Abstract
Introduction:
Thoracolumbar spine trauma is the most common site of spinal cord injury, with clinical and epidemiological importance.
Materials and Methods:
We performed a comprehensive literature review on the management and treatment of TLST.
Results:
Currently, computed tomography is frequently used as the primary diagnostic test in TLST, with magnetic resonance imaging used in addition to assess disc, ligamentous, and neurological injury. The Thoracolumbar Injury Classification System is a new injury severity score created to help the decision-making process between conservative versus surgical treatment. When decision for surgery is made, early procedures are feasible, safe, can improve outcomes, and reduce healthcare costs. Surgical treatment is individualized based on the injury characteristics and surgeon's experience, as there is no evidence-based for the superiority of one technique over the other.
Conclusions:
The correct management of TLST involves multiple steps, such as a precise diagnosis, classification, and treatment. The TLICS can improve care and communication between spine surgeons, resulting in a more standardized treatment.
Keywords: Classification, diagnosis, spinal cord injury, thoracolumbar spine trauma, treatment
INTRODUCTION
Thoracolumbar spine trauma represents the most common area fractured in the spine.[1] In a large series of 3,142 patients with traumatic spinal fractures, Wang et al., 2012, reported that 54.9% of the patients had fractures in the thoracolumbar spine. Interesting, cervical spine injuries were more common after traffic accidents, whereas lumbar injuries were more commonly associated to falls. Patients with complete neurological deficits (American Spinal Injury Association Impairment Scale — A) generally have thoracic fractures, possibly due to a small canal diameter when compared to the cervical or lumbar spine.[1]
Although grouped together, TLST is comprised of injures to the more rigid thoracic spine (T1-T10), the flexible and transitional thoracolumbar junction (T11-L2), more susceptible to injuries, and the lumbar spine L3-5. Early diagnosis and adequate management may improve patients’ outcome and decrease its inherent disability. In this paper, we review the primary concepts in the diagnosis, classification, and treatment of TLST.
DIAGNOSTIC IMAGING
After hospital admission and clinical stabilization, patients with potential spine trauma should be screened radiologically. Plain radiographs, although valuable, have limitations regarding three-dimensional (3D) visualization, patient positioning, body habitus, and overlapping bony anatomy in certain spinal regions, such as the thoracic spine, cervicothoracic, and or craniocervical junction. In this context, computed tomography scans are available in almost all trauma centers and provide rich and detailed information about the bony anatomy as well as spine alignment, faster and more accurately than plain radiographs.[2] Helical CT scan reconstruction has accuracy in detects, thoracolumbar fractures of almost 99% (95% confidence interval (CI) 96-100%), compared with 87% of standard radiographs (95% CI 82-92%) in high-risk trauma patients.[3] Moreover, as the CT scan is generally performed for thoracic and abdominal trauma, spine evaluation can typically be accomplished without significant increase in time.
Magnetic resonance is recommended, especially in patients with neurological deficits, in the evaluation of the disc, ligaments, and neural elements. In other injuries, principally thoracolumbar burst fractures, MR has been used to obtain information about the status of the posterior ligamentous complex, a critical determinant of surgical decision-making.[4,5,6] In some centers, MR is part of routine evaluation of detected spinal fractures. However, the literature reveals conflicting results from the use of MR in TLST.
In trying to analyze the importance of the MR findings in diagnosis and treatment of TLST; Pizones et al., 2011, performed a prospective evaluation of 33 patients. Using plain radiographs and CT scan, they classified injuries according to the AO classification system.[7] Then, a MR protocol was performed, and patients were reclassified according to the AO system and the Thoracolumbar Injury Classification System. There were 41 fractures diagnosed using plain X-rays and CT scans. MR identified nine additional vertebral fractures not seen on X-ray or CT, increasing the number to 50. The addition of the MR information changed the diagnosis in 40% of the patients and discovered 18 additional occult injuries. The AO classification changed from A to B in 24% of the patients and therapeutic management changed in 16% of the cases. Based on these findings, the authors concluded that MR was important to classify and also in the surgical decision-making of TLST.
Similar results were presented by Winklhofer et al., 2012.[8] The authors reported that, after a primary analysis of 100 consecutive patients with fracture on CT scan, MR changed the AO classification in 31% of them and the TLICS in 33%. Moreover, CT and MR changed the TLICS from values <5 to ≥5 in 24%, changing the proposed treatment from nonoperative to operative management. From an initial 162 fractures detected on CT scan, a total of 196 injuries were identified using a MR. Based on these findings, the authors suggested that MR may improve the detection of fractures as well as influence classification system and treatment chosen. The findings of these two papers suggest that MR have a higher sensitivity than conventional imaging in the diagnosis of TLST.
Vaccaro et al., 2009, performed a prospective study to assess the accuracy of the MR in diagnosis PLC injury in patients with TLST and compared these findings to intraoperative surgical findings.[9] Sensitivity for various PLC components ranged from 79% to 90% whereas specificity ranged from 53% to 65% (ligamentum flavum). Patients with more severe trauma had more agreement between MR evaluation and intraoperative findings. The authors concluded that the PLC status could not be determined by MR in isolation.
A similar study evaluating the status of the PLC with MR also comparing it with intraoperative findings was performed by Rihn et al., 2010.[5] The authors concluded that interpretation of MR findings had a high negative predictive value and sensitivity of up to 100%, but had a low specificity, ranging from 51.5 to 80.5%. Moreover, the relatively low positive predictive value and specificity for MR imaging in evaluating the PLC status, may lead to the overdiagnosis of injuries and overtreatment of stable lesions. These studies identify that MR, while highly sensitive, does not yet provide the high specificity required of definitive diagnostic testing. Therefore, even though promising, the validity of the MR findings in the clinical context (patient's outcome) yet needs to be proven with greater evidence.[6]
CLASSIFICATION
Classification of spine injuries is critical in the decision-making process, evaluating medical interventions, and ultimately predicting patient outcomes. The ideal classification should be reproducible, precise (accurate) and comprehensive, including all types of injuries.[5] Moreover, it should infer patient outcome and help in the decision-making process.
Throughout the years, many traumatic classification systems for the thoracic and lumbar spine have been proposed. Many authors, such as Nicoll, Holdsworth, Louis, and Denis, have contributed to the evolution of fracture classification.[10,11,12,13] Newer systems have derived many elements from the previous ones, with progress based on changes in scientific knowledge and author's personal views. However, if the ideal system exists, it would be not necessary to create newer ones.
In 1994, Magerl et al., proposed one of the most cited systems to classify thoracolumbar spine injuries.[14] Also known as AO Classification system, categories are established according to the injury mechanism, considering prognostic aspects regarding healing, in a progressive scale of instability. The three main categories include [Table 1]:
Table 1.
Three main categories of the Magerl/AO classification system
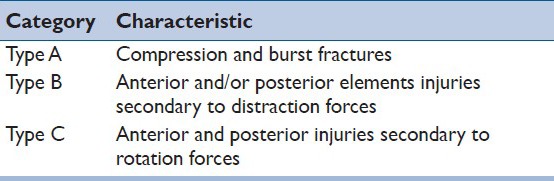
Type A: Vertebral body compression injuries secondary to compression forces, predominantly in the vertebral body. Posterior elements fractures are absent or represented by lamina or spinous process fractures. Magerl, et al., presumed that there was no disruption of the posterior ligamentous complex in type A injuries.
Type B: Injuries with transverse disruption and elongation of the posterior and/or anterior elements in distraction.
Type C: Injuries secondary to rotation or translation, most of the times with concomitant type A and C injuries. Shear injuries associated with torsion also can be present in this group.
These injuries were then reclassified accord to morphological criteria in groups and subgroups of more than 50 subtypes. The severity of the injury pattern increases from A to C and from 1 to 3.
This system has been criticized by some authors for its high complexity, its low reliability, and it lack of consideration of the neurological status.[15,16] It is also criticized for being based on plain radiographs, a less accurate diagnostic tool that CT scan with reconstruction and MR.[15,16,17] Even despite all of these potential pitfalls, the classification has still been used in clinical practice in many centers around the world, providing a common fracture language, and helping surgeons in their surgical decision making.
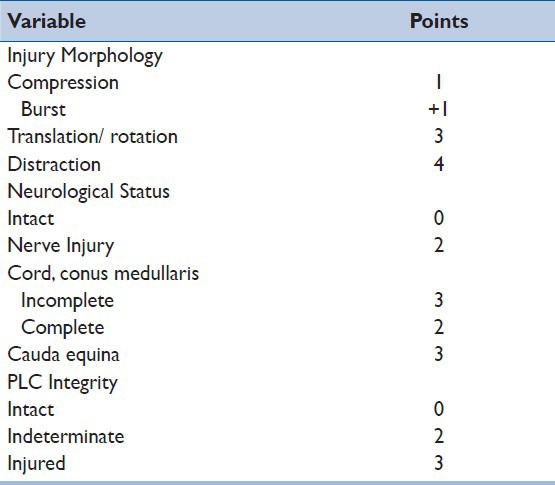
In 2005, the TLICS, a new system was proposed by the Spine Trauma Study Group to help surgeons to treat thoracic and lumbar spine fractures.[15] Three major characteristics associated with patient outcome (neurological and spinal stability) were considered and evaluated. These three main characteristics are: 1) injury morphology, 2) integrity of the posterior ligamentous complex (PLC), and 3) neurological status.
The characteristics are evaluated in isolation, and after summation, a final injury description and injury severity score is obtained, guiding treatment. Three or less points suggest that conservative treatment can be used. Five or more points suggest that surgery is the best option for treatment and patients with four points can be treated conservatively or surgically, according to surgeon's preference and considering other factors, such as additional injuries, patient age, preexisting spinal disease, and patient preference [Table 2].
Table 2.
Thoracolumbar injury classification and severity score (TLICS)
As reproducibility is one of the most important factors in a classification system, assessing its reliability is of paramount importance. Blauth et al., analyzed the interobserver reliability of the AO classification system in 14 fractures in 22 different institutions responsible for treatment of spinal trauma.[18] Inter-observer agreement for the 14 cases studied was 67% (range 41-91%) when using the three main categories of the system (A, B, or C), with a kappa coefficient of 0.33 (fair reliability). The reliability coefficient decreases by increasing the number of injury subgroups. Similar results were obtained by Oner, who reported fair reproducibility (kappa coefficient of 0.34) when classifying the AO fractures using CT scan and moderate reproducibility (kappa coefficient of 0.42) when MRI was used. Based on these studies, we may conclude that the AO system has limitation based its reliability, especially in the subgroups.
Koh et al., evaluated the reliability of the TLICS.[19] Three surgeons reviewed charts of 114 TLST and retrospectively evaluate the injury severity score. This process was repeated after 4 weeks and compared to the first evaluation. Substantial agreement was obtained regarding intrarater reliability on total score and injury morphology and almost perfect reliability regarding neurological status and PLC. When interrater reliability was evaluated, substantial agreement on injury morphology and integrity of the PLC, moderate agreement on total score, and almost perfect agreement on neurological status was reported.
Lewkonia et al., 2012, performed a study of 54 spine cases selected from a chart review, accessed in two occasions by 11 experts using the TLICS.[20] As a result, they obtained a good interobserver agreement with the TLICS (kappa coefficient of 0.73-0.74), emphasizing that the PLC was the least reliable factor. As a conclusion, the TLICS proposal treatment was in concordance with the treatment proposal by the experts.
Based on this good reliability and reproducibility, we may infer that the TLICS can be used to assess and compare results between different institutions.
Some considerations should be made before applying the TLICS system[15,21]:
Morphology is better characterized by CT scan reconstruction in sagittal, axial, and coronal plane and extra information can be obtained with the use of MRI.[7,8]
Distraction and rotational injuries will, by definition, have an associated PLC injury, with exception of pure chance fractures and some extension-distraction injuries. Given this relationship, patients with distraction and rotational injuries receive 3 (rotation) or 4 (distraction) points for morphology as well as 3 points for PLC disruption, being treated surgically regarding of the neurological status.
Unlike the AO system, the TLICS allows for a definition of stable and unstable burst fractures, based on integrity of the PLC. Assessment of the PLC status in burst fractures can be made using MRI (high signal intensity on T2 sequence or short tau inversion recovery (STIR) in the region of PLC elements) or indirect signs, such as diastasis of the facet joints.[22] Surgical treatment is generally recommended for unstable injuries, while stable burst fractures may be treated conservatively.
In burst fractures without neurological deficits, decrease in vertebral body height, local kyphosis, or canal compromise should not be considered independently in the decision-making for surgery. While these findings have been widely described, they do not correlate with PLC injury, as stated in recent studies, and have not been correlated with long-term patient outcomes.[23,24]
Probably the most controversial aspect of the TLICS system is the evaluation of the PLC. Although clearly disrupted in severe fracture dislocations and rotational injuries, in milder cases, such as burst fractures without deficits, PLC disruption can be suggested by diastasis of the facet joints, spacing of the spinous process, or injury detected at the MR. Even though it can be injured, as mentioned before, there are no prospective studies accessing the prognostic value of these findings in patient's outcome.
PHARMACOLOGICAL TREATMENT
The use of methylprednisolone in acute closed spinal cord injury has been widely accepted after publication of the National Acute Spinal Cord Injuries Study II.[25] However, many methodological biases were pointed later by other authors, suggesting that the benefits of corticosteroids were minimal when compared to the potential catastrophic complications.[26,27,28] Furthermore, the studies included both cervical and thoracolumbar spinal cord injuries without independent data reporting. In this context, the use of methylprednisolone is not accepted as a standard therapy in many centers.
CONSERVATIVE TREATMENT
Nonsurgical treatment is proposed when the TLICS score is 3 or less points, typically compression or burst fractures in patients without neurological deficits and without PLC injury.[29] An illustrative case is presented in Figure 1.
Figure 1.
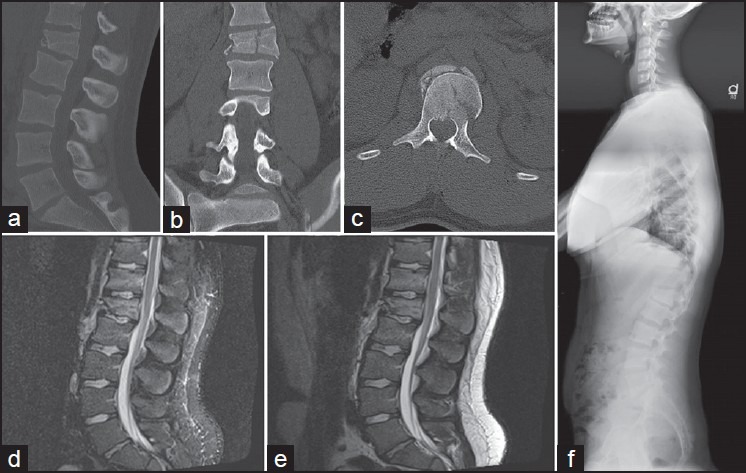
This woman fell from a height and had a L1 fracture. She was neurologically intact. (a-c) Sagittal, coronal, and axial computed tomography scan, respectively; showing a fracture with decrease of body height and a sagittal split. (d and e) T2 sequence magnetic resonance without evident posterior ligament complex disruption. The total Thoracolumbar Injury Classification System Score was 2 points morphology +0 points for PLC +0 for neurological status; total of 2 points. She was treated with a brace for 6 weeks with good clinical outcome. A panoramic lateral plain radiograph is shown (f)
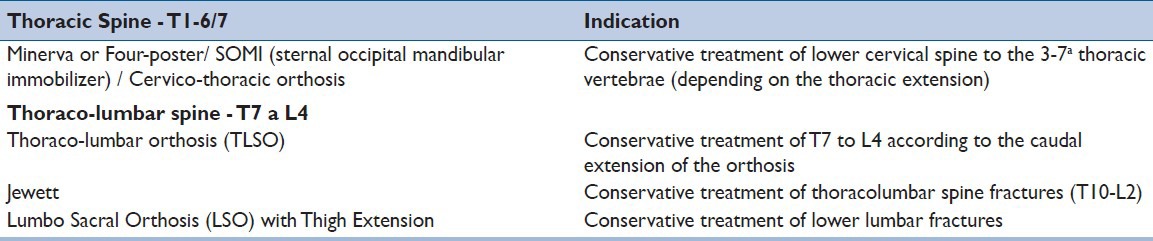
As a treatment option, a spinal orthosis can be used with early ambulation for 6-12 weeks.[16] Upright radiographs are obtained prior hospital discharge to verify fracture stability and spinal alignment, as well as occult ligamentous injury by patient's position. The potential benefits of an orthosis are improvements in acute pain and function well as restricting patients from more vigorous physical activities.[30] The orthoses are prescribed according to the site of injury, as shown in Table 3. However, effectiveness of bracing in stable fractures has been debated.[31]. Bailey et al., 2009, compared patient's outcome after burst fracture treated with or without a brace. The authors included just only AO type A3 in their study, from T11 to L2, in patients under 60 years, with kyphotic deformity of less than 35° and without neurological injury.[30] Outcome was assessed using the Roland-Morris Disability Questionnaire at 3 months. Sixty-nine patients were followed at 3 months and 47 at 1 year. There were no differences between patients’ outcome in both groups. Four patients required surgical intervention (three in the brace group and one in the non-brace). They concluded that brace using did not affect patient's outcome regarding pain control and function. Similar results were found by Shamji et al., 2012, who also performed a prospective randomized controlled trial comparing the relationship between bracing versus no-bracing in the treatment of stable thoracolumbar burst fractures.[32] Patients with stable burst fractures (AO Type A3) from T12 to L2 were randomized. Both groups had similar level of injury, canal compromise and anterior loss of vertebral body height and sagittal Cobb angle. At 6 months of follow-up, there were no differences in anterior loss of vertebral body height, kyphotic progression or self-reported clinical outcomes. As a conclusion, the authors state that stable burst fractures can be treated without brace.
Table 3.
Indication for orthosis according to Injury Site in the thoracic and lumbar spine
Outpatient visits are scheduled according to surgeon experience and preference. It is generally our practice to see patients 7 days after hospital discharge, then 15 days and 2-3 months after injury, with upright plain radiographs obtained at each visit. The brace, when used, is typically removed gradually at 6-12 weeks of treatment. Patients are then seen annually in the following 3-5 years, according to clinical and radiological status.
SURGICAL TREATMENT
Surgery is recommended in patients with a TLICS of 5 or more points. Generally, these patients have unstable burst fractures, burst fractures with neurological deficits, or distraction/rotational injuries with or without neurological injury.[29] An illustrative case is presented in Figure 2.
Figure 2.
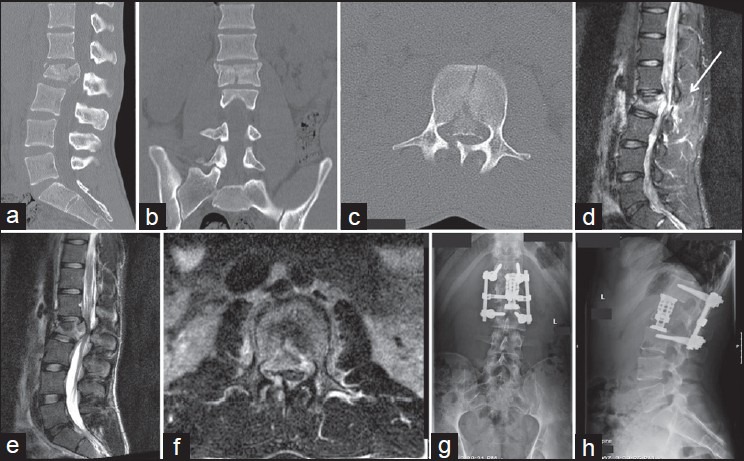
This 14-year-old girl was involved in a car accident. She had impairment of strength in her lower limbs (1/5 left lower limb and 2/5 right lower limb) with normal sensibility and rectal tone. (a) Sagittal CT scan showing a L2 burst fracture with canal compression. (b) Coronal CT scan reconstruction showing loss of vertebral body height. (c) Axial CT scan with canal compression and laminar fracture. (d-f) T2 sequence magnetic resonance imaging suggestion PLC disruption (see white arrow). TLICS Score was 2 points (burst) +2 points for suspect PLC disruption (MR) +2 points for neurological status (root injury) = total 6 points; surgical treatment was performed with a combined approach (g and h)
Timing for surgery
Surgery is typically recommended as soon as possible, based on patient's concurrent injuries, hemodynamic stability, and hospital resources (operating room and staff availability). In thoracic fractures, early surgery is associated with reduction of number of days on a ventilator, as well as in the intensive care unit and in hospital.[33] Potential benefits also included decrease of secondary complications of immobilization, such as atelectasis, pneumonia, and decubitus ulcers. However, there is no evidence available to determine the effect of timing of surgery in mortality.
Patients with neurological injury are typically decompressed and stabilized within 24 h of admission.[34] The benefits of early surgery in patients with neurological deficits with thoracolumbar spine fractures remain to be proven, even though surgery with less than 24 h improves patients’ outcome in cervical spinal cord injuries.[35]
Approaches
As a general rule, patients with PLC injury require posterior instrumentation and fusion. Among patients with neurological injury, decompression may be obtained in some patients through spinal realignment. Others will require direct decompression of the neural elements either through an anterior or posterior approach.[15,36,37] Patients with burst fractures have been treated either anteriorly, posteriorly, or using combined approaches without clinical evidence to suggest superiority of either approach.[36,38]
Posterior-only, transpedicular, or costotransversectomy approaches can be used to obtain a circumferential decompression of the neural elements. Posterior-only approach variations, such as percutaneous surgery or paraspinal approaches sparing the posterior muscles have been successfully used in treatment of unstable burst fractures.[39] Confirming the high number of techniques variations, Hwang et al., 2012, published a series of 46 patients with burst fractures treated surgically with posterior fixation with and without fusion.[40] They obtained similar results in both groups, suggesting that posterolateral fusion may be unnecessary in the treatment of burst fractures treated by a posterior approach. Even kyphoplasty has been used for treatment of TLST, with some studies suggesting benefits in improvement and maintenance of radiological parameters, such as body height and local kyphosis, although these advantages are not clearly correlated to patient's outcome.[41]
Less invasive techniques are also used for anterior approaches. Thoracoscopic techniques can be used in the thoracolumbar junction, with detachment of the diaphragm and access of the retroperitoneal space, allowing decompression and fixation with specific implants. This technique, though implemented in few centers, has reported good results and potential benefits, such as less postoperative pain and less blood loss.[42]
Among patients with thoracolumbar burst fractures, the superiority of anterior versus posterior approach of burst fractures remains unclear.[36,43] In this case, surgeons should choose their approach based on personal experience and preferences, but with a goal of early neurological decompression and stabilization.
Postoperative care and outcome
Early mobilization is recommended to avoid complications associated with extended bed rest. A supporting brace may or may not be used based on surgeon experience. It is our practice to generally not use braces in patients with internally stabilized injuries. Early physical rehabilitation is also recommended, before and after hospital discharge. In patients with neurological deficits, a multidisciplinary support including urological and psychological assessment is necessary.
Neurological status at admission is probably the most important factor related to patient's outcome. Harrop et al., 2011, published a series of 95 patients followed by at least 1 year with spinal cord injury.[44] They report that after 1 year, patients with lumbar (conus) impairment had up to 90% of chances in improvement of one or more ASIA level, compared to just 22.4% of thoracic (T4-9) and thoracolumbar (T10-12) injuries. Patients with complete deficits (ASIA A) had about only 7.7% of improvement compared with 95.2% of ASIA D patients. ASIA B had 66.7% improvement rate and ASIA C 84.6%. The worse chance of neurological improvement was in patients with complete deficits in the thoracic spine and the best chances of recovery were in patients with lumbar and incomplete cord injuries.
CONCLUSIONS
After initial clinical stabilization, early radiological diagnosis of thoracic and lumbar spine trauma can be made based on CT scan reconstruction, complemented by an MR at surgeon's discretion. Using an accepted classification system, such as the TLICS, surgeons can more consistently choose the best treatment option for their patients. Conservative treatment with close clinical radiological follow-up is recommended for patients with stable injury patterns (e.g., TLICS of three or less points, AO type A fractures without deficits), with or without a brace. Early surgery is recommended for burst fractures with deficits or unstable, distraction, and rotational injuries (e.g., TLICS of 5 or more points, AO B and C fractures). Although there are a multitude of surgical techniques, posterior reconstruction are recommend in cases of PLC injury, whereas anterior or posterior approaches can be used in burst fractures without PLC disruption.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Wang H, Zhang Y, Xiang Q, Wang X, Li C, Xiong H, et al. Epidemiology of traumatic spinal fractures: Experience from medical university-affiliated hospitals in Chongqing, China, 2001-2010. J Neurosurg Spine. 2012;17:459–68. doi: 10.3171/2012.8.SPINE111003. [DOI] [PubMed] [Google Scholar]
- 2.Looby S, Flanders A. Spine trauma. Radiol Clin North Am. 2011;49:129–63. doi: 10.1016/j.rcl.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 3.Hauser CJ, Visvikis G, Hinrichs C, Eber CD, Cho K, Lavery RF, et al. Prospective validation of computed tomographic screening of the thoracolumbar spine in trauma. J Trauma. 2003;55:228–34. doi: 10.1097/01.TA.0000076622.19246.CF. [DOI] [PubMed] [Google Scholar]
- 4.Emery SE, Pathria MN, Wilber RG, Masaryk T, Bohlman HH. Magnetic resonance imaging of posttraumatic spinal ligament injury. J Spinal Disord. 1989;2:229–33. [PubMed] [Google Scholar]
- 5.Rihn JA, Yang N, Fisher C, Saravanja D, Smith H, Morrison WB, et al. Using magnetic resonance imaging to accurately assess injury to the posterior ligamentous complex of the spine: A prospective comparison of the surgeon and radiologist. J Neurosurg Spine. 2010;12:391–6. doi: 10.3171/2009.10.SPINE08742. [DOI] [PubMed] [Google Scholar]
- 6.van Middendorp JJ, Patel AA, Schuetz M, Joaquim AF. The precision, accuracy and validity of detecting posterior ligamentous complex injuries of the thoracic and lumbar spine: A critical appraisal of the literature. Eur Spine J. 2013;22:461–74. doi: 10.1007/s00586-012-2602-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pizones J, Izquierdo E, Alvarez P, Sánchez-Mariscal F, Zúñiga L, Chimeno P, et al. Impact of magnetic resonance imaging on decision making for thoracolumbar traumatic fracture diagnosis and treatment. Eur Spine J. 2011;20:390–6. doi: 10.1007/s00586-011-1913-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winklhofer S, Thekkumthala-Sommer M, Schmidt D, Rufibach K, Werner CM, Wanner GA, et al. Magnetic resonance imaging frequently changes classification of acute traumatic thoracolumbar spine injuries. Skeletal Radiol. 2013;42:779–86. doi: 10.1007/s00256-012-1551-x. [DOI] [PubMed] [Google Scholar]
- 9.Vaccaro AR, Rihn JA, Saravanja D, Anderson DG, Hilibrand AS, Albert TJ, et al. Injury of the posterior ligamentous complex of the thoracolumbar spine: A prospective evaluation of the diagnostic accuracy of magnetic resonance imaging. Spine. 2009;34:E841–7. doi: 10.1097/BRS.0b013e3181bd11be. [DOI] [PubMed] [Google Scholar]
- 10.Nicoll EA. Fractures of the dorso-lumbar spine. J Bone Joint Surg Br. 1949;31:376–94. [PubMed] [Google Scholar]
- 11.Holdsworth F. Fractures, dislocations and fractures-dislocations of the spine. J Bone Joint Surg Am. 1970;52:1534–51. [PubMed] [Google Scholar]
- 12.Louis R. Unstable fractures of the spine. III. Instability. A Theories concerning instability. Rev Chir Orthop Reparatrice Appar Mot. 1977;63:423–5. [PubMed] [Google Scholar]
- 13.Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine (Phila Pa 1976) 1983;8:817–31. doi: 10.1097/00007632-198311000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Magerl F, Aebi M, Gertzbein SD, Harms J, Nazarian S. A comprehensive classification of thoracic and lumbar injuries. Eur Spine J. 1994;3:184–201. doi: 10.1007/BF02221591. [DOI] [PubMed] [Google Scholar]
- 15.Vaccaro AR, Lehman RA, Jr, Hurbelt PA, Anderson PA, Harris M, Hedlund R, et al. A new classification of thoracolumbar injuries: The importance of injury morphology, the integrity of the posterior ligamentous complex, and neurologic status. Spine (Phila Pa 1976) 2005;30:2325–33. doi: 10.1097/01.brs.0000182986.43345.cb. [DOI] [PubMed] [Google Scholar]
- 16.Vaccaro AR, Lee JY, Schweitzer KM, Jr, Lim MR, Baron EM, Oner FC, et al. Assessment of injury to the posterior ligamentous complex in thoracolumbar spine trauma. Spine J. 2006;6:524–8. doi: 10.1016/j.spinee.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 17.Vaccaro AR, Lim MR, Hurlbert RJ, Lehman RA, Jr, Harrop J, Fisher DC, et al. Spine Trauma Study Group. Surgical decision making for unstable thoracolumbar spine injuries: Results of a consensus panel review by the Spine Trauma Study Group. J Spinal Disord Tech. 2006;19:1–10. doi: 10.1097/01.bsd.0000180080.59559.45. [DOI] [PubMed] [Google Scholar]
- 18.Blauth M, Bastian L, Knop C, Lange U, Tusch G. Inter-observer reliability in the classification of thoraco-lumbar spinal injuries. Orthopade. 1999;28:662–81. doi: 10.1007/s001320050397. [DOI] [PubMed] [Google Scholar]
- 19.Koh YD, Kim DJ, Koh YW. Reliability and Validity of Thoracolumbar Injury Classification and Severity Score (TLICS) Asian Spine J. 2010;4:109–17. doi: 10.4184/asj.2010.4.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewkonia P, Paolucci EO, Thomas K. Reliability of the thoracolumbar injury classification and severity score and comparison with the denis classification for injury to the thoracic and lumbar spine. Spine (Phila pa 1976) 2012;37:2161–7. doi: 10.1097/BRS.0b013e3182601469. [DOI] [PubMed] [Google Scholar]
- 21.Joaquim AF, Fernandes YB, Cavalcante RA, Fragoso RM, Honorato DC, Patel AA. Evaluation of the thoracolumbar injury classification system in thoracic and lumbar spinal trauma. Spine (Phila Pa 1976) 2011;36:33–6. doi: 10.1097/BRS.0b013e3181c95047. [DOI] [PubMed] [Google Scholar]
- 22.Lee JY, Vaccaro AR, Schweitzer KM, Jr, Lim MR, Baron EM, Rampersaud R, et al. Assessment of injury to the thoracolumbar posterior ligamentous complex in the setting of normal-appearing plain radiography. Spine J. 2007;7:422–7. doi: 10.1016/j.spinee.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 23.Radcliff K, Su BW, Kepler CK, Rubin T, Shimer AL, Rihn JA, et al. Correlation of posterior ligamentous complex injury and neurological injury to loss of vertebral body height, kyphosis, and canal compromise. Spine (Phila Pa 1976) 2012;37:1142–50. doi: 10.1097/BRS.0b013e318240fcd3. [DOI] [PubMed] [Google Scholar]
- 24.Radcliff K, Kepler CK, Rubin TA, Maaieh M, Hilibrand AS, Harrop J, et al. Does the load-sharing classification predict ligamentous injury, neurological injury, and the need for surgery in patients with thoracolumbar burst fractures. Clinical article? J Neurosurg Spine. 2012;16:534–8. doi: 10.3171/2012.3.SPINE11570. [DOI] [PubMed] [Google Scholar]
- 25.Bracken MB, Shepard MJ, Collins WF, Holford TR, Young W, Baskin DS, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322:1405–11. doi: 10.1056/NEJM199005173222001. [DOI] [PubMed] [Google Scholar]
- 26.Nesathurai S. Steroids and spinal cord injury: Revisiting the NASCIS 2 and NASCIS 3 trials. J Trauma. 1998;45:1088–93. doi: 10.1097/00005373-199812000-00021. [DOI] [PubMed] [Google Scholar]
- 27.Botelho RV, Daniel JW, Boulosa JL, Colli BO, Farias Rde L, Moraes OJ, et al. Effectiveness of methylprednisolone in the acute phase of spinal cord injuries--a systematic review of randomized controlled trials. Rev Assoc Med Bras. 2009;55:729–37. doi: 10.1590/s0104-42302009000600019. [DOI] [PubMed] [Google Scholar]
- 28.Coleman WP, Benzel D, Cahill DW, Ducker T, Geisler F, Green B, et al. A critical appraisal of the reporting of the National Acute Spinal Cord Injury Studies (II and III) of methylprednisolone in acute spinal cord injury. J Spinal Disord. 2000;13:185–99. doi: 10.1097/00002517-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Joaquim AF, Patel AA. Relationships between the AO Spine and the Thoracolumbar Injury Classification System (TLICS): An analysis of the literature. J Spinal Cord Med. 2013 doi: 10.1179/2045772313Y.0000000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bailey CS, Dvorak MF, Thomas KC, Boyd MC, Paquett S, Kwon BK, et al. Comparison of thoracolumbosacral orthosis and no orthosis for the treatment of thoracolumbar burst fractures: Interim analysis of a multicenter randomized clinical equivalence trial. J Neurosurg Spine. 2009;11:295–303. doi: 10.3171/2009.3.SPINE08312. [DOI] [PubMed] [Google Scholar]
- 31.Giele BM, Wiertsema SH, Beelen A, van der Schaaf M, Lucas C, Been HD, et al. No evidence for the effectiveness of bracing in patients with thoracolumbar fractures. Acta Orthop. 2009;80:226–32. doi: 10.3109/17453670902875245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shamji MF, Roffey DM, Young DK, Reindl R, Wai EK. A pilot evaluation of the role of bracing in stable thoracolumbar burst fractures without neurologic deficit. J Spinal Disord Tech. 2012 doi: 10.1097/BSD.0b013e31826eacae. [DOI] [PubMed] [Google Scholar]
- 33.Bellabarba C, Fisher C, Chapman JR, Dettori JR, Norvell DC. Does early fracture fixation of thoracolumbar spine fractures decrease morbidity or mortality? Spine (Phila Pa 1976) 2010;35:S138–45. doi: 10.1097/BRS.0b013e3181d830c1. [DOI] [PubMed] [Google Scholar]
- 34.Furlan JC, Noonan V, Cadotte DW, Fehlings MG. Timing of decompressive surgery of spinal cord after traumatic spinal cord injury: An evidence-based examination of pre-clinical and clinical studies. J Neurotrauma. 2011;28:1371–99. doi: 10.1089/neu.2009.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fehlings MG, Vaccaro A, Wilson JR, Singh A, W Cadotte D, Harrop JS, et al. Early versus delayed decompression for traumatic cervical spinal cord injury: Results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS) PLoS One. 2012;7:e32037. doi: 10.1371/journal.pone.0032037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wood KB, Bohn D, Mehbod A. Anterior versus posterior treatment of stable thoracolumbar burst fractures without neurologic deficit: A prospective, randomized study. J Spinal Disord Tech. 2005;18:S15–23. doi: 10.1097/01.bsd.0000132287.65702.8a. [DOI] [PubMed] [Google Scholar]
- 37.Oner FC, Wood KB, Smith JS, Shaffrey CI. Therapeutic decision making in thoracolumbar spine trauma. Spine (Phila Pa 1976) 2010;35:S235–44. doi: 10.1097/BRS.0b013e3181f32734. [DOI] [PubMed] [Google Scholar]
- 38.P Oprel P, Tuinebreijer WE, Patka P, den Hartog D. Combined anterior-posterior surgery versus posterior surgery for thoracolumbar burst fractures: A systematic review of the literature. Open Orthop J. 2010;4:93–100. doi: 10.2174/1874325001004010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang XZ, Tian W, Liu B, Li Q, Zhang GL, Hu L, et al. Comparison of a paraspinal approach with a percutaneous approach in the treatment of thoracolumbar burst fractures with posterior ligamentous complex injury: A prospective randomized controlled trial. J Int Med Res. 2012;40:1343–56. doi: 10.1177/147323001204000413. [DOI] [PubMed] [Google Scholar]
- 40.Hwang JU, Hur JW, Lee JW, Kwon KY, Lee HK. Comparison of posterior fixation alone and supplementation with posterolateral fusion in thoracolumbar burst fractures. J Korean Neurosurg Soc. 2012;52:346–52. doi: 10.3340/jkns.2012.52.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korovessis P, Repantis T, Petsinis G, Iliopoulos P, Hadjipavlou A. Direct reduction of thoracolumbar burst fractures by means of balloon kyphoplasty with calcium phosphate and stabilization with pedicle-screw instrumentation and fusion. Spine (Phila Pa 1976) 2008;33:E100–8. doi: 10.1097/BRS.0b013e3181646b07. [DOI] [PubMed] [Google Scholar]
- 42.Beisse R. Endoscopic surgery on the thoracolumbar junction of the spine. Eur Spine J. 2010;19:S52–65. doi: 10.1007/s00586-009-1124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin B, Chen ZW, Guo ZM, Liu H, Yi ZK. Anterior approach versus posterior approach with subtotal corpectomy, decompression, and reconstruction of spine in the treatment of thoracolumbar burst fractures: A prospective randomized controlled study. J Spinal Disord Tech. 2011 doi: 10.1097/BSD.0b013e3182204c53. [DOI] [PubMed] [Google Scholar]
- 44.Harrop JS, Naroji S, Maltenfort MG, Ratliff JK, Tjoumakaris SI, Frank B, et al. Neurologic improvement after thoracic, thoracolumbar, and lumbar spinal cord (conus medullaris) injuries. Spine (Phila Pa 1976) 2011;36:21–5. doi: 10.1097/BRS.0b013e3181fd6b36. [DOI] [PubMed] [Google Scholar]


