Abstract
Background:
Current treatment guidelines support the role of lifestyle modification, in terms of increasing the quantity and quality of physical activity to achieve target glycemia in patients with type 2 diabetes mellitus.
Objective:
To assess the effect of structured exercise training and unstructured physical activity interventions on glycemic control.
Materials and Methods:
This was a randomized six-month exercise intervention study conducted with previously inactive 279 patients of type 2 diabetes mellitus. Before randomization, all enrolled T2DM participants (n: 300; 30 to 60 year old, having diabetes for more than a year with HbA1c levels of 6.5% or higher) entered a one-month run-in phase to reduce dropout and maintain adherence.
Results:
A recommendation to increase physical activity was beneficial (0.14% HbA1c reduction; P = 0.12), but was not bringing significantly declines in HbA1c, whereas, structured exercise training is associated with a significant HbA1c decline of 0.59%. (P = 0.030). In a subgroup analysis limited to participants with a baseline HbA1c value > 7%, both the unstructured (0. 48%; P = 0.04) and structured exercise training (0.77%; P < 0.01) groups experienced significant decline in HbA1c Vs the control, whereas among participants with baseline hemoglobin A1c values less than 7%, significant reduction occurred only in the structured exercise training group. Changes in blood pressure; total cholesterol, HDL-cholesterol (high-density lipoprotein), LDL-cholesterol (low-density lipoprotein) and the atherogenic index factors did not statistically significantly differ within (baseline to follow-up) and among groups.
Conclusion:
Supervised structured training was more efficacious than unstructured activity in achieving declines in HbA1c. Although both structured and unstructured training provide benefits, only the former was associated with significant reductions in HbA1c levels. Therefore, T2DM patients should be stimulated to participate in specifically designed exercise intervention programs.
Keywords: Hemoglobin A1c, physical activity, structured exercise, type 2 diabetes mellitus
INTRODUCTION
Diabetes, a global escalating public health problem, primarily because of the increasing prevalence, is estimated to affect 285 million individuals worldwide[1] (Approximately 90% have type 2 diabetes mellitus) and causes hundreds of billions of dollars of economic damage each year. Global estimates for the year 2030 predict a further growth of almost 50%.[2] In 2000, it is estimated that 2.8% of world's population had diabetes mellitus and that by 2030 this number will be 4.4% of the world's population. According to WHO[2] the ‘top’ three countries in terms of the number of type 2 diabetes mellitus (T2DM) individuals with diabetes are India (31.7 million in 2000; 79.4 million in 2030), China (20.8 million in 2000; 42.3 million in 2030) and the US (17.7 million in 2000; 30.3 million in 2030). This increase is a warning sign for Indian health care system to be vigilant for adequate diabetes mellitus management.
Type 2 diabetes mellitus is a complex and pleomorphic metabolic disorder, characterized by defects in insulin secretion and insulin action which lead to hyperglycemia.[3] With the onset of this chronic condition and the associated co-morbidities (hypertension, abdominal obesity, dyslipidemia and insulin resistance; together termed as metabolic syndrome), a life-long reduction in quality of life and premature mortality due to micro and macro-vascular complications can be expected.[4] Genetic background and environment factors are likely to be important in determining susceptibility to associated micro and macro-vascular complications, but exposure of tissues to chronic hyperglycemia is the main initiating factor. Thus, the primary therapeutic goal is to reduce plasma hyperglycemia. For many years, it is generally accepted that exercise is a cornerstone of diabetes management, along with dietary and pharmacological interventions. Based on a number of large randomized controlled trials, current guidelines from the American Diabetes Association (ADA)[5] acknowledge the therapeutic strength of exercise intervention. Although, there are guidelines on treatment patterns for T2DM patients, it appears that most patients with T2DM are treated pharmacologically.[6] It is not known whether this is a reflection of poor adherence to lifestyle modifications in T2DM patients or because clinician's perceptions of and experiences with lifestyle recommendations and interventions are less effective (for whatsoever reasons) for managing T2DM patients.
Presently available literature regarding the effects of lifestyle modification, in terms of increasing the quantity and quality of physical activity, on overall glycemic control independently or in combination with hypoglycemic drugs are scarce and less conclusive. The majority of studies which supplement weight reduction and exercise programs with appropriate drug therapy have been done on western population. There is a paucity of such studies in Indian population more so specifically in Gujarat population. In India, considering the economic/time constraint and other factors (such as availability of suitable training center, awareness etc), structured exercise training may be available only to a subset of patients with type 2 diabetes, increased physical activity (unstructured activity) is more feasible and can be easily followed.
Therefore, with the hypothesis that short-term six month regular physical activity (structured exercise/unstructured activity) undertaken by T2DM patients, with minimal diet modification may boost glycemic control, an attempt has been made in this study to assess the effect of structured exercise training and unstructured activity interventions on glycemic control along with other biochemical and anthropometric parameters in T2DM patients.
Primary outcome for the result was change in hemoglobin A1c value from baseline to the end of the intervention and secondary outcomes were measures of anthropometry, plasma lipid levels and blood pressure. In addition, we assessed the proportion of T2DM patients in whom cardiovascular risk factors (CVRFs)/profile were not under control as established by the NCEP-ATP III (National Cholesterol Education Program-Adult Treatment Panel III) guidelines.[7]
MATERIALS AND METHODS
This was a randomized six month exercise intervention study conducted from October 2011 to July 2012 (Box 1 shows the flow of participants from enrollment to follow–up) [Figure 1]. Before randomization, all enrolled T2DM participants (n: 300) entered a one-month run-in phase to reduce dropout and maintain adherence. Participants performed 15 min of aerobic exercise[8] and one set of nine resistance exercises[8] {four upper body exercises (bench press, seated row, shoulder press, and pull down), 3 leg exercises (leg press, extension and flexion), abdominal crunches and back extensions}, at moderate intensity[8] under professional trainer and physiotherapist. Only persons who attended >75% of the scheduled 24 run-in sessions were eligible for randomization. The study protocol was approved by the human research ethical committee and informed consent was obtained (after providing a detailed study overview) from all the patients before enrolling into the study.
Figure 1.
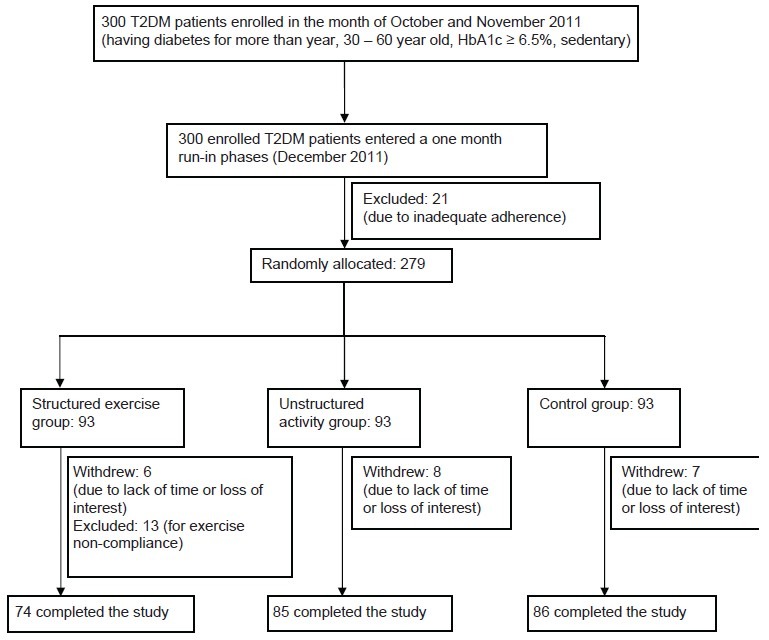
Participant flow
Inclusion criteria for enrollment includes sedentary, 30 to 60 year old adults of either sex with type 2 diabetes mellitus (for more than a year) of HbA1c levels of 6.5% or higher, residing in and around Ahmedabad (Gujarat), and attending the diabetic clinics at B. J. Medical College and Civil Hospital, Ahmedabad (Gujarat). Sedentary was defined as not exercising more than 20 min on three or more days a week. The history of diabetes mellitus was based on patient self report of a prior physician diagnosis. Patients with overt albuminuria, congestive cardiac failure, preexisting macro-vascular condition, any severe illness (such as malignancy, severe infection, respiratory disease, kidney disease, liver disease), impairment of speech, hearing, vision or cognition, suffering from a serious diabetes complication, using insulin, changes during the previous three months in oral hypoglycemic agents, continuous or periodic use of corticosteroids, pregnant females or who had given birth within the preceding six weeks, orthopedic constraints or any musculoskeletal injury or joint or peripheral vascular disease sufficient to impede exercise or who had participated in regular physical exercise (more than two 30 min sessions/week of moderate/vigorous aerobic exercise or one 30 min session/week of resistance training) during the preceding six months, serious exertion hypertension or any medical condition that prevented participants from adhering to the protocol or exercising safely, lack of approval by physician and patients showing disinterest were excluded from the study.
After one-month run-in phase, participants were randomly allocated (randomization sequence was computer generated) in equal numbers to the structured exercise training, unstructured activity and control groups, stratified by sex and age. Structured exercise training was defined as an intervention in which patients were engaged in planned, individualized and supervised exercise programs (combination of aerobic and resistance exercise). Unstructured activity was defined as an intervention in which patients were not engaged in supervised exercise training, but received advice to increase the “physical activity” which refers to any bodily movement produced by skeletal muscles that results in an expenditure of energy and includes a walk for 45 min/day, broad range of occupational, leisure and daily activities and control group in which patients reverted to pre-study physical activity levels. Later two groups were asked to maintain the said activity during the six-month study period.
Participants, physiotherapist and trainers could not feasibly be blinded to group assignment after randomization, but the main study outcomes were measured by blinded objective methods. Dietician recommended (based on standard guidelines)[6,9] a diet (which was not greatly different from individual's routine diet) to all participants that would not cause weight loss to minimize dietary variability among groups. Physicians were requested not to alter the medications (antihypertensive, lipid-altering, or hypoglycemic) during the six month intervention unless it was medically necessary and if any change occurs throughout the study, it was well documented.
Structured exercise training group participants followed an exercise plan consists of a combination of aerobic and resistance exercise, conducted under supervision of professional trainer and physiotherapist. Participants exercised six-times weekly,[6,10] Each session lasted for 45 min and training progressed gradually in intensity. Each exercise session had a five minute warm-ups and cool-down period, consisting of very light exercises and stretching, to allow a gradual warming/cooling of the muscles. During 12th week the exercise dose was reduced by one-third to provide a recuperation week.
Nine resistance exercises were performed on three non-consecutive days/week using weight-stacked machines (Multi Station Gym Khare Enterprises Pvt. Ltd, Sangli.). {The weight loading was set at 70-85% one repetition maximum, determined for each exercise at week 0 to assess the muscle strength.[11] One repetition maximum is defined and determined as: Following a low-intensity warm-up, participants performed four trials (separated by a one-minute resting interval) using varying moderate-heavy weights to determine the highest weight that could be lifted with only one repetition through the full range of motion with correct technique}. Each session consisting of two sets of four upper body exercises, three sets of three leg exercises and two sets each of abdominal crunches and back extensions (with one-min rest between sets). Each set consisted of 12 repetitions. Weight or resistance was increased by 5 to 10 pounds when the participant was able to complete 12 repetitions for each set of exercises on 2 consecutive exercise sessions while maintaining proper form. On other three days of week, aerobic activities were performed on a bicycle ergometer or treadmill. At week 0, participants self-selected a walking/cycling pace and the grade increased by 2% every 2 min until exhaustion. The same speed/grade was used for baseline and then the intensity was increased on a weekly basis.
All patients were studied as outpatient. Patients were interviewed for medical and nutritional history. Present and past history of each case was recorded in detail regarding their general information i.e., name, age, sex, address, religion, occupation, economic status, nutritional and personal habits, education, medication and history suggestive of any systemic illness. Each patient was then examined for various anthropometric parameters: Weight (Kg) and height (meters) were measured (using Omron digital body weight scale HN-286 and SECA 206 wall mounted metal tapes respectively). Body mass index (BMI) was calculated by weight (Kg)/height squared (m2).[12] Waist circumference was assessed in the standing position, midway between the highest point of the iliac crest and the lowest point of the costal margin in the midaxillary line. Hip circumference was measured at the level of the femoral greater trochanter. Total body fat (%) was measured using bioelectrical impedance analyzer (Omron HBF-362). All anthropometric measures reflect the average of 3 measurements (measured by same person on same instrument to avoid inter-instrument and inter personal variation). Blood pressure was measured three times in the seated position after 10 min of rest with a standard manual mercury sphygmomanometer (Diamond Deluxe Industrial Electronics and Products). The recorded pressure of the three measurements was averaged. Patients were assigned to a category of hypertensive status according to the Seventh Report of the Joint National Committee, JNC 7.[13] Age was defined as the age at the time of interview (though no documentary proof had been entertained) and the date of diagnosis of diabetes mellitus was obtained from the patient.
After an overnight fast of 12 h, venous sampling was done at baseline and at 6 m (post-intervention). Serum and plasma was separated by centrifugation of blood sample and were subjected for analytical procedures. Glucose (Glucose oxidase method, CV %: 3.4),[14] cholesterol (Cholesterol oxidase method, CV %: 3.9),[15] triglycerides (Enzymatic method, CV %: 3.6),[16] high-density lipoprotein cholesterol (HDL-C) (Phosphpotungstic method, CV %: 4.7)[17] low-density lipoprotein cholesterol (LDL-C) (CV %: 3.6)[18] and HbA1c (Immunoturbidimetric method, CV %: 3.9),[19] were measured in fully automated analyzer (Bayer express plus). Quality was controlled using standard solutions. To ensure safety, participants had monthly visits with a physician who reviewed glucose levels to identify hypoglycemic risk (HbA1c, fasting and 2 h post prandial glucose levels was assessed monthly for the same).
The prevalence of the metabolic syndrome was assessed according to the NCEP-ATP III criteria;[7] when three or more of the following five conditions were present: Abdominal obesity (waist circumference >102 cm in men, >88 cm in women); serum triglycerides equal to or greater than 150 mg/dl; HDL cholesterol less than 39 mg/dl in men and 45 mg/dl in women; systolic blood pressure equal to or greater than 130 mm Hg and/or diastolic blood pressure equal to or greater than 85 mm Hg; and fasting plasma glucose >110 mg/dl or use of hypoglycemic medication. In addition, we assessed the proportion of participants in whom CVRFs were not under control as established by the NCEP-ATP III guidelines.[7]
All analyses were performed using SPSS statistical software (SPSS, version 15.0). Prior to hypothesis testing, data were examined for normality. Non–normally distributed variables were logarithmically transformed before analysis. Two-group comparisons were made using X2 or Fisher's exact tests (when any expected cell frequency was <5) for categorical variables and Student's t-tests or one-way ANOVA for continuous variables. For all analyses, two-sided probability values < 0.05 were considered statistically. Power and sample size calculation yielded 61 participants/group. It was based on a predicted HbA1c difference of 0.65 HbA1c units with an SD of effect of 1.2 HbA1c units, α=0.05, 1− β=0.85 and an expected dropout rate of 15%. This study exceeded this sample size.
RESULTS
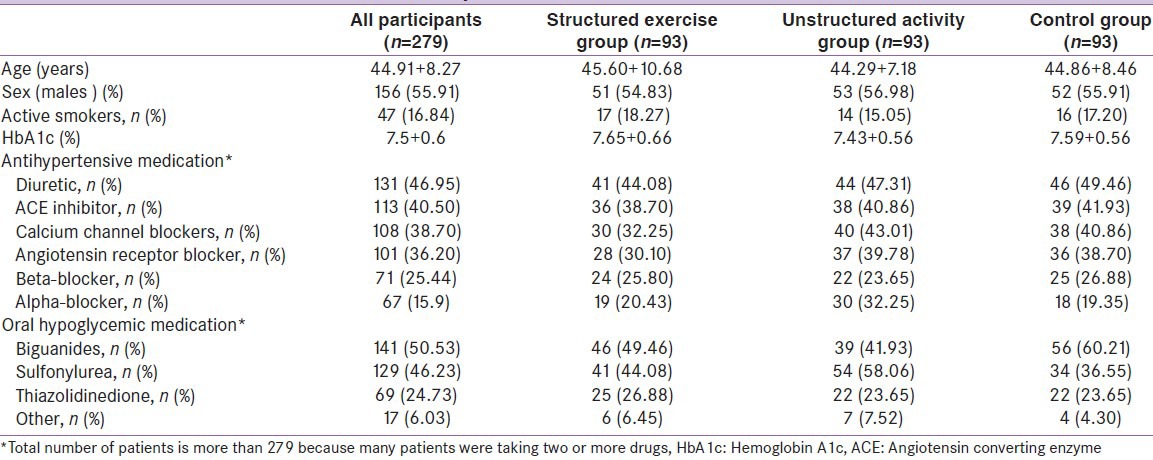
We enrolled 300 patients (56% males) with T2DM, 21 were excluded during run-in phase due to inadequate adherence. Of the 279 randomized participants, 21 withdrew {due to lack of time or loss of interest (8 from unstructured activity group; 6 from structured exercise training group and seven from control group)} and 13 participants were excluded for exercise noncompliance (Compliance with structured exercise training was defined as 75% of prescribed sessions and was determined as the number of exercise sessions completed at the prescribed training loads per total number of prescribed sessions). There was no difference between exercises versus non-exercise groups in the number of participants who withdrew (P > 0.66). From baseline to end, the median exercise training attendance was 84%. Table 1 shows the clinical characteristics of the randomized patients. One of every 6 patients reported to actively smoke. The mean reported duration of diabetes mellitus in the randomized participants was 6.1 + 4.2 years with a HbA1c level of 7.5 + 0.6% and there were no significant differences in age, duration of the disease, weight, BMI, and sex distribution between groups (P > 0.46) at baseline. Elevated body weight was a concomitant health disorder for most patients. Ninety two percent showed a body mass index equal to or greater than 25.0 kg/m2, and 56% of the patients (95% confidence interval [CI], 51-61%) were obese (BMI > 30.0 kg/m2). Baseline systolic blood pressure was well controlled, most likely because of the high percentage of patients taking medications. Only 51 (18.27%) of the patients were not receiving antihypertensive medications. Two or more antihypertensive drugs were being taken by 57% of the patients and 12% were treated with four different blood-pressure lowering drugs. As for glycemic control, biguanides and sulfonylurea group of drugs were taken by most patients.
Table 1.
Clinical characteristics of randomized patients at baseline
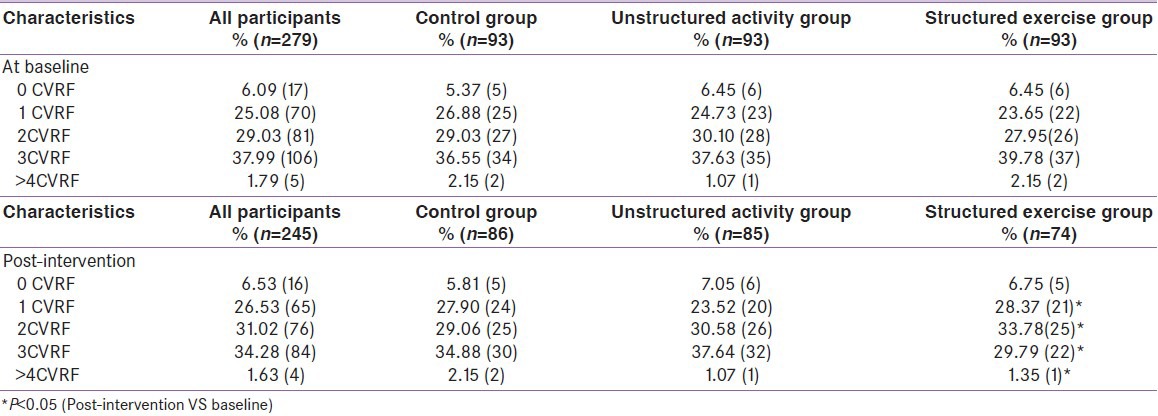
Tables 2 and 3 shows overall results for baseline, post- intervention and change in various primary and secondary outcome parameters across the groups respectively, providing details on within group changes and intergroup analyses. Unstructured activity alone was not found to have a statistically significant effect on HbA1c reduction (0.14%, 95% CI, 0.09-0.22%; P = 0.12). Structured exercise training reduced HbA1c by 0.59% (95% CI, 0.52-0.68; P = 0.03), which is considered both statistically and clinically significant. The absolute change in HbA1c in the structured exercise training group Vs the control group was 0.69% (95% CI, 0.62-0.75%; P = 0.02) whereas in unstructured activity group changes in HbA1c was not significant compared with those in the control group 0.24% (95% CI, 0.19 - 0.31%; P = 0.09). In a subgroup analysis limited to participants with a baseline HbA1c value >7%, both the unstructured (0. 48% (95% CI, 0.42-0.54%; P = 0.04) and structured exercise training (0.77% (95% CI, 0.69 -0.83%; P < 0.01)) groups experienced significant decline in HbA1c Vs the control, whereas among participants with baseline hemoglobin A1c values less than 7%, significant reduction occurred only in the structured exercise training group. Even after excluding 17 participants due to any changes in oral hypoglycemic medications, results were similar to those of the overall study sample.
Table 2.
Baseline and post-intervention values of primary and secondary outcomes variables
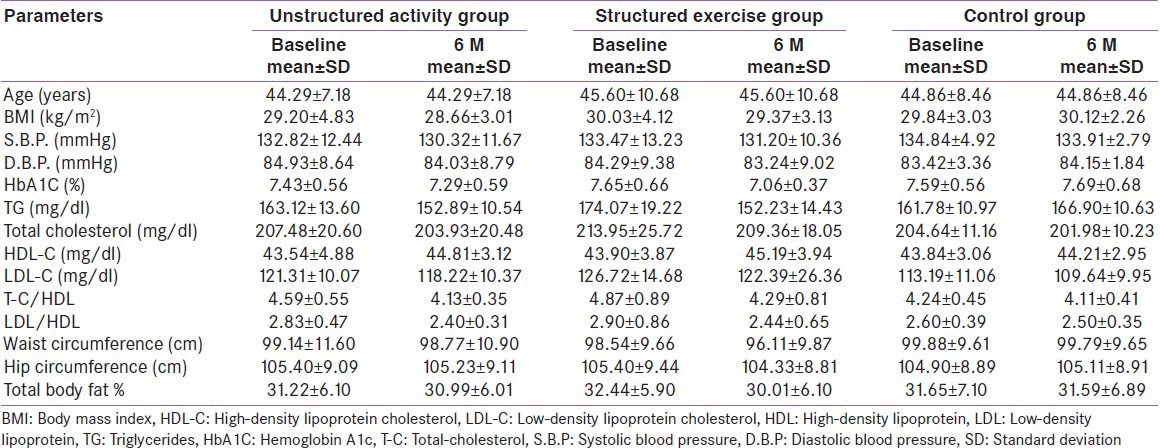
Table 3.
Baseline to post-intervention changes in primary and secondary outcomes variables
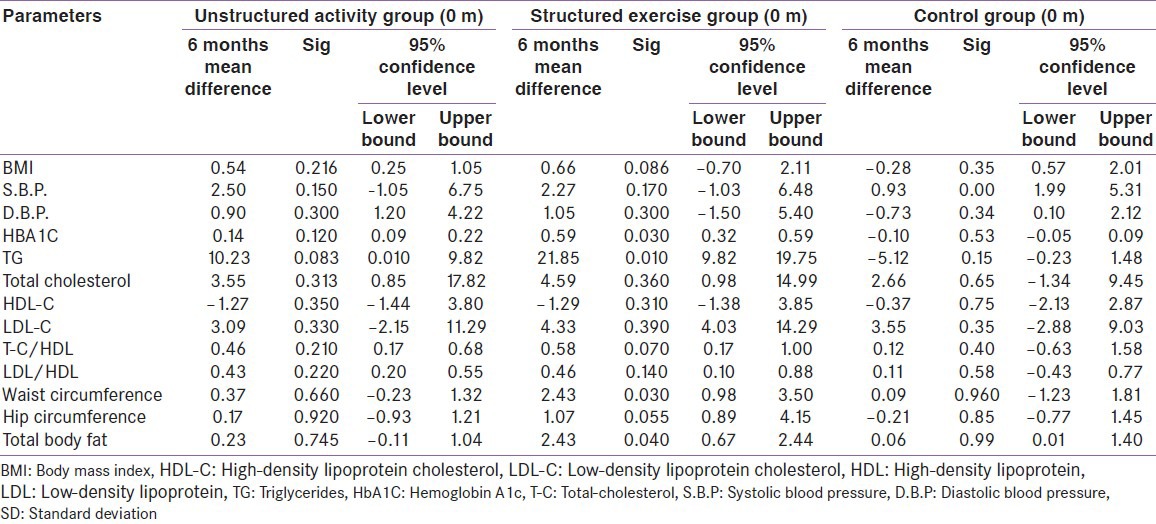
Changes in blood pressure; total cholesterol, HDL cholesterol, LDL-cholesterol and the atherogenic index factors (total cholesterol/HDL-cholesterol and LDL-cholesterol/HDL-cholesterol ratio) did not statistically significantly differ within (baseline to follow-up) and among groups. However, structured exercise was related to a significant decrease of 21.85 mg/dl post-intervention (95% CI, 13.82-29.75; P < 0.05) in triglycerides.
The average baseline to post-intervention changes in body weight were −0.5kg (P = 0.79) in the control group, −3.7 kg (P = 0.21) in the structured exercise training group and 1.4 kg (P = 0.48) in the unstructured activity group. In the body mass index comparisons for the exercise groups Vs control group, no significant post-intervention differences were found. However, total body fat percentage did show significant improvement in structured exercise training group (P = 0.04), as did waist circumference (P = 0.030).
At baseline, the metabolic syndrome was diagnosed in 71.9% (95% CI, 67.1-76.8%) of participants, as per NCEP-ATP III criteria.[7] Most patients showed at least one uncontrolled CVRF [Figure 2 and Table 4]. In fact, only 17 patients (6.09%, 95% CI, 5.7-8.8%) had all CVRFs under control. Approximately two thirds (192 patients, 68.81%; 95% CI, 64.6-72.9%) showed two or more uncontrolled CVRFs. Of the CVRFs, lipids were not optimally controlled in 60% of the patients (95% CI, 55-65%); diabetes was not optimally controlled (HbA1c < 7.5%) in 61% (95% CI, 53-67%); arterial hypertension was not optimally controlled in 36% (95% CI, 27-46%); weight was not optimally controlled in 56% (95% CI, 45-55%); and 17% (95% CI, 13-20%) were active smokers. Intergroup differences in prevalence of metabolic syndrome and CVRFs were not statistically significant. Baseline to post-intervention changes in the prevalence of CVRFs were significant only in structured exercise training group (>4CVRFs: 2.15% Vs 1.35%, P < 0.05; 3CVRFs: 39.78% Vs 29.79%, P < 0.05; 2CVRF: 27.95% Vs 33.78%, P < 0.05) [Table 4], even here changes in proportion was mainly because of significant improved glycemic control.
Figure 2.
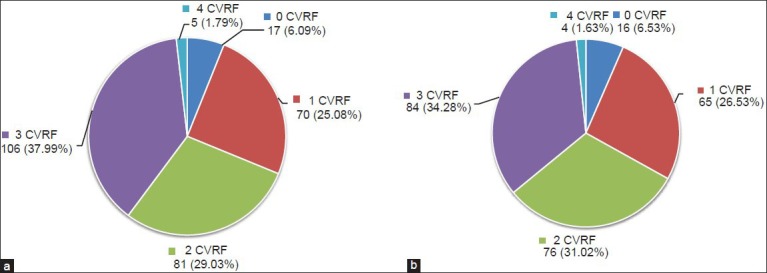
(a): At baseline; (b): Post-intervention: Number of uncontrolled CVRFs. Values are number of patients (%)
Table 4.
Number of uncontrolled cardiovascular risk factors
DISCUSSION
Based on a number of large randomized controlled trials, current clinical guidelines[5,20,21] acknowledge the therapeutic strength of exercise intervention. Despite this recommendation, it is estimated that only 30-40% of people with T2DM[22] engage in regular exercise program indicating that their rate of participation is significantly below.
The primary finding from this randomized, exercise trial involving individuals with type 2 diabetes is that supervised structured (combination of aerobic and resistance exercises) exercise training were more efficacious than unstructured activity (increased physical activity) in achieving declines in HbA1c. Although both structured and unstructured training provide benefits, only the former was associated with significant reductions in HbA1c levels. Though in subgroup analysis (in either the structured or the unstructured group), we found that exercise-induced improvements in glycemic control were greater among persons with higher baseline hemoglobin A1c values (>7%). Among persons with lower baseline hemoglobin A1c values (<7%), only combined aerobic and resistance training improved values; unstructured training alone did not. The potential importance of good glycemic control for the reduction of cardiovascular disease risk was supported in a meta-regression study,[23] which demonstrated an exponential relationship between fasting glucose concentrations and the incidence of cardiovascular events. Furthermore, cumulative benefit across other outcomes was also greater in the structured training group compared with other participating groups, thus may results in substantial improvement in CVD risk factors.
To our knowledge, this is the first randomized trial in Gujarat population involving individuals with type 2 diabetes to assess the effect of structured exercise training and unstructured activity interventions on glycemic control and to determine whether unstructured activity is associated with similar declines in HbA1c as compared with those associated with structured exercise. Our results demonstrate that in patients with type 2 diabetes, a recommendation to increase physical activity was beneficial (0.14% HbA1c reduction), but was not bringing significantly declines in HbA1c, whereas, structured training is associated with a HbA1c decline of 0.59%. (P = 0.030). A reduction in HbA1c of this magnitude is clinically significant and close to the difference between conventional and intensive glucose-lowering therapy in the United Kingdom Prospective Diabetes Study (UKPDS).[24] In the UKPDS, subjects receiving intensive treatment with insulin or sulfonylurea's had HbA1c averaging 0.9% below the conventional treatment (7.0% vs 7.9%; P < 001) and had significant reduction in diabetes-related clinical end points (40.9 Vs 46 events per 1000 patient-years; P =0.03).[25,26]
For each 1% increase in the level of HbA1c, the relative risk of CVD increases by 1.18%,[27] whereas each 1% decrease in HbA1c levels is associated with a 37% reduction in micro-vascular complications and a 14% reduction in myocardial infarctions.[28] Thus, our observed 0.59% reduction in HbA1c levels might be expected to produce 21% decrease in risk of micro-vascular complications and an 8% reduction in cardiovascular disease risk.
Because HbA1c reduction in type 2 diabetes is associated with improved insulin resistance[20] and increased physical workout (structured exercise/unstructured activity) have distinct mechanisms to elicit these effects,[20] it is expected that these interventions would result in greater metabolic effects, virtually bringing beneficial effects on all aspects of metabolic syndrome, one way or other. However, we did not find statistical support for the existence of a relationship between these exercise approaches (short term structured/unstructured) and an improved weight/BMI, or blood pressure, total cholesterol, HDL-C and LDL-C levels. There are two possible reasons for this discrepancy. Firstly, because many patients were on antihypertensive and lipid lowering drugs, and over the last decade, both lipid lowering therapy[29,30] and blood pressure lowering therapies[31,32] have been proven effective to improve cardiovascular outcome in T2DM patients. The effectiveness of these drugs may explain why the additive benefits of intensive glycemic control were not observed in this study. Similar results were obtained in even large and long-term clinical trials such as ACCORD, ADVANCE and VADT.[33] Other reason may be that exercise approaches employed in this study were of short duration. To achieve greater changes, perhaps exercise interventions prescribing higher levels of exercise quantity might be necessary[34] to positively affect body composition (weight/BMI) and lipid levels in individuals with T2DM on antihypertensive and/or lipid lowering drugs. Despite this, one of the important outcomes of this study is that exercise does not need to reduce body weight to have a beneficial impact on glycemic control. Exercise training decreases hepatic and muscle insulin resistance and increases glucose disposal through a number of mechanisms that would not necessarily be associated with body weight changes. The mechanisms were extensively reviewed by Ivy et al.,[35] and include increased post-receptor insulin signaling,[36] increased glucose transporter protein and messenger RNA,[37] increased activity of glycogen synthase[38] and hexokinase,[39] decreased release and increased clearance of free fatty acids,[35] increased muscle glucose delivery due to increased muscle capillary density[39,40,41] and changes in muscle composition favoring increased glucose disposal.[42,43]
Nonetheless, this study has few limitations. Firstly, sampling may not be representative to all T2DM patients as the participants were probably more adherent to exercise and healthier on average than the general population with type 2 diabetes, as we excluded patients who were receiving insulin or who had advanced diabetes complications thus findings cannot be generalized to patients who cannot or do not wish to undertake exercise programs. Secondly, effect of different medications (antihypertensive, hypoglycemic and lipid lowering) were not taken into account, and fact is that a significant number of patients were receiving antihypertensive and lipid lowering drugs along with hypoglycemic medication and it well known that different drugs individually or in combination affect glucose metabolism (e.g., diuretics and beta blockers negatively affect glucose metabolism. In contrast, angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers, calcium antagonists, and alpha-receptor blockers are thought to be metabolically neutral.). Still, the results of our study are particularly relevant, as our trial was not designed to study effects of different medications, rather our study have contributed to disclose metabolic changes in T2DM patients on routinely prescribed drugs. Thirdly, the proxy definition of diabetes mellitus was used in the study and auto antibodies screening such as Anti-GAD analyses was not assessed for patients. Fourthly, unstructured activity group did not receive a similar amount of supervision. Still, taken together, these results provide important information for clinical practice. Future research should include longer interventions with better quantification of body composition changes.
CONCLUSION
This RCT demonstrates important findings regarding the prescription of exercise training. First, unstructured activity and structured training, each are associated with HbA1c decrease, but the magnitude of this reduction is different across the two training modalities. Second, the extent of HbA1c reduction is positively related to the baseline value of HbA1c, combined aerobic and strength training, may be the preferred first-line treatment option for individuals with lower baseline HbA1c values, but if glycemic control is poor, then even increased physical activity would also improve the hemoglobin A1c value, but the structured training having combination of aerobic and resistance exercise would be better. Based on this, it is concluded that T2DM patients should be stimulated to participate in specifically designed exercise intervention programs. More clinical research is warranted to establish the efficacy of exercise intervention in a more differentiated approach for type 2 diabetes subpopulations within different stages of the disease and various levels of co-morbidity. In the interim, our analysis adds support to the idea that exercise is a cornerstone of diabetes therapy and findings of this study strongly support the ADA guidelines[20] recommendation that in the treatment regimen of T2DM, optimal physical activity programs consist of regular physical activity combined with structured training must be added to supplement the effect of pharmacological intervention.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Shaw JE, Sicree RA, Zimmet PZ. Diabetes Atlas-Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.Ferrannini E. Insulin resistance versus insulin deficiency in non-insulin-dependent diabetes mellitus: Problems and prospects. Endocr Rev. 1998;19:477–90. doi: 10.1210/edrv.19.4.0336. [DOI] [PubMed] [Google Scholar]
- 4.Pavkov ME, Bennett PH, Knowler WC, Krakoff J, Sievers ML, Nelson RG. Effect of youth-onset type 2 diabetes mellitus on incidence of end-stage renal disease and mortality in young and middle-aged Pima Indians. JAMA. 2006;296:421–6. doi: 10.1001/jama.296.4.421. [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association: Standards of medical care in diabetes-2011. Diabetes Care. 2011;34:S11–61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Diabetes Association: Standards of Medical Care in diabetes-2010. Diabetes Care. 2010;33:S11–61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Executive summary: The 3rd report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 8.Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C. Physical Activity/Exercise and Type 2 Diabetes. Diabetes Care. 2004;27:2518–39. doi: 10.2337/diacare.27.10.2518. [DOI] [PubMed] [Google Scholar]
- 9.National Nutrition Committee CDA. Guidelines for the nutritional management of diabetes mellitus in the new millennium: A position statement by the Canadian Diabetes Association. Can J Diabetes Care. 1999;23:56–69. [Google Scholar]
- 10.van Dijk JW, Tummers K, Stehouwer CD, Hartgens F, van Loon LJ. Exercise Therapy in Type 2 Diabetes. Is daily exercise required to optimize glycemic control. Diabetes Care. 2012;35:948–54. doi: 10.2337/dc11-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verdijk LB, Van Loon L, Meijer K, Savelberg HH. One-repetition maximum strength test represents a valid means to assess leg strength in vivo in humans. J Sports Sci. 2009;27:59–68. doi: 10.1080/02640410802428089. [DOI] [PubMed] [Google Scholar]
- 12.World health organization expert consultation. Appropriate body mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 13.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 14.Trinder P. Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J Clin Pathol. 1969;22:158–61. doi: 10.1136/jcp.22.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allain CC, Poon LS, Chan CSG, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–5. [PubMed] [Google Scholar]
- 16.Fossati P, Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem. 1982;28:2077–80. [PubMed] [Google Scholar]
- 17.Burstein M, Morfin R. Precipitation of alpha lipoproteins in serum by sodium phosphotungstate in the presence of magnesium chloride. Life Sci. 1969;8:345–8. doi: 10.1016/0024-3205(69)90056-3. [DOI] [PubMed] [Google Scholar]
- 18.Assmann G, Jabs HU, Kohnert U, Nolte W, Schriewer H. LDL-cholesterol determination in blood serum following precipitation of LDL with polyvinyl sulfate. Clin Chim Acta. 1984;140:77–83. doi: 10.1016/0009-8981(84)90153-0. [DOI] [PubMed] [Google Scholar]
- 19.Metus P, Ruzzante N, Bonvicini P, Meneghetti M, Zaninotto M, Plebani M. Immunoturbidimetric assay of glycated hemoglobin. J Clin Lab Anal. 1999;13:5–8. doi: 10.1002/(SICI)1098-2825(1999)13:1<5::AID-JCLA2>3.0.CO;2-S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, et al. American College of Sports Medicine; American Diabetes Association. Exercise and type 2 diabetes: The American College of Sports Medicine and the American Diabetes Association: Joint position statement. Diabetes Care. 2010;33:147–67. doi: 10.2337/dc10-9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Micheli A. Italian standards for diabetes mellitus 2007: Executive summary. Acta Diabetol. 2008;45:107–27. doi: 10.1007/s00592-008-0030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plotnikoff RC, Johnson ST, Loucaides CA, Bauman AE, Karunamuni ND, Pickering MA. Population-based estimates of physical activity for adults with type 2 diabetes: A cautionary tale of potential confounding by weight status. J Obes. 2011;1:1–5. doi: 10.1155/2011/561432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. Diabetes Care. 1999;22:233–40. doi: 10.2337/diacare.22.2.233. [DOI] [PubMed] [Google Scholar]
- 24.UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 25.UK Prospective Diabetes Study Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–65. [PubMed] [Google Scholar]
- 26.King P, Peacock I, Donnelly R. The UK Prospective Diabetes Study (UKPDS): Clinical and therapeutic implications for type 2 diabetes. Br J Clin Pharmacol. 1999;48:643–8. doi: 10.1046/j.1365-2125.1999.00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, et al. Meta-analysis: Glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004;141:421–31. doi: 10.7326/0003-4819-141-6-200409210-00007. [DOI] [PubMed] [Google Scholar]
- 28.Church TS, Blair SN, Cocreham S, Neil Johannsen BS, Johnson W, Kramer K, et al. Effects of Aerobic and Resistance Training on Hemoglobin A1c Levels in Patients With Type 2 Diabetes. JAMA. 2010;304:2253–62. doi: 10.1001/jama.2010.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, et al. CARDS investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): Multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–96. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 30.Scott R, O’Brien R, Fulcher G, Pardy C, D’Emden M, Tse D, et al. Effects of fenofibrate treatment on cardiovascular disease risk in 9,795 individuals with type 2 diabetes and various components of the metabolic syndrome: The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetes Care. 2009;32:493–8. doi: 10.2337/dc08-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel A, MacMahon S, Chalmers J, Neal B, Woodward M, Billot L, et al. ADVANCE Collaborative Group. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): A randomised controlled trial. Lancet. 2007;70:829–40. doi: 10.1016/S0140-6736(07)61303-8. [DOI] [PubMed] [Google Scholar]
- 32.Turner R, Holman R, Stratton I, Cull C, Frighi V, Manley S, et al. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. Br Med J. 1998;317:703–13. [PMC free article] [PubMed] [Google Scholar]
- 33.Skyler JS, Bergenstal R, Bonow RO, Buse J, Deedwania P, Gale EM, et al. Intensive glycemic control and the prevention of cardiovascular events: Implications of the ACCORD, ADVANCE, and VA diabetes trials: A position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care. 2009;32:187–92. doi: 10.2337/dc08-9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kraus WE, Houmard JA, Duscha BD, Knetzger KJ, Wharton MB, McCartney JS, et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347:1483–92. doi: 10.1056/NEJMoa020194. [DOI] [PubMed] [Google Scholar]
- 35.Ivy JL, Zderic TW, Fogt DL. Prevention and treatment of non-insulin-dependent diabetes mellitus. Exerc Sport Sci Rev. 1999;27:1–35. [PubMed] [Google Scholar]
- 36.Dela F, Handberg A, Mikines KJ, Vinten J, Galbo H. GLUT4 and insulin receptor binding and kinase activity in trained human muscle. J Physiol. 1993;469:615–24. doi: 10.1113/jphysiol.1993.sp019833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dela F, Ploug T, Handberg A, Petersen LN, Larsen JJ, Mikines KJ, et al. Physical training increases muscle GLUT4 protein and mRNA in patients with NIDDM. Diabetes. 1994;43:862–5. doi: 10.2337/diab.43.7.862. [DOI] [PubMed] [Google Scholar]
- 38.Ebeling P, Bourey R, Koranyi L, Tuominen JA, Groop LC, Henriksson J, et al. Mechanism of enhanced insulin sensitivity in athletes: Increased blood flow, muscle glucose transport protein (GLUT-4) concentration, and glycogen synthase activity. J Clin Invest. 1993;92:1623–31. doi: 10.1172/JCI116747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coggan AR, Spina RJ, Kohrt WM, Holloszy JO. Effect of prolonged exercise on muscle citrate concentration before and after endurance training in men. Am J Physiol. 1993;264:E215–20. doi: 10.1152/ajpendo.1993.264.2.E215. [DOI] [PubMed] [Google Scholar]
- 40.Mandroukas K, Krotkiewski M, Hedberg M, Wroblewski Z, Bjorntorp P, Grimby G. Physical training in obese women: Effects of muscle morphology, biochemistry and function. Eur J Appl Physiol Occup Physiol. 1984;52:355–61. doi: 10.1007/BF00943363. [DOI] [PubMed] [Google Scholar]
- 41.Saltin B, Henriksson J, Nygaard E, Andersen P, Jansson E. Fiber types and metabolic potentials of skeletal muscles in sedentary man and endurance runners. Ann N Y Acad Sci. 1977;301:3–29. doi: 10.1111/j.1749-6632.1977.tb38182.x. [DOI] [PubMed] [Google Scholar]
- 42.Andersson A, Sjödin A, Olsson R, Vessby B. Effects of physical exercise on phospholipid fatty acid composition in skeletal muscle. Am J Physiol. 1998;274:E432–8. doi: 10.1152/ajpendo.1998.274.3.E432. [DOI] [PubMed] [Google Scholar]
- 43.Krotkiewski M, Björntorp P. Muscle tissue in obesity with different distribution of adipose tissue: Effects of physical training. Int J Obes. 1986;10:331–41. [PubMed] [Google Scholar]


