anatomical reorganization due to a brain tumor. To confirm this principle, we compared the results of fMRI with intraoperative brain mapping of M1, and found that this principle of symmetry in fMRI totally agreed with the results of brain mapping.[30] We therefore used this principle of symmetry in fMRI for the localization of M1 instead of brain mapping.
DTI and image analysis of motor tracts
DTI and image analysis were performed as described previously.[25] Briefly, standard imaging gradients were used with a maximum strength of 23 mT/m and a slew rate of 50 mT/m/ms. The DTI acquisition sequence used single-shot, spin-echo echo-planar imaging with the following parameters: TE, 127.6 ms; acquisition matrix, 128 × 128; and field of view, 24 × 24 cm.[22] Contiguous 5-mm-thick slices were acquired, covering the entire brain, with a b value of 1000 mm2/s in 30 noncollinear directions. The reconstructed voxel size was 1.88 × 1.88 × 4.00 mm3. DTI acquisition time for a total of 61 images was approximately 10 min. Diffusion tensor eigenvalues (λ1, λ2, λ3) and eigenvectors (ε1, ε2, ε3) were calculated from DTI data, and fractional anisotropy (FA) maps[2] were generated according to the Tensorlines (TL) algorithm, as a combination of the Tensor Deflection (TEND) algorithm at low FA[16] and streamlines tracking (STT) at high FA.[7] These data were obtained with DTI Analyzer software (IDL version 5.6; Research Systems, Boulder, CO), and a termination criterion (threshold of 0.1) was used for the analysis.
To visualize individual motor tracts by DTI, the areas activated by corresponding motor functions were used as seed points in fMRI. Motor tracts were constructed using the following three ROIs: The activated area within or near M1, the posterior limb of the internal capsule, and the cerebral peduncle. The relationship between motor tracts and brain tumors was categorized as either type A [Figure 1a], indicating that motor tracts were in close proximity to and compressed by the tumor or type B, indicating that motor tracts were distant to the tumor [Figure 1b].
Figure 1.
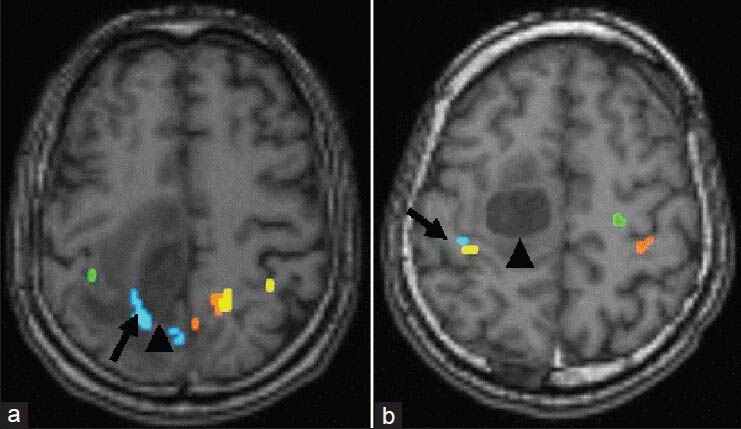
(a) A 61-year-old male with metastatic brain tumor (arrow) located in the right M1 and PMA. Axial DTI images were constructed during left-hand clenching (green), left-foot flexion (blue), right-hand clenching (yellow) and right-foot flexion (orange) during fMRI. Motor tracts were constructed using the following three ROIs: Activated area in the M1 on fMRI, posterior limb of the internal capsule, and the cerebral peduncle. Arrows indicate the motor tract of the foot running in close proximity to the brain tumor (arrowhead), identifying this case as type a. (b) A 34-year-old male with metastatic brain tumor located in the right M1 and PMA. Axial DTI images were constructed by left-hand clenching (blue), elbow flexion (yellow), right-hand clenching (red), and elbow flexion (green) as described previously. Motor tracts of the left hand and elbow (arrow) run distant to the brain tumor (arrowhead), identifying this case as type b
Awake tumor resection
Both mapping and awake tumor resection were performed as previously described.[26] Briefly, patients were positioned supine or in a half-sitting position and given rigid head fixation (Sugita headrest; Mizuho Medical, Tokyo, Japan) after administration of a local anesthetic agent (1% xylocaine with epinephrine and 0.75% anapain) at the pin sites and regional field block sites. Under general anesthesia with propofol, dexmedetomidine, or remifentanil, a laryngeal mask was placed with or without intubation, the skin was infiltrated with the same local anesthetic agent and incised, and neuronavigated craniotomy and incision of the dura was performed.
After removal of the laryngeal airway or suspension of anesthetic agent, oxygen was administered via the laryngeal mask, and cortical mapping was performed by stimulating the cortex with a modified Ojemann stimulator.[4] To avoid inducing intraoperative seizure, a low stimulus intensity was used (3-5 mA, 60-Hz biphasic square-wave pulse of 1 ms/phase for 4 s duration). The tumor was removed in the usual fashion. Adequacy of motor function such as tongue swinging, eye closing, hand clenching, elbow flexion, knee flexion, and foot flexion were continuously assessed during tumor removal.[26] In the sensory-related area, sensory functions such as tactile sensation and deep sensation were continuously evaluated. Tumor removal was assisted by a neuronavigation system (Stealth, Medtronic Sofamor Danek, Osaka, Japan). We started the resection away from eloquent cortex, moving progressively closer. If neurological deficit occurred at any point during the resection, the operation was interrupted, and neurological function was assessed over the next 5 min. If the neurological deficit did not recover, the operation was terminated. Following completion of tumor resection, intravenous anesthesia was administered using propofol. After closure of the dura, the bone flap was replaced, and the skin was closed in the usual manner.
When awake surgery could not be continued due to complications such as epilepsy or severe somnolence, which indicated failure of awake surgery, surgery was performed under general anesthesia with intubation through the laryngeal mask. The tumor was removed by repeated internal decompression and dissection of the tumor margins. Following completion of tumor resection, the dura was closed, the bone flap replaced, and the skin closed in the usual manner. The degree of resection of brain tumors was categorized as either gross total removal (GTR) or partial removal (PR).
Evaluation of paresis
Paresis was evaluated intraoperatively, immediately after surgery and one month after surgery, and the results were compared with preoperative motor deficit. Deterioration of paresis during surgery included both transient and permanent deterioration.
Statistical analysis
Chi-square and univariate logistic regression analysis were used to evaluate clinical and intraoperative parameters related to worsened paresis at one month after surgery. These parameters consisted of the following: Outcome of awake surgery (success or failure); intraoperative deterioration of paresis; intraoperative complications; immediate worsened postoperative paresis; preoperative motor deficit; extent of resection (GTR or PR); and tumor location, histology and size. Multivariate logistic regression analysis was performed to evaluate significant independent factors of worsened paresis at one month after surgery to develop a predictive model regarding tumor histology and location. Odds ratios with 95% confidence intervals were computed. A value of P < 0.05 was considered significant. Statistical analyses were performed using JMP 8.0 statistical software (SAS Institute, Tokyo, Japan).
RESULTS
Worsened paresis one month after surgery
Worsened paresis was defined as deteriorated motor deficit immediately and one month after awake surgery compared with the baseline preoperative status. For worsened paresis during surgery, both unresolved and transient deterioration of motor function compared with preoperative status were included.
Of the 102 cases of awake surgery, worsened paresis at one month of follow-up occurred in eight cases. Whereas 4 of 96 cases (4%) of successful awake surgery showed worsened paresis at one month, 4 of 6 cases (67%) of failed awake surgery showed worsened paresis at one month. Worsened paresis after one month was significantly associated with failure of awake surgery (χ2, P < 0.0001; Table 1). Causes of failure in the six failed cases were as follows: Air embolism in two cases, epilepsy in one, motor neglect in one, severe somnolence in one, and no wakeup in one.
Table 1.
Neurological outcome based on clinical parameters
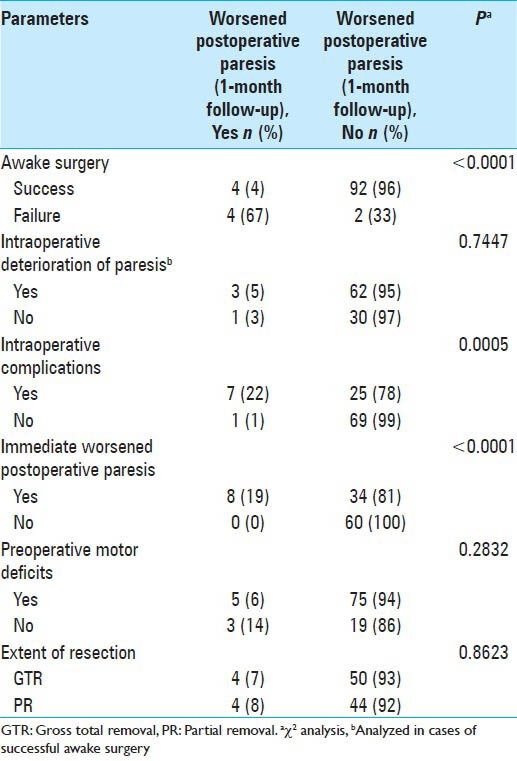
Seven of 32 cases (22%) with intraoperative complications showed worsened paresis at one month of follow-up, although 1 of 70 cases (1%) without intraoperative complications showed worsened paresis at one month Worsened paresis after one month was significantly associated with intraoperative complications (χ2, P = 0.0005; Table 1).
Eight of 42 cases (19%) with worsened paresis immediately after surgery showed worsened paresis at one month; however, no cases without worsened paresis immediately after surgery showed worsened paresis at one month. Worsened neurological deficit after one month was significantly associated with worsened paresis immediately after surgery (χ2, P < 0.0001; Table 1).
Intraoperative deterioration of paresis, preoperative neurological deficit, and extent of resection were not significantly associated with worsened paresis at one month after surgery [Table 1].
Worsened neurological deficit immediately after surgery
Of the 102 cases of awake surgery, worsened paresis immediately after surgery occurred in 42 cases. Although 33 of 65 cases (51%) with intraoperative deterioration of paresis showed worsened paresis immediately after surgery, 5 of 31 cases (16%) without intraoperative deterioration of paresis showed worsened paresis immediately after surgery. Worsened paresis immediately after surgery was significantly associated with intraoperative deterioration of paresis (χ2, P = 0.0007; Table 2).
Table 2.
Neurological outcomes based on clinical parameters
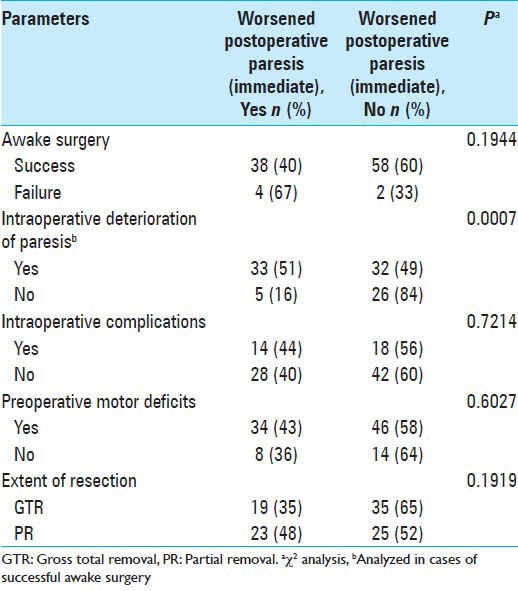
Failure of awake surgery, intraoperative complications, preoperative neurological deficit, and extent of resection were not significantly associated with worsened paresis immediately after surgery [Table 2].
Worsened paresis during surgery
Among the 102 cases of awake surgery, worsened paresis during surgery occurred in 65 cases. Whereas 56 of 76 cases (74%) with preoperative motor deficit showed worsened paresis during surgery, nine of 20 cases (45%) without preoperative motor deficit showed worsened paresis during surgery. Worsened paresis during surgery was significantly associated with preoperative motor deficit (χ2, P = 0.0175; Table 3).
Table 3.
Neurological outcomes based on clinical parameters
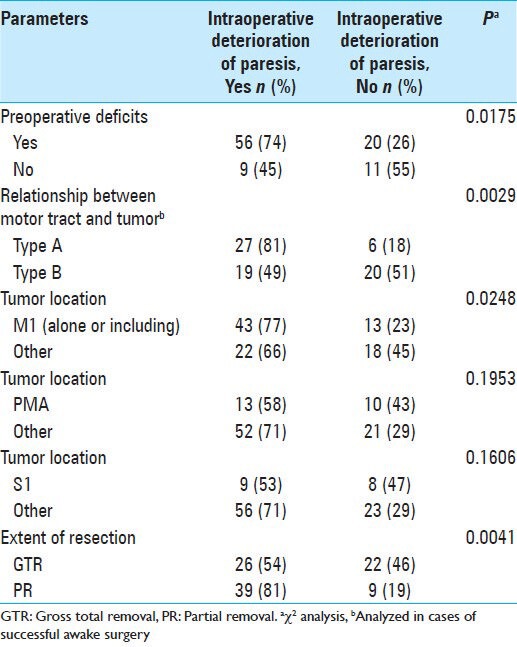
In addition, among 72 cases in which the relationship between motor tract and the tumor type was analyzed, 27 of 33 cases (81%) of type A and 19 of 39 cases (49%) of type B showed worsened paresis during surgery. Worsened paresis during surgery was significantly associated with type A tumor (χ2, P = 0.0029; Table 3).
While 43 of 56 cases (77%) with the tumor located within or including M1 showed worsened paresis during surgery, 22 of 40 cases (66%) with the tumor at other locations showed worsened paresis during surgery. Worsened paresis during surgery was significantly associated with the brain tumor located within or including M1 (χ2, P = 0.0248; Table 3).
Although 26 of 48 cases (54%) of GTR showed worsened paresis during surgery, 39 of 48 cases (81%) of PR displayed worsened paresis during surgery. Worsened paresis during surgery was significantly associated with use of PR (χ2, P = 0.0041; Table 3).
Tumors located in PMA or S1 were not significantly associated with worsened paresis during surgery [Table 3].
Relationship between worsened paresis one month after surgery and tumor histology, location, and size
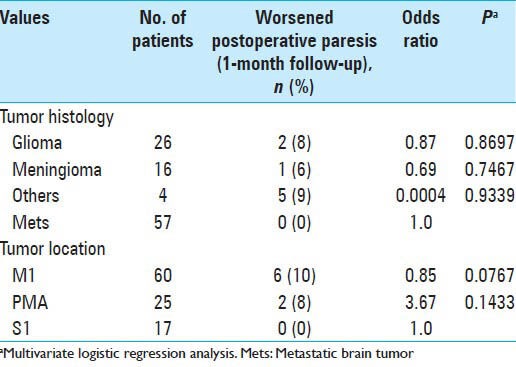
Multivariate logistic regression analysis showed that tumor histology, including glioma, meningioma, and metastatic brain tumor, was not significantly associated with worsened paresis at one month of follow-up. Similarly, tumor locations including M1, PMA, and S1 were not significantly associated with worsened paresis at one month of follow-up [Table 4]. Furthermore, there was no significant association between worsened paresis after one month and tumor size.
Table 4.
Neurological outcomes based on clinical parameters
DISCUSSION
The present study demonstrated that worsened paresis at one month follow-up was significantly related to failure of awake surgery, intraoperative complications, and worsened paresis immediately after surgery [Table 1]. Worsened paresis immediately after surgery was, in turn, significantly associated with worsened paresis during surgery [Table 2]. Worsened paresis during surgery was significantly related to preoperative motor deficit, type A tumor [Figure 1], brain tumor located within or including M1 and PR [Table 3]. Neither tumor histology, location nor size was significantly associated with deterioration of paresis at one month [Table 4]. It is to be noted that awake craniotomy for brain lesions within or near M1 might improve survival of patients, because the survival of patients with astrocytoma Gr IV in this series was 23 months on average, whereas typical survival in these patients is about one year (data not shown).
Failure of awake surgery was significantly associated with deterioration of paresis at one month of follow-up in awake surgery for brain tumors within or near M1. Failure of awake craniotomy has been reported to increase postoperative morbidity, in agreement with our findings.[17] Causes of failure of awake surgery in our study were severe somnolence, epilepsy, air embolism, no wake up, and motor neglect. To prevent the complications of severe somnolence or no wake up, more stringent patient selection, and procedures of the awake surgery protocol would be required.[5] Since anticonvulsive drugs such as phenytoin may cause somnolence, we load the patient with such drugs 2 weeks before surgery and check for drug side effects.[8] As for epilepsy, adequate loading of anticonvulsive drug before surgery would prevent epilepsy during surgery, although epilepsy was not easily controllable during tumor removal when the tumor was located within or including M1 or when the patient experienced epilepsy before surgery according to our experience.[17] Air embolism appears to be closely related to the sitting position.[1,10,15,24] The half-sitting position was needed to prevent deterioration of motor function when the tumor was located near the falx, possibly because the sitting position avoided compression of M1 located between the tumor and falx.[27] In this position, care should be taken regarding signs of air embolism, such as hypoxia, reduction in end-tidal CO2 and persistent cough in patients in a sitting position. When such signs are noted, the position of the patient's head should be lowered and compression of the cervical vein performed to increase venous pressure. With proper positioning of the patient and anesthetic adjustment, most of the complications discussed earlier can be avoided.
As for motor neglect, this finding was noted in a case with brain tumor located between the right M1 and S1, possibly due to the damage to the nerve fibers connecting M1 with S1.[29] In this case, we switched from monitoring of motor function to motor-evoked potentials. In summary, a meticulous protocol and careful procedures for awake craniotomy may help prevent the failure of awake surgery. Regarding intraoperative complications, in addition to the causes of failure described earlier, vomiting sometimes occurred. This can be controlled using antiemetic drugs.
Worsened paresis immediately after surgery was significantly associated with worsened paresis at one month of follow-up. To prevent the deterioration of motor function immediately after surgery, preoperative fMRI and DTI, intraoperative neuronavigation and cortical and subcortical mapping for motor function may be important.[6,9,14,21,25,31,32] Kim et al. reported that negative mapping of eloquent areas resulted in a low incidence of neurological deficit, whereas positive mapping of eloquent areas was associated with increased postoperative deficits due to the close proximity of the tumor to the functional cortex.[14] To improve this problem, continuous clinical evaluation by a professional neurocognitive team during awake surgery is recommended, since awake surgery can be suspended immediately upon deterioration of function, potentially allowing recovery and preservation of function.[17,18,28] A team comprising a neuropsychologist, speech therapist, neuroradiologist, and neurosurgeon thus evaluates patients before, during, and after awake craniotomy in our hospital.
Worsened paresis immediately after surgery, as one of the factors significantly associated with worsened paresis at one month of follow-up, was significantly associated with worsened paresis during surgery. Worsened paresis during surgery was significantly related to preoperative motor deficit, type A tumor, brain tumor located within or including M, and use of PR. In cases with preoperative motor deficit, type A tumor, or brain tumor located within or including M1, important motor-related nerve cells such as the pyramidal cells of Betz in layer V of M1 or the pyramidal tract that runs from M1 may sustain damage or undergo severe compression by the tumor (type A), resulting in preoperative motor deficit. PR was significantly related to intraoperative worsening of motor function, possibly because we stopped removing the tumor when deterioration of motor function during surgery occurred and did not improve within 5 min, resulting in PR.[29] Therefore, in cases with type A tumor, meticulous care should be taken to remove the brain tumor, and the surgery should be stopped immediately upon deterioration of motor function.
In conclusion, successful awake surgery and prevention of deterioration of paresis immediately after surgery without intraoperative complications may help prevent worsened paresis at one month of follow-up. Factors associated with worsening of motor function during awake surgery were preoperative motor deficit, type A tumor and tumor location in M1, possibly leading to PR of the tumor.
ACKNOWLEDGMENTS
This work was supported by the Japanese Foundation for Multidisciplinary Treatment of Cancer.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2013/4/1/149/122003
Contributor Information
Nobusada Shinoura, Email: shinoura@cick.jp.
Akira Midorikawa, Email: green@tamacc.chuo - u.ac.jp.
Ryoji Yamada, Email: r.yamada@cick.jp.
Taijun Hana, Email: wolves_in_witer@yahoo.co.jp.
Akira Saito, Email: akirannms@gmail.com.
Kentaro Hiromitsu, Email: mitsuhiromitsu@gmail.com.
Chisato Itoi, Email: ichigodecember@yahoo.co.jp.
Syoko Saito, Email: a12.m7jf@g.chuo - u.ac.jp.
Kazuo Yagi, Email: yagi@hs.tmu.ac.jp.
REFERENCES
- 1.Balki M, Manninen PH, McGuire GP, El-Beheiry H, Bernstein M. Venous air embolism during awake surgery in a supine patient. Can J Anaesth. 2003;50:835–8. doi: 10.1007/BF03019383. [DOI] [PubMed] [Google Scholar]
- 2.Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–67. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bello L, Gallucci M, Fava M, Carrabba G, Giussani C, Acerbi F, et al. Intraoperative subcortical language tract mapping guides surgical removal of gliomas involving speech areas. Neurosurgery. 2007;60:67–80. doi: 10.1227/01.NEU.0000249206.58601.DE. [DOI] [PubMed] [Google Scholar]
- 4.Berger MS, Kincaid J, Ojemann GA, Lettich E. Brain mapping techniques to maximize resection, safety, and seizure control in children with brain tumors. Neurosurgery. 1989;25:786–92. doi: 10.1097/00006123-198911000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Carrabba G, Venkatraghavan L, Bernstein M. Day surgery awake craniotomy for removing brain tumours: Technical note describing a simple protocol. Minim Invasive Neurosurg. 2008;51:208–10. doi: 10.1055/s-2008-1073132. [DOI] [PubMed] [Google Scholar]
- 6.Chacko AG, Thomas SG, Babu KS, Daniel RT, Chacko G, Prabhu K, et al. Awake craniotomy and electrophysiological mapping for eloquent area tumours. Clin Neurol Neurosurg. 2013;115:329–34. doi: 10.1016/j.clineuro.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 7.Conturo TE, Lori NF, Cull TS, Akbudak E, Snyder AZ, Shimoi JS, et al. Tracking neuronal fiber pathways in the living human brain. Proc Natl Acad Sci U S A. 1999;96:10422–7. doi: 10.1073/pnas.96.18.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Craig S. Phenytoin poisoning. Neurocrit Care. 2005;3:161–70. doi: 10.1385/NCC:3:2:161. [DOI] [PubMed] [Google Scholar]
- 9.De Benedictis A, Moritz-Gasser S, Duffau H. Awake mapping optimizes the extent of resection for low-grade gliomas in eloquent areas. Neurosurgery. 2010;66:1074–84. doi: 10.1227/01.NEU.0000369514.74284.78. [DOI] [PubMed] [Google Scholar]
- 10.Deogaonkar A, Avitsian R, Henderson JM, Schubert A. Venous air embolism during deep brain stimulation surgery in an awake supine patient. Stereotact Funct Neurosurg. 2005;83:32–5. doi: 10.1159/000085024. [DOI] [PubMed] [Google Scholar]
- 11.Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Comparing functional (PET) images: The assessment significant change. J Cereb Blood Flow Metab. 1991;11:690–9. doi: 10.1038/jcbfm.1991.122. [DOI] [PubMed] [Google Scholar]
- 12.Haglund MM, Berger MS, Shamseldin M, Lettich E, Ojemann GA. Coritcal localization of temporal lobe language sites in patients with gliomas. Neurosurgery. 1994;34:567–76. doi: 10.1227/00006123-199404000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Ilmberger J, Ruge M, Kreth FW, Briegel J, Reulen HJ, Tonn JC. Intraoperative mapping of language functions: A longitudinal neurolinguistic analysis. J Neurosurg. 2008;109:583–92. doi: 10.3171/JNS/2008/109/10/0583. [DOI] [PubMed] [Google Scholar]
- 14.Kim SS, McCutcheon IE, Suki D, Weinberg JS, Sawaya R, Lang FF, et al. Awake craniotomy for brain tumors near eloquent cortex: Correlation of intraoperative cortical mapping with neurological outcomes in 309 consecutive patient. Neurosurgery. 2009;64:836–45. doi: 10.1227/01.NEU.0000342405.80881.81. [DOI] [PubMed] [Google Scholar]
- 15.Kumar R, Goyal V, Chauhan RS. Venous air embolism during microelectrode recording in deep brain stimulation surgery in an awake supine patient. Br J Neurosurg. 2009;23:446–8. doi: 10.1080/02688690902775538. [DOI] [PubMed] [Google Scholar]
- 16.Lazar M, Weinstein DM, Tsuruda JS, Hasan KM, Arfanakis K, Meyerand ME, et al. White matter tractography using diffusion tensor deflection. Hum Brain Mapp. 2003;18:306–21. doi: 10.1002/hbm.10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nossek E, Matot I, Shahar T, Barzilai O, Rapoport Y, Gonen T, et al. Failed awake craniotomy: A retrospective analysis in 44 patients undergoing craniotomy for brain tumor. J Neurosurg. 2013;118:243–9. doi: 10.3171/2012.10.JNS12511. [DOI] [PubMed] [Google Scholar]
- 18.Pereira LC, Oliveira KM, L’Abbate GL, Sugai R, Ferreira JA, da Motta LA. Outcome of fully awake craniotomy for lesions near the eloquent cortex: Analysis of a prospective surgical series of 79 supratentorial primary brain tumors with long follow-up. Acta Neurochir (Wien) 2009;151:1215–30. doi: 10.1007/s00701-009-0363-9. [DOI] [PubMed] [Google Scholar]
- 19.Peruzzi P, Bergese SD, Viloria A, Puente EG, Abdel-Rasoul M, Chiocca EA. A retrospective cohort-matched comparison of conscious sedation versus general anesthesia for supratentorial glioma resection. J Neurosurg. 2011;114:633–9. doi: 10.3171/2010.5.JNS1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Picht T, Kombos T, Gramm HJ, Brock M, Suess O. Multimodal protocol for awake craniotomy in language cortex tumour surgery. Acta Neurochir (Wien) 2006;148:127–37. doi: 10.1007/s00701-005-0706-0. [DOI] [PubMed] [Google Scholar]
- 21.Pinksker MO, Nabavi A, Mehdorn HM. Neuronavigation and resection of lesions located in eloquent brain areas under local anesthesia and neuropsychological-neurophysiological monitoring. Minim Invasive Neurosurg. 2007;50:281–4. doi: 10.1055/s-2007-985825. [DOI] [PubMed] [Google Scholar]
- 22.Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current- induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med. 2003;49:177–82. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- 23.Sacko O, Lauwers-Cances V, Brauge D, Sesay M, Brenner A, Roux FE. Awake craniotomy vs surgery under general anesthesia for resection of supratentorial lesions. Neurosurgery. 2011;68:1192–9. doi: 10.1227/NEU.0b013e31820c02a3. [DOI] [PubMed] [Google Scholar]
- 24.Scuplak SM, Smith M, Harkness WF. Air embolism during awake craniotomy. Anaesthesia. 1995;50:338–40. doi: 10.1111/j.1365-2044.1995.tb04611.x. [DOI] [PubMed] [Google Scholar]
- 25.Shinoura N, Suzuki Y, Yamada R, Kodama T, Takahashi M, Yagi K. Fibers connecting the primary motor and sensory area play a role in grasp stability of the hand. Neuroimage. 2005;25:936–41. doi: 10.1016/j.neuroimage.2004.12.060. [DOI] [PubMed] [Google Scholar]
- 26.Shinoura N, Yamada R, Kodama T, Suzuki Y, Takahashi M, Yagi K. Preoperative fMRI, tractography and continuous task during awake surgery for maintenance of motor function following surgical resection of metastatic tumor spread to the primary motor area. Minim Invasive Neurosurg. 2005;48:85–90. doi: 10.1055/s-2004-830227. [DOI] [PubMed] [Google Scholar]
- 27.Shinoura N, Yamada R, Kodama T, Suzuki Y, Takahashi M, Yagi K. Association of motor deficits with head position during awake surgery for resection of medial motor area brain tumors. Minim Invasive Neurosurg. 2005;48:315–21. doi: 10.1055/s-2005-915627. [DOI] [PubMed] [Google Scholar]
- 28.Shinoura N, Yoshida M, Yamada R, Tabei Y, Saito K, Suzuki Y, et al. Awake surgery with continuous task for resection of brain tumors in the primary motor area. J Clin Neurosci. 2009;16:188–94. doi: 10.1016/j.jocn.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Shinoura N, Yoshida M, Yamada R, Tabei Y, Saito K, Suzuki Y, et al. Combined damage to the right hemispheric hand area in the primary motor and sensory area plays a critical role in motor hemineglect. Eur Neurol. 2010;63:17–23. doi: 10.1159/000258636. [DOI] [PubMed] [Google Scholar]
- 30.Shinoura N, Yamada R, Tabei Y, Saito K, Suzuki Y, Yagi K. Advantages and disadvantages of awake surgery for brain tumors in the primary motor cortex: Institutional experience and review of literature. Br J Neurosurg. 2011;25:218–24. doi: 10.3109/02688697.2010.505671. [DOI] [PubMed] [Google Scholar]
- 31.Spena G, Nava A, Cassini F, Pepoli A, Bruno M, D’Agata F, et al. Preoperative and intraoperative brain mapping for the resection of eloquent-area tumors. A prospective analysis of methodology, correlation, and usefulness based on clinical outcomes. Acta Neurochir. 2010;152:1835–46. doi: 10.1007/s00701-010-0764-9. [DOI] [PubMed] [Google Scholar]
- 32.Taylor MD, Bernstein M. Awake craniotomy with brain mapping as the routine surgical approach to treating patients with supratentorial intraaxial tumors: A prospective trial of 200 cases. J Neurosurg. 1999;90:35–41. doi: 10.3171/jns.1999.90.1.0035. [DOI] [PubMed] [Google Scholar]
- 33.White LE, Andrews TJ, Hulette C, Richards A, Groelle M, Paydarfar J, et al. Structure of the human sensorimotor system. II: Lateral symmetry. Cereb Cortex. 1997;7:31–47. doi: 10.1093/cercor/7.1.31. [DOI] [PubMed] [Google Scholar]


