Abstract
Context:
Low testosterone levels are associated with an atherogenic lipid profile and may contribute to the pathogenesis of atherosclerosis.
Aims:
Our study aimed to investigate the relationship between serum total testosterone (TT) levels and lipid profile in angiographically confirmed coronary artery disease (CAD) in men.
Settings and Design:
This is a case-control hospital-based study at Teaching Hospital, Karapitiya, Galle, Sri Lanka.
Materials and Methods:
Two hundred and six men, 103 with angiographically proven CAD and 103 healthy men as a control group were studied. The serum levels of TT and lipids were assessed.
Statistical Analysis:
Data were analyzed using Minitab software (version 15 for Windows).
Results:
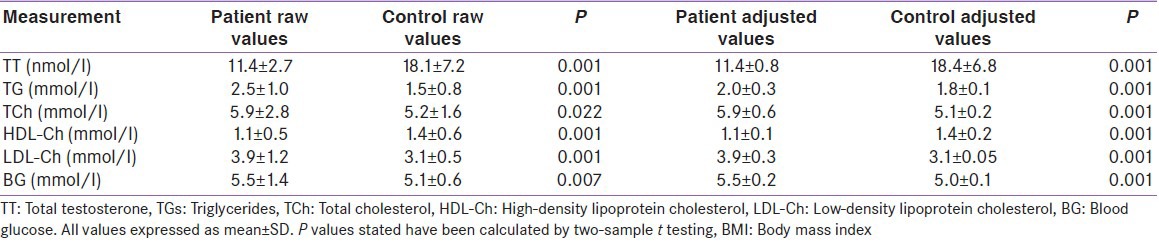
The mean concentrations of lipid parameters of patients and controls were as follows: Serum total cholesterol (TCh), 5.9 ± 2.8 vs. 5.2 ± 1.6 mmol/l (P = 0.022), low-density lipoprotein cholesterol (LDL-Ch), 3.9 ± 1.2 vs. 3.1 ± 0.5 mmol/l (P = 0.001), high-density lipoprotein cholesterol (HDL-Ch), 1.1 ± 0.5 vs. 1.4 ± 0.6 mmol/l (P = 0.001), and TGs, 2.0 ± 1.0 vs. 1.5 ± 0.8 mmol/l (P = 0.001); lipid levels were significantly different between the two groups. The mean levels of TT in the patients and controls were 11.4 ± 2.7 vs. 18.1 ± 7.2 nmol/l (P = 0.001), significantly different. Among CAD patients, a significant positive association was found between testosterone and HDL-Ch (r = 0.623, P = 0.001), whereas a negative association was found with LDL-Ch (r = -0.579, P = 0.001).
Conclusions:
Low levels of TT in men with CAD that appear together with an atherogenic lipid milieu may be involved in the pathogenesis of CAD. The observed association between testosterone and HDL-Ch suggests a protective effect of the hormone.
Keywords: Coronary artery disease, lipid profile, testosterone
INTRODUCTION
Coronary artery disease (CAD) occurs more frequently in men than in women before menopause. Despite various hypotheses, the reason for this phenomenon remains unknown.[1] However, androgens are thought to be responsible for the higher prevalence of CAD in men. Sex hormones have shown to regulate lipoprotein metabolism and are likely to influence the development of CAD.[2] The difference may result from the negative influence of androgens and the positive influence of estrogens on the lipid profile.[3]
On the other hand, numerous studies have shown that a deficiency of endogenous testosterone, dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS) in men is associated with CAD and its risk factors as metabolic syndrome: Insulin resistance, atherogenic lipid profile, hypertension, and visceral obesity.[4,5,6,7,8] Moreover, physiological concentrations of endogenous testosterone exhibited a positive correlation with high-density lipoprotein cholesterol (HDL-Ch) levels.[9,10,11,12,13] Most of the studies done were on healthy men and the literature on the relationship of testosterone with lipid profile in patients with CAD is scarce.
Therefore our study aimed to investigate the relationship between serum total testosterone (TT) levels and lipid parameters in angiographically confirmed CAD patients.
MATERIALS AND METHODS
Study setting
Patients with angiographically proven CAD who were admitted for coronary artery bypass graft were selected from the Cardiothoracic Unit, Teaching Hospital, Karapitiya. A group of controls who were in the same age range were recruited from the surgical units of Teaching Hospital, Karapitiya.
Study population and data collection
This clinical protocol was approved by the Ethical Review Committee of Faculty of Medicine, University of Ruhuna, Galle, Sri Lanka, and was conducted according to the ethical guidelines outlined in the Declaration of Helsinki. The case group (age ranged from 30 to 70 years) included 103 consecutive male patients with angiographically diagnosed CAD, awaiting coronary artery bypass graft. The exclusion criteria were as follows: History of recent surgery or major trauma (within 3 months) or history of acute coronary syndrome in the past 3 months, malignancy, chronic inflammatory disorders, current acute severe infections (C-reactive protein level more than 10 mg/dl), dementia or any structural damage to the central nervous system, rrenal dysfunction (was defined as a serum creatinine concentration more than 2 mg/dl (177 mmol/l), chronic liver disease, alcohol dependency based on the CAGE[14] criteria and current therapy with drugs that can interact with serum testosterone.
A group of controls were selected from patients awaiting minor surgeries who were in the same age range without clinically manifested CAD or major CAD risk factors (diabetes mellitus, hypertension, dyslipidemia/hypercholesterolemia, death of a first-degree relative with a premature myocardial infarction, smoking) and with normal ECG. The other exclusion criteria used in the selection of the case group were also applicable to the controls.
An interviewer-administered questionnaire was used to collect the following information: Socio-demographic data; risk factors such as hypertension, diabetes mellitus, cigarette smoking, hyperlipidemia, family history of coronary heart disease, previous myocardial infarction and medications; Weight, height, waist circumference, hip circumference; and systolic and diastolic blood pressures (mean of three consecutive measurements) were measured. The same investigator performed all measurements using the same instruments.
Sample collection and biochemical investigation
Blood samples for analysis were obtained in the morning after overnight fast. Separated sera were aliquoted and stored in a freezer at -70°C until analysis for testosterone.
Fasting plasma glucose, serum total cholesterol (TCh), triglycerides (TGs), HDL-Ch and LDL-Ch were estimated by enzyme-based colorimetric methods using commercial kits (ProDia International UAE, European authorized representative: ID consulting service e.K. Korbach/Germany).
The serum levels of TT were measured using a commercial EIA kit (Pathozyme Testosterone; Omega Diagnostics Ltd, Omega House, Hillfoots Business Village, Alva FK12 5DQ, Scotland, UK.; Star Fax 1000), according to the manufacturer's instructions and with the lowest detection limit of 0.06 ng/ml and a coefficient of variation ≤10%.
Statistical analysis
Data were analyzed using Minitab version 15 for Windows. Categorical baseline data displayed in Table 1 were analyzed by χ2 -test. Numerical data, displayed in Table 2, comparing cases and controls were analyzed by two-sample t-tests for independent samples assuming unequal variance. Adjusted means of hormone and lipid levels were calculated using multiple regression in which the dependent variables were TT and lipids, and the independent variables were age, BMI and case/control status. Pearson correlation coefficient was used to assess the relationship between serum TT concentrations and lipid parameters. All values were expressed as mean (± standard deviation) or percentages. Statistical significance was defined as P < 0.05.
Table 1.
Baseline characteristics in cases vs controls

Table 2.
Comparison of laboratory findings before and after adjustment for age and BMI
RESULTS
The study recruited 206 male patients, which included 103 angiographically proven CAD patients awaiting coronary artery bypass graft and 103 otherwise healthy patients awaiting minor surgery. The CAD patients were in the stable phase of CAD and no patients had a myocardial infarction within the last three months. As shown in Table 1, there were significant differences in mean age, BMI, waist circumference, hip circumference and waist-hip ratio, history of risk factors, and medication among the two groups (all P < 0.05).
Smoking was reported in 78.6% and 32% of the CAD patient group and the control group, respectively, but CAD patients who were awaiting coronary artery bypass graft had been ex-smokers for about 3 months and control group individuals had given up smoking more than one year ago. The traditional risk factors of CAD were more prevalent among the cases compared with the controls, and 95.1% of the CAD patients were on statin treatment.
According to Table 2, TT level, fasting blood glucose levels and level of lipids (TGs, TCh, HDL-Ch and LDL-Ch) among the two groups were significantly different (all P < 0.05).
The TT, FBS and lipid parameters between the two groups remained significantly different after adjustment for clinical covariates [Table 2]. A significant negative correlation was seen between total cholesterol and testosterone (r = -0.280; P = 0.004) [Table 3 and Figure 1a] following adjustments of covariates, but the negative relationship between TT and TGs remained insignificant (r = −0.060; P = 0.540 [Table 3]) in the case group. A significant positive correlation was found between the serum levels of HDL-Ch and testosterone (r = 0.893; P = 0.001 [Table 3 and Figure 1b]), whereas a negative correlation was observed between LDL-Ch and testosterone (r = -0.987; P = 0.001) [Table 3 and Figure 1c] in the patient group. It showed that the correlation had become stronger following the adjustments.
Table 3.
Relationship between serum TT levels and lipid levels in CAD patients
Figure 1.
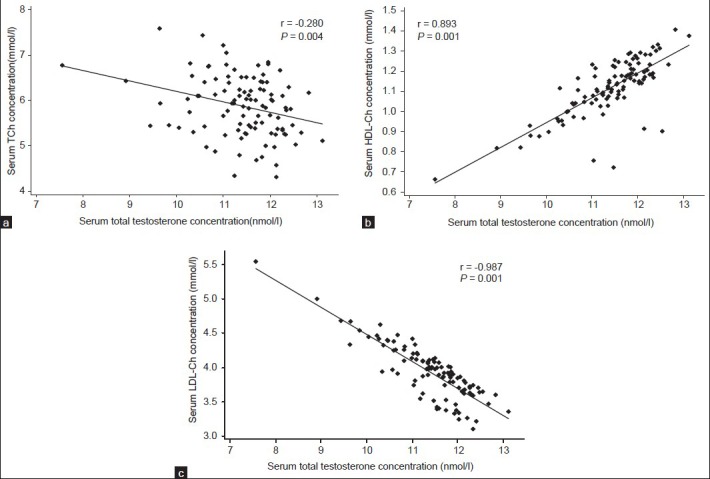
Correlation between serum concentration of TT and concentrations of: Total cholesterol (TCh)(a), HDL-Ch (b), and LDL-Ch (c) in CAD HDL-Ch:High density lipoprotein cholesterol, LDL-Ch:Low density lipoprotein cholesterol, CAD: Coronary artery disease
In the control group, significant positive correlations were observed between testosterone and TGs (r = 0.714; P = 0.001), TCh (r = 0.571; P = 0.001), HDL-Ch (r = 0.997; P = 0.001) [Figure 2] and LDL-Ch (r = 0.995; P = 0.001).
Figure 2.
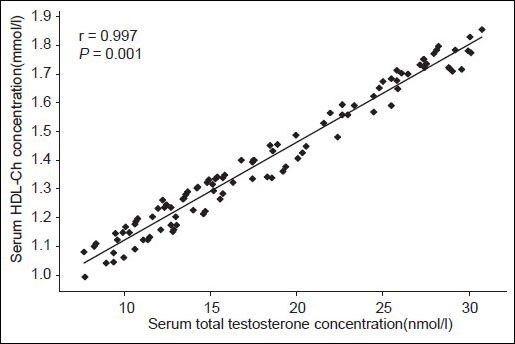
Correlation between serum TT and HDL-Ch in controls TT: Total testosterone, HDL-Ch: High density lipoprotein cholesterol
DISCUSSION
In this case-control study, consistent significant differences in serum testosterone and lipid parameters were observed between the case group and control group, which remained unchanged following adjustments for covariates. It was shown that there was a significant positive correlation between the serum levels of HDL-Ch and testosterone, whereas a significant negative correlation was elicited between LDL-Ch and testosterone in patients with CAD. In the control group, a significant positive correlation was observed between testosterone and HDL-Ch.
There are several ways by which testosterone may affect lipid metabolism and atherosclerosis. One proposed mechanism is that testosterone acts through modulation of lipid profile, supported by findings of a favorable relationship with HDL-Ch. Our finding of a potentially anti-atherogenic effect of endogenous natural testosterone within the physiological range, by modulating the HDL cholesterol level, is in agreement with earlier reports from studies done on healthy men.[9,10,11,12,13] Among other studies, TT and sex hormone-binding globulin are associated with a beneficial lipid profile particularly with high HDL-Ch levels in men.[15,16]
It has been shown that healthy men with lower levels of plasma testosterone tend to have lower levels of HDL-Ch and lipoprotein lipase.[17,18] By contrast, one study showed a significant negative association of testosterone with HDL cholesterol.[19]
There are studies where intramuscular administration of near-physiological doses of testosterone resulted in a decrease in total and LDL cholesterol levels without significantly affecting HDL cholesterol levels.[20,21,22] Testosterone supplementations in men with hypo-gonadotropic hypogonadism were shown to increase the levels of total and LDL-Ch, but increased the HDL-LDL ratio.[23]
On the other hand, the significance of endogenous testosterone for the lipid parameter in patients with established CAD needs to be further evaluated, as some epidemiological studies assessing cardiovascular events did not firmly establish a definite link of testosterone with development of CAD.[24] Moreover, data on the relationship of testosterone with lipid profile in patients with CAD are scarce. Therefore our study adds new knowledge in this area.
In contrast to our study, a previous study reported significant associations of estradiol and lipids in a group of patients with CAD,[25] but revealed no association between TT and lipid levels.
Several explanations can be postulated for the effects of testosterone on lipids. Testosterone may directly relate with HDL-Ch levels, perhaps by regulating the activities of enzymes involved in HDL-Ch metabolism. Alternatively, the relationship of testosterone with lipids may be due to peripheral conversion to estradiol. In another way, hormone and lipid levels may simply be markers of altered metabolic pathways: Hepatic metabolism or inter-conversion of hormones, and synthesis or degradation of cholesterol.
CONCLUSIONS
In conclusion, TT was low in patients with CAD compared with controls. There was a significant positive association between serum TT and HDL-Ch in both groups. A negative association was found between testosterone and LDL-Ch in patients with CAD. This reflected that low levels of testosterone appear to create an atherogenic lipid milieu.
Limitations
Unavailability of facilities and the financial constraints in the Sri Lankan context have limited the number of assays performed such as sex hormone-binding globulin and an index of free and bio-available testosterone.
Footnotes
Source of Support: UGC, Sri Lanka
Conflict of Interest: No
REFERENCES
- 1.BHF statistics database. 2010. [Last acceses on 2012 Dec 1]. Available at: http://www.bhf.org.uk/research/heart-statistics.aspx .
- 2.Nathan L, Shi W, Dinh H, Mukherjee TK, Wang X, Lusis AJ, et al. Testosterone inhibits early atherogenesis by conversion to estradiol: Critical role of aromatase. Proc Natl Acad Sci USA. 2001;98:3589–93. doi: 10.1073/pnas.051003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson KM, Castelli WP, Levy D. Cholesterol and mortality: 30 years of follow-up from the Framingham Heart Study. J Am Med Assoc. 1989;257:2176–84. doi: 10.1001/jama.257.16.2176. [DOI] [PubMed] [Google Scholar]
- 4.English KM, Mandour O, Steeds RP, Diver MJ, Jones TH, Channer KS. Men with coronary artery disease have lower levels of androgens than men with normal coronary angiograms. Eur Heart J. 2000;21:890–94. doi: 10.1053/euhj.1999.1873. [DOI] [PubMed] [Google Scholar]
- 5.Phillips GB, Pinkernell BH, Jing TY. The association of hypotestosteronemia with coronary artery disease in men. Arterioscler Thromb. 1994;14:701–06. doi: 10.1161/01.atv.14.5.701. [DOI] [PubMed] [Google Scholar]
- 6.Phillips GB, Jing TY, Resnick LM, Barbagallo M, Laragh JH, Sealey JE. Sex hormones and haemostatic risk factors for coronary heart disease in men with hypertension. J Hypertens. 1993;1:699–702. doi: 10.1097/00004872-199307000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Zumoff B, Strain GW, Miller LK, Rosner W, Senie R, Seres DS, et al. Plasma free and non sex hormone binding globulin bound testosterone are decreased in obese men in proportion to their degree of obesity. J Clin Endocrinol Metab. 1990;71:929–31. doi: 10.1210/jcem-71-4-929. [DOI] [PubMed] [Google Scholar]
- 8.Haffner SM, Karhapaa P, Mykkanen L, Laakso M. Insulin resistance, body fat distribution and sex hormones in men. Diabetes. 1994;43:212–9. doi: 10.2337/diab.43.2.212. [DOI] [PubMed] [Google Scholar]
- 9.Khaw KT, Barrett-Connor E. Endogenous sex hormones, high density lipoprotein cholesterol and other lipoprotein fractions in men. Arterioscler Thromb. 1991;11:489–94. doi: 10.1161/01.atv.11.3.489. [DOI] [PubMed] [Google Scholar]
- 10.Dai WS, Gutai JP, Kuller LH, Laporte RE, Falvo-Gerard L, Caggiula A. Relation between high-density lipoprotein cholesterol and sex hormone concentrations in men. Am J Cardiol. 1984;53:1259–63. doi: 10.1016/0002-9149(84)90075-4. [DOI] [PubMed] [Google Scholar]
- 11.Hamalainen E, Adlercreutz H, Ehnholm C, Puska P. Relationships of serum lipoproteins and apoproteins to sex hormones and to the binding capacity of sex hormone binding globulin in healthy Finnish men. Metabolism. 1986;35:535–41. doi: 10.1016/0026-0495(86)90011-9. [DOI] [PubMed] [Google Scholar]
- 12.Zmuda JM, Cauley JA, Kriska A, Glynn NW, Gutai JP, Kuller LH. Longitudinal relation between endogenous testosterone and cardiovascular disease risk factors in middle-aged men. A 13-year follow-up of former multiple risk factor intervention trial participants. Am J Epidemiol. 1997;146:609–17. doi: 10.1093/oxfordjournals.aje.a009326. [DOI] [PubMed] [Google Scholar]
- 13.Van Pottelbergh I, Braeckman L, De Bacquer D, De Backer G, Kaufman JM. Differential contribution of testosterone and estradiol in the determination of cholesterol and lipoprotein profile in healthy middle-aged men. Atherosclerosis. 2003;166:95–102. doi: 10.1016/s0021-9150(02)00308-8. [DOI] [PubMed] [Google Scholar]
- 14.Dhalla S, Kopec JA. The CAGE questionnaire for alcohol misuse: A review of reliability and validity studies. Clin Invest Med. 2007;30:33–41. doi: 10.25011/cim.v30i1.447. [DOI] [PubMed] [Google Scholar]
- 15.Gyllenborg J, Rasmussen SL, Borch-Johnsen K, Heitmann BL, Skakkebaek NE, Juul A. Cardiovascular risk factors in men: The role of gonadal steroids and sex hormone-binding globulin. Metabolism. 2001;50:882–8. doi: 10.1053/meta.2001.24916. [DOI] [PubMed] [Google Scholar]
- 16.Bataille V, Perret B, Evans A, Amouyel P, Arveiler D, Ducimetiere P, et al. Sex hormone -binding globulin is a major determinant of the lipid profile: The PRIME study. Atherosclerosis. 2005;179:369–73. doi: 10.1016/j.atherosclerosis.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 17.Nordoy A, Asbjorn A, Dag T. Sex hormones and high density lipoproteins in healthy males. Atherosclerosis. 1979;34:431–6. doi: 10.1016/0021-9150(79)90067-4. [DOI] [PubMed] [Google Scholar]
- 18.Gutai J, LaPorte R, Kuller L, Dai W, Flavo-Gerard L, Caggiula A. Plasma testosterone, high density lipoprotein cholesterol and other lipoprotein fractions. Am J Cardiol. 1981;48:897–02. doi: 10.1016/0002-9149(81)90356-8. [DOI] [PubMed] [Google Scholar]
- 19.Semmens J, Rouse I, Beilin LJ, Masarei JR. Relationship of plasma HDL-cholesterol to testosterone, estradiol, and sex-hormone-binding globulin levels in men and women. Metabolism. 1983;32:428–32. doi: 10.1016/0026-0495(83)90002-1. [DOI] [PubMed] [Google Scholar]
- 20.Zgliczynski S, Ossowski M, Slowinska-Srzednicka J, Brzezinska A, Zgliczynski W, Soszynski P, et al. Effect of testosterone replacement therapy on lipids and lipoproteins in hypogonadal and elderly men. Atherosclerosis. 1996;121:35–43. doi: 10.1016/0021-9150(95)05673-4. [DOI] [PubMed] [Google Scholar]
- 21.Tenover JS. Effects of testosterone supplementation in the aging male. J Clin Endocrinol Metab. 1992;75:1092–8. doi: 10.1210/jcem.75.4.1400877. [DOI] [PubMed] [Google Scholar]
- 22.Morley JE, Perry HM, Kaiser FE, Kraenzle D, Jensen J, Houston K, et al. Effects of testosterone replacement in old hypogonadal males: A preliminary study. J Am Geriatr Soc. 1993;41:149–52. doi: 10.1111/j.1532-5415.1993.tb02049.x. [DOI] [PubMed] [Google Scholar]
- 23.Ozata M, Yildirimkaya M, Bulur M, Yilmaz K, Bolu E, Corakci A, et al. Effects of gonadotropin and testosterone treatments on lipoprotein (a), high density lipoprotein particles, and other lipoprotein levels in male hypogonadism. J Clin Endocrinol Metab. 1996;81:3372–8. doi: 10.1210/jcem.81.9.8784099. [DOI] [PubMed] [Google Scholar]
- 24.Cauley JA, Gutai JP, Kuller LH, Dai WS. Usefulness of sex steroid hormone levels in predicting coronary artery disease in men. Am J Cardiol. 1987;60:771–7. doi: 10.1016/0002-9149(87)91021-6. [DOI] [PubMed] [Google Scholar]
- 25.Wranicz JK, Cygankiewicz I, Rosiak M, Kula P, Kula K, Zareba W. The relationship between sex hormones and lipid profile in men with coronary artery disease. Int J Cardiol. 2005;101:105–10. doi: 10.1016/j.ijcard.2004.07.010. [DOI] [PubMed] [Google Scholar]


