Abstract
Background:
The simultaneous occurrence of multiple intracranial neoplasms has been reported, especially in genetic familial syndromes and after cranial irradiation. In the absence of these etiologic factors, some reports showed simultaneous occurrence of glioblastoma and meningioma but the association between gliosarcoma and meningioma is unknown.
Case Description:
We report a case of a 51-year-old woman with synchronous gliosarcoma and meningioma in whom extensive immunohistochemical characterization and molecular profile was performed. The gliosarcoma recurred 21 months after the first resection, reaching 3 years of overall survival. A molecular characterization of all three lesions was performed. None of the lesions showed the presence of mutations in TP53 and BRAF genes. MGMT analysis showed the presence of loss of expression associated with promoter hypermethylation in both gliosarcoma lesions. EGFR overexpression and gene amplification was found only in the recurrent gliosarcoma.
Conclusion:
The immunohistochemistry and molecular data of this unique case, suggest the distinct clonal origin of meningioma and gliosarcoma lesions, and the association of MGMT methylation with the presumable favorable prognosis observed.
Keywords: EGFR, gliosarcoma, long survival, MGMT, meningioma, synchronous
INTRODUCTION
Gliosarcoma is a glioblastoma variant characterized by a biphasic tissue pattern with alternate areas displaying glial and mesenchymal differentiation.[17] It was originally described in 1895 by Stroebe et al. and comprises approximately 2% of all glioblastoma.[17,28] The sarcomatous areas commonly resemble fibrosarcoma, but may show a variety of lines of mesenchymal differentiation, such as osteogenic, chondrogenic, adipogenic, smooth, and skeletal muscle.[2,24] The occurrence of similar genetic alterations in both glial and mesenchymal components supports the concept of a monoclonal origin of the metaplastic mesenchymal differentiation from the astrocytic component.[11,12,28,29] However, the molecular mechanisms governing this mesenchymal differentiation are still unclear. Interestingly, a recent study report the isolation of gliosarcoma stem cells, which were able to further undergo glial and mesenchymal differentiation.[9] Meningiomas are the most common extra-axial neoplasms and the second most common primary tumors of the central nervous system, accounting for 24-30% of all brain tumors.[17,31]
The occurrence of simultaneous brain tumors of different histological natures in the absence of hereditary syndromes or prior exposure to ionizing radiotherapy is rare.[8] Nevertheless, several reports described the concurrent association of meningioma and gliomas, mainly glioblastomas.[7,10,23,33,36] Herein, we report a case of a long survival gliosarcoma with a synchronous meningioma. Due to the exceptionality of the case, we performed an immunohistochemical and molecular characterization of the lesions, in order to better understand their biology.
CASE REPORT
A previously healthy 51-year-old woman, with no family history of cancer, was admitted in another institution in October 2003 with a history of dysarthria and left hemibody paresthesias followed by generalized tonic-clonic seizure. On physical examination the patient was full awake with mild dysarthria and a grade 4 left hemiparesis with brachiofacial predominance. She had no signs of intracranial hypertension. The Karnofsky performance status (KPS) was 70. A computed tomography (CT) scan was performed showing a right frontal cortico-subcortical hypodense area resembling a secondary lesion in nature. Primary neoplasm investigation was negative and the patient was referred to our institution (Hospital S. João, Porto; Portugal). A magnetic resonance imaging (MRI) was done and revealed a right frontal parasagittal and well-demarcated hyperintense lesion with homogeneous contrast-enhancement and a second lesion in the posterior right frontal lobe with poorly demarcated borders and heterogeneous contrast-enhancement. The patient underwent a right frontal craniotomy with gross total removal of the two lesions in December 2003. There was no complication and the patient was discharged with a grade 4 hemiparesis and a complete recovery of the dysarthria. The KPS at discharge was 80. The pathological examination revealed two distinct lesions being the anterior a meningioma and the posterior a gliosarcoma [Figure 1]. The patient received postoperative radiotherapy with a total dose of 60 Gy given in 30 fractions with margin of 1 cm in the area of the gliosarcoma. The patient remained asymptomatic until September 2005 when she developed dysarthria and worsening of the hemiparesis. The MRI showed a regrowth of the initial gliosarcoma and the patient was re-operated with macroscopically complete removal of the recurrent gliosarcoma [Figure 1]. The patient recovered again from the neurological deficits and started temozolomide treatment with 150 mg/m2 followed by 200 mg/m2. In November 2005, the patient showed progressive neurological deterioration with headaches, disorientation episodes, and paresis worsening with left arm plegia. The MRI showed an early new regrowth with no indication for surgical removal and the patient underwent palliation with dexamethasone. In June 2006 a new MRI showed a growth of the right frontal lesion with involvement of the basal ganglia and corpus callosum, crossing the midline and development of hydrocephalus. The patient died in October 2006 after a progressive neurological deterioration. The overall survival was 36 months. No postmortem examination was performed.
Figure 1.
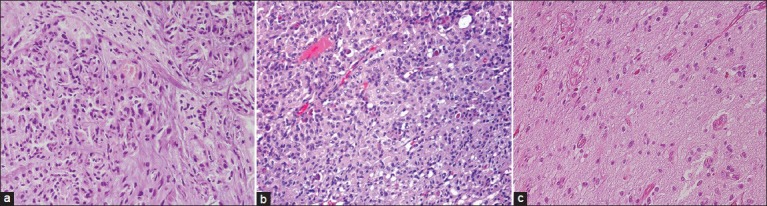
Hemotoxylin and eosin staining of the three lesions; (a) primary gliosarcoma (×200); (b) meningioma (×200); (c) recurrent gliosarcoma (×200)
Pathological findings
Histological examination of the initial anterior lobular frontal and posterior lobular frontal lesions revealed two clear distinct tumors: A meningioma (M) depicting positivity for epithelial membrane antigen (EMA) staining and a gliosarcoma (GS) exhibiting typical features of intermingled GFAP and reticuline neoplastic regions. The analysis of recurrent lesion showed the presence of a gliosarcoma (GS-R) with a more prominent glial component.
In order, to determine the presence of mutations in the TP53 (exons 5-8) and BRAF (exon 11 and 15) genes, DNA was isolated from the formalin-fixed and paraffin-embedded tissues of all three lesions. The screening of mutations was carried out by polymerase chain reaction (PCR)-single-strand conformational polymorphism (PCR-SSCP), followed by direct DNA sequencing, as previously described.[27,29] None of lesions showed TP53 or BRAF mutations [Table 1].
Table 1.
Molecular and immunohistochemical analysis of the reported case
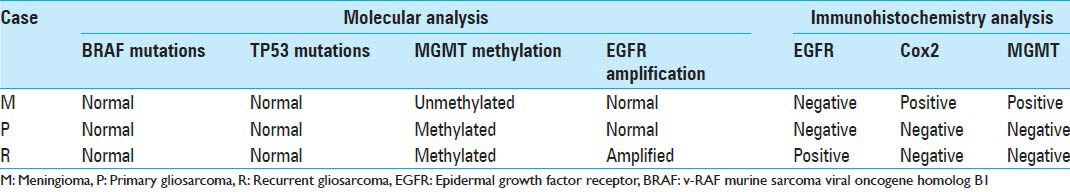
We further investigated for the presence of MGMT protein expression by immunohistochemistry, using the mouse anti-MGMT monoclonal antibody (dilution 1:400; clone MT3.1, Chemicon International) and correlated with MGMT gene promoter hypermethylation, assessed by methylation-specific PCR (MSP) as previously described.[6,21] As illustrated in Figure 2, MGMT methylation was found in both primary and recurrent gliosarcoma. The meningioma lesion was negative for MGMT methylation [Table 1 and Figure 2]. In concordance with methylation status, MGMT expression was only present in the meningioma and absent in both primary and recurrent gliosarcoma [Table 1 and Figure 3]. Next, we analyzed the presence of EGFR alterations at both gene and protein levels by CISH (EGFR spot-Light amplification probes from Zymed® Laboratories Inc., South San Francisco, CA, USA) and immunohistochemistry (anti-EGFR polyclonal antibody, dilution 1:100, clone 31G7, Zymed® Laboratories Inc., San Francisco, CA, USA), respectively.[30,39] The meningioma and primary gliosarcoma were negative for EGFR protein expression [Table 1 and Figure 3]. EGFR immunohistochemistry showed a focal region with strong immunoreactivity in the recurrent gliosarcoma. In both meningioma and primary gliosarcoma, it was observed the presence of 2 signals per neoplastic cell, representing absence of EGFR gene copy number alterations [Table 1 and Figure 3]. EGFR gene amplification, represented by clusters of more than 5 signals per neoplastic cell, was observed only in the recurrent gliosarcoma, in the same region with strong focal positivity for EGFR protein [Table 1 and Figure 3].
Figure 2.
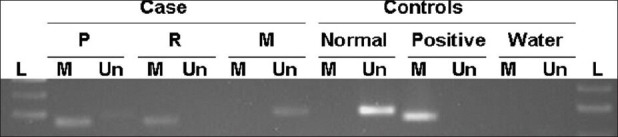
Methylation-specific PCR (MSP) analyses of the MGMT promoter of the three lesions; P (primary gliosarcoma); R (recurrent gliosarcoma); M (meningioma). MSP controls reactions consisted of blood-extracted DNA from a cancer-free individual to use as umethylated DNA control (Un.), and a CpGenome™ Universal Methylated DNA (Chemicon International) as methylated DNA control (m). PCR reactions in the absence of DNA (water) were performed as negative controls for both the unmethylated and methylated reactions
Figure 3.
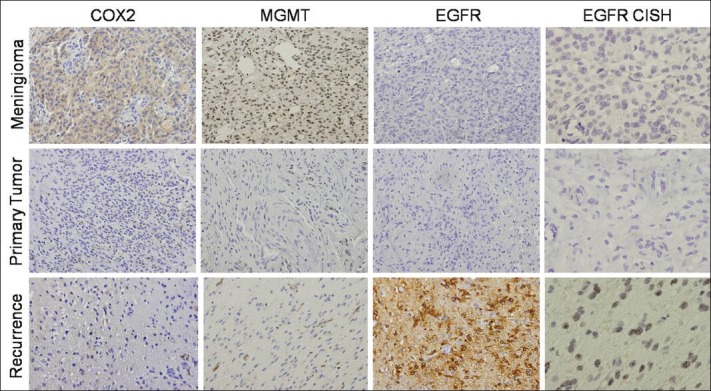
Imunohistochemistry and Chromogenic In Situ Hybridization (CISH) analysis of the three lesions. COX2 immunohistochemistry (×200) positive for the meningioma lesion and negative for the primary and recurrent gliosarcoma. MGMT staining (×200) was only positive for the meningioma. EGFR immunostaining was negative in primary gliosarcoma and meningioma, with recurrent gliosarcoma exhibiting strong positivity. CISH analysis of EGFR confirmed these findings after EGFR amplification
Finally, we studied the expression of COX-2 by immunohistochemistry, using the anti-COX-2 monoclonal antibody (dilution 1:50, clone SP21, Neomarkers, Fremont, CA, USA).[4] We observed positivity only in meningioma lesion [Figure 3]. Both gliosarcomas (primary and recurrent) were negative for COX-2 expression [Table 1 and Figure 3].
DISCUSSION
Gliosarcomas are rare tumors of the central nervous system.[11,17,28] In general, the epidemiology and natural history of gliosarcoma appears to be similar to the glioblastoma, mainly primary glioblastoma.[14] Both show a propensity to affect elderly patients, with a median age of diagnosis over 60 years. With the current standard treatment, which includes adjuvant radiotherapy with concomitant temozolomide, gliosarcoma patients have a median survival time of approximately 14 months.[11] Parameters such as age of presentation, extension of tumor resection and adjuvant radio therapy (RT) are also significantly associated with gliosarcoma patient's survival.[14] They tend to occur in the temporal lobe and some of them are well-circumscribed.[14]
It is not frequent the concurrent occurrence of central nervous system neoplasms outside familial tumor syndromes or previous to radiotherapy.[38,40] Several authors have previously reported synchronous glioma and meningioma. Literature review shows that glioblastoma is the most frequent glioma subtype associated.[7,8,15,20,23,32,37,38] The occurrence of gliosarcoma and meningioma was rarely reported.[13,25,40] The explanation of this simultaneous occurrence of two primary distinct brain tumors is not clear. Some authors suggested that this occurrence is most likely a casual coincidence,[5,15,36,37] whereas others hypothesized that they can be the result of common etiological route.[38] In the present study, the observation of distinct molecular characteristics displayed by the two tumors, namely MGMT, COX-2 and EGFR profile, suggest that both tumors evolved through distinct pathways.
It is known that gliosarcomas can present with different imaging features. The lesions can be similar to glioblastoma with a central necrotic area surrounded by a ring of heterogeneous contrast enhancement, as occurred in our patient, or as homogeneous hyperdense lesions resembling meningiomas. Those that resemble meningiomas tend to have better prognosis.[19,34] In our case, the presence of distinct histological and molecular features in both meningioma and primary gliosarcoma, exclude the possibility of monoclonal origin of both tumors.
MGMT is a DNA repair enzyme that removes promutagenic methyl groups from the O-6 position of guanine induced by alkylating agents such as temozolomide.[6,26] Regulation of MGMT expression is an epigenetic event directly dependent on MGMT promoter methylation status. Although not completely consensual, MGMT promoter methylation has been associated with better survival in glioblastoma patients and has prognostic value.[6,26] Our case has an unusual good behavior with a 36 months survival. The methylation of MGMT is present in both primary and recurrent gliosarcoma, thus potentially contributing to the long survival of the patient.
Some studies suggested the involvement of the p53 pathway in the development of meningiomas and gliosarcomas.[1,3,28] In our case we did not find any mutation in the TP53 gene. EGFR alterations, namely gene amplification, are reported to be infrequent in gliosarcomas and meningiomas.[18,28] In other glioma subtypes, mainly glioblastomas it can be associated with worse clinical outcome.[16] Interestingly, in the present case, EGFR amplification and overexpression was only present in the recurrent tumor and this change could explain the aggressive behavior of the recurrent gliosarcoma. Meningiomas are strongly positive for COX-2, that can be a predictor of shortest outcome.[22,35] In gliomas COX-2 overexpression can also occur, however, this is usually less common than in meningiomas.[35] Interestingly, in the present case, COX-2 expression was only present in the meningioma lesion.
CONCLUSION
We report for the first time the occurrence of a synchronous meningioma and gliosarcoma with a long survival. We observed the absence of mutations in TP53 and BRAF genes. MGMT loss of expression/function by promoter methylation was only found in both primary and recurrent gliosarcoma, which could in part explain the good prognosis of the patient. EGFR gene overexpression/amplification was present only in the recurrent gliosarcoma supporting its association with tumor aggressiveness. Overall, immunohistochemistry and molecular data suggest the distinct clonal origin of meningioma and gliosarcoma lesions.
ACKNOWLEDGMENTS
Olga Martinho is a recipient of a fellowship grant from the Portuguese Ministry of Science (SFRH/BD/36463/2007). This project was sponsored, in part, by Schering-Plough Farma (Portugal). The funding institutions had no role in the study design, data collection and analysis, interpretation of the results, the preparation of the manuscript, or the decision to submit the manuscript for publication.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2013/4/1/151/122229
Contributor Information
Paulo Linhares, Email: paulojlinhares@yahoo.com.
Olga Martinho, Email: olgamartinho@ecsaude.uminho.pt.
Bruno Carvalho, Email: bmfcarvalho@gmail.com.
Lígia Castro, Email: stirling.carpenter@gmail.com.
José Manuel Lopes, Email: jmlopes@ipatimup.pt.
Rui Vaz, Email: ruimcvaz@gmail.com.
Rui Manuel Reis, Email: rreis@ecsaude.uminho.pt.
REFERENCES
- 1.Al-Khalaf HH, Lach B, Allam A, Hassounah M, Alkhani A, Elkum N, et al. Expression of survivin and p16(INK4a)/Cdk6/pRB proteins and induction of apoptosis in response to radiation and cisplatin in meningioma cells. Brain Res. 2008;1188:25–34. doi: 10.1016/j.brainres.2007.10.074. [DOI] [PubMed] [Google Scholar]
- 2.Alatakis S, Stuckey S, Siu K, McLean C. Gliosarcoma with osteosarcomatous differentiation: Review of radiological and pathological features. J Clin Neurosci. 2004;11:650–6. doi: 10.1016/j.jocn.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Amatya VJ, Takeshima Y, Inai K. Methylation of p14(ARF) gene in meningiomas and its correlation to the p53 expression and mutation. Mod Pathol. 2004;17:705–10. doi: 10.1038/modpathol.3800111. [DOI] [PubMed] [Google Scholar]
- 4.Baltazar F, Filho AL, Pinheiro C, Moreira MA, Queiroz GS, Oton GJ, et al. Cyclooxygenase-2 and epidermal growth factor receptor expressions in different histological subtypes of cervical carcinomas. Int J Gynecol Pathol. 2007;26:235–41. doi: 10.1097/pgp.0b013e31802f1996. [DOI] [PubMed] [Google Scholar]
- 5.Chen G, Gao X, Liao Y, Xu B. A glioblastoma adjacent to a meningioma. Br J Neurosurg. 2010;24:718–9. doi: 10.3109/02688697.2010.487132. [DOI] [PubMed] [Google Scholar]
- 6.Costa BM, Caeiro C, Guimaraes I, Martinho O, Jaraquemada T, Augusto I, et al. Prognostic value of MGMT promoter methylation in glioblastoma patients treated with temozolomide-based chemoradiation: A Portuguese multicentre study. Oncol Rep. 2010;23:1655–62. doi: 10.3892/or_00000808. [DOI] [PubMed] [Google Scholar]
- 7.Dario A, Marra A, Cerati M, Scamoni C, Dorizzi A. Intracranial meningioma and astrocytoma in the same patient. Case report and review of the literature. J Neurosurg Sci. 1995;39:27–35. [PubMed] [Google Scholar]
- 8.Davis GA, Fabinyi GC, Kalnins RM, Brazenor GA, Rogers MA. Concurrent adjacent meningioma and astrocytoma: A report of three cases and review of the literature. Neurosurgery. 1995;36:599–604. doi: 10.1227/00006123-199503000-00023. [DOI] [PubMed] [Google Scholar]
- 9.deCarvalho AC, Nelson K, Lemke N, Lehman NL, Arbab AS, Kalkanis S, et al. Gliosarcoma stem cells undergo glial and mesenchymal differentiation in vivo. Stem Cells. 2010;28:181–90. doi: 10.1002/stem.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goyal A, Singh AK, Sinha S, Tatke M, Singh D, Gupta V. Simultaneous occurrence of meningioma and glioma in brain: Report of two cases. J Clin Neurosci. 2003;10:252–4. doi: 10.1016/s0967-5868(02)00345-4. [DOI] [PubMed] [Google Scholar]
- 11.Han SJ, Yang I, Ahn BJ, Otero JJ, Tihan T, McDermott MW, et al. Clinical characteristics and outcomes for a modern series of primary gliosarcoma patients. Cancer. 2010;116:1358–66. doi: 10.1002/cncr.24857. [DOI] [PubMed] [Google Scholar]
- 12.Han SJ, Yang I, Tihan T, Prados MD, Parsa AT. Primary gliosarcoma: Key clinical and pathologic distinctions from glioblastoma with implications as a unique oncologic entity. J Neurooncol. 2010;96:313–20. doi: 10.1007/s11060-009-9973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jager B, Schuhmann MU, Schober R, Kortmann RD, Meixensberger J. Induction of gliosarcoma and atypical meningioma 13 years after radiotherapy of residual pilocytic astrocytoma in childhood. Pediatr Neurosurg. 2008;44:153–8. doi: 10.1159/000113120. [DOI] [PubMed] [Google Scholar]
- 14.Kozak KR, Moody JS. Giant cell glioblastoma: A glioblastoma subtype with distinct epidemiology and superior prognosis. Neuro Oncol. 2009;11:833–41. doi: 10.1215/15228517-2008-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee EJ, Chang CH, Wang LC, Hung YC, Chen HH. Two primary brain tumors, meningioma and glioblastoma multiforme, in opposite hemispheres of the same patient. J Clin Neurosci. 2002;9:589–91. doi: 10.1054/jocn.2002.1086. [DOI] [PubMed] [Google Scholar]
- 16.Lo HW. EGFR-targeted therapy in malignant glioma: Novel aspects and mechanisms of drug resistance. Curr Mol Pharmacol. 2010;3:37–52. doi: 10.2174/1874467211003010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lusis EA, Chicoine MR, Perry A. High throughput screening of meningioma biomarkers using a tissue microarray. J Neurooncol. 2005;73:219–23. doi: 10.1007/s11060-004-5233-y. [DOI] [PubMed] [Google Scholar]
- 19.Maiuri F, Stella L, Benvenuti D, Giamundo A, Pettinato G. Cerebral gliosarcomas: Correlation of computed tomographic findings, surgical aspect, pathological features, and prognosis. Neurosurgery. 1990;26:261–7. [PubMed] [Google Scholar]
- 20.Marra A, Ramponi G, Grimaldi G. Simultaneous occurrence of right supratentorial meningioma and glioblastoma multiforme. Case report. Acta Neurochir (Wien) 1977;36:83–91. doi: 10.1007/BF01405989. [DOI] [PubMed] [Google Scholar]
- 21.Nakasu S, Fukami T, Baba K, Matsuda M. Immunohistochemical study for O6-methylguanine-DNA methyltransferase in the non-neoplastic and neoplastic components of gliomas. J Neurooncol. 2004;70:333–40. doi: 10.1007/s11060-004-9170-6. [DOI] [PubMed] [Google Scholar]
- 22.Nathoo N, Barnett GH, Golubic M. The eicosanoid cascade: Possible role in gliomas and meningiomas. J Clin Pathol. 2004;57:6–13. doi: 10.1136/jcp.57.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nestler U, Schmidinger A, Schulz C, Huegens-Penzel M, Gamerdinger UA, Koehler A, et al. Glioblastoma simultaneously present with meningioma--report of three cases. Zentralbl Neurochir. 2007;68:145–50. doi: 10.1055/s-2007-981673. [DOI] [PubMed] [Google Scholar]
- 24.Ozolek JA, Finkelstein SD, Couce ME. Gliosarcoma with epithelial differentiation: Immunohistochemical and molecular characterization. A case report and review of the literature. Mod Pathol. 2004;17:739–45. doi: 10.1038/modpathol.3800109. [DOI] [PubMed] [Google Scholar]
- 25.Paueksakon P, Blacklock JB, Powell SZ, Goodman JC. September 2003: A 79-year-old female with right frontal lobe mass. Brain Pathol. 2004;14:113–5. doi: 10.1111/j.1750-3639.2004.tb00506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piperi C, Themistocleous MS, Papavassiliou GA, Farmaki E, Levidou G, Korkolopoulou P, et al. High incidence of MGMT and RARbeta promoter methylation in primary glioblastomas: Association with histopathological characteristics, inflammatory mediators and clinical outcome. Mol Med. 2010;16:1–9. doi: 10.2119/molmed.2009.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reis RM, Hara A, Kleihues P, Ohgaki H. Genetic evidence of the neoplastic nature of gemistocytes in astrocytomas. Acta Neuropathol. 2001;102:422–5. doi: 10.1007/s004010100452. [DOI] [PubMed] [Google Scholar]
- 28.Reis RM, Konu-Lebleblicioglu D, Lopes JM, Kleihues P, Ohgaki H. Genetic profile of gliosarcomas. Am J Pathol. 2000;156:425–32. doi: 10.1016/S0002-9440(10)64746-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reis RM, Martins A, Ribeiro SA, Basto D, Longatto-Filho A, Schmitt FC, et al. Molecular characterization of PDGFR-alpha/PDGF-A and c-KIT/SCF in gliosarcomas. Cell Oncol. 2005;27:319–26. doi: 10.1155/2005/347863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reis-Filho JS, Pinheiro C, Lambros MB, Milanezi F, Carvalho S, Savage K, et al. EGFR amplification and lack of activating mutations in metaplastic breast carcinomas. J Pathol. 2006;209:445–53. doi: 10.1002/path.2004. [DOI] [PubMed] [Google Scholar]
- 31.Riemenschneider MJ, Perry A, Reifenberger G. Histological classification and molecular genetics of meningiomas. Lancet Neurol. 2006;5:1045–54. doi: 10.1016/S1474-4422(06)70625-1. [DOI] [PubMed] [Google Scholar]
- 32.Rieske P, Zakrzewska M, Biernat W, Bartkowiak J, Zimmermann A, Liberski PP. Atypical molecular background of glioblastoma and meningioma developed in a patient with Li-Fraumeni syndrome. J Neurooncol. 2005;71:27–30. doi: 10.1007/s11060-004-9181-3. [DOI] [PubMed] [Google Scholar]
- 33.Rotondo M, Parlato C, Zotta DC, Moraci A. Simultaneous multiple brain tumors of different histological nature. Report of two cases. J Neurosurg Sci. 1990;34:57–60. [PubMed] [Google Scholar]
- 34.Salvati M, Caroli E, Raco A, Giangaspero F, Delfini R, Ferrante L. Gliosarcomas: Analysis of 11 cases do two subtypes exist? J Neurooncol. 2005;74:59–63. doi: 10.1007/s11060-004-5949-8. [DOI] [PubMed] [Google Scholar]
- 35.Shono T, Tofilon PJ, Bruner JM, Owolabi O, Lang FF. Cyclooxygenase-2 expression in human gliomas: Prognostic significance and molecular correlations. Cancer Res. 2001;61:4375–81. [PubMed] [Google Scholar]
- 36.Spallone A, Santoro A, Palatinsky E, Giunta F. Intracranial meningiomas associated with glial tumours: A review based on 54 selected literature cases from the literature and 3 additional personal cases. Acta Neurochir (Wien) 1991;110:133–9. doi: 10.1007/BF01400681. [DOI] [PubMed] [Google Scholar]
- 37.Strong AJ, Symon L, MacGregor BJ, O’Neill BP. Coincidental meningioma and glioma. Report of two cases. J Neurosurg. 1976;45:455–8. doi: 10.3171/jns.1976.45.4.0455. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki K, Momota H, Tonooka A, Noguchi H, Yamamoto K, Wanibuchi M, et al. Glioblastoma simultaneously present with adjacent meningioma: Case report and review of the literature. J Neurooncol. 2010;99:147–53. doi: 10.1007/s11060-009-0109-9. [DOI] [PubMed] [Google Scholar]
- 39.Viana-Pereira M, Lopes JM, Little S, Milanezi F, Basto D, Pardal F, et al. Analysis of EGFR overexpression, EGFR gene amplification and the EGFRvIII mutation in Portuguese high-grade gliomas. Anticancer Res. 2008;28:913–20. [PubMed] [Google Scholar]
- 40.Welsh JS, Thurman SA, Howard SP. Thymoma and multiple malignancies: A case of five synchronous neoplasms and literature review. Clin Med Res. 2003;1:227–32. doi: 10.3121/cmr.1.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]


