Abstract
Background:
Almost 15% of India's urban adult populace now lives with type 2 diabetes. This study aimed to characterize the eating patterns, knowledge, beliefs, and determinants of food choice, and assess associations with the metabolic health among urban Asian Indians with type 2 diabetes.
Materials and Methods:
A cross-sectional study of 258 individuals (mean age 55.7 ± 10 years; body mass index 27.1 ± 4.8 kg/m2; diabetes duration 10.1 ± 6.5 years) attending two out-patient clinics in New Delhi, India. Food-related information was collected during a semi-structured interview. Clinical, anthropometric, and biochemical data were recorded.
Results:
Beliefs related to health and diabetes played a role determining food choice and dietary patterns; erroneous views were associated with the poor food choices and greater metabolic perturbations. Average consumption of fruits/vegetables was low. Intakes were positively associated with intentions to manage diabetes; inversely associated with the waist circumference and negatively correlated with one's degree of personal responsibility for food choice. Household saturated fat usage was common. High fat intakes were positively associated with the taste preference, ratings of perceived “health-value;” waist circumference, glycosylated haemoglobin percentage (HbA1c%) and lipids.
Conclusions:
Strategies to enhance diabetes control among Asian Indians are required and should encourage fruit/vegetable intake, personal accountability, and consider individual beliefs and preferences. Greater emphasis and resources directed to regular dietary and behavioral counseling may assist.
Keywords: Asian Indian, behavior, belief, diabetes, food choice
INTRODUCTION
Worldwide rates of obesity and type 2 diabetes have increased at alarming speed, and nowhere this is more evident than among the city-dwelling middle classes of India.[1] Current estimates by the International Diabetes Federation suggest nationwide prevalence rates of 9.2% among Indians aged 20-79 years.[2] While rural populations bare approximately 3% of the burden, in the order of 15% of India's adult urban populace are now believed to have type 2 diabetes.[3]
Asian Indians appear genetically predisposed, most likely through the interplay of multiple susceptible genes;[4] however, recent environmental and behavioral change appears to be the primary driver of the increasing prevalence of obesity, insulin resistance, and type 2 diabetes.[5] Most notably, India's rapidly developing economy has driven the expansion of urbanized zones, altered social and employment patterns, and provided greater access to high fat processed foods that are often consumed outside of the home.[6]
Economic advancement has also changed food availability and eating patterns. In recent decades, refined vegetable oils have become more plentiful and affordable and an increasing number of meals and snacks are being consumed out of the home.[6] Fast- and snack-foods are generally high in fat, and also commonly contain trans fats, both of which can contribute to insulin resistance.[7] City-dwellers in particular appear to consume a greater percentage of energy from fat (32%) compared to rural areas (17%).[8]
Aberrant dietary patterns are associated with the diabetes onset and progression and once established, food choice and eating patterns play a pivotal role in the diabetes management.[9] Although studies among Northern Indians are lacking, investigation of Southern Indians with type 2 diabetes have however, demonstrated low adherence to dietary regimes. In the multi-center DiabCare Asia-India Study, only 4% of 2269 participants followed a specific dietary therapy.[10] In two urban South Indian groups of similar size to the current study, around one- third of participants were adhering to any dietary prescription.[11,12] Adherence to a diabetic regimen was positively related to dietary counseling and optimistic, pro-active health-related attitudes.[12] Both studies emphasized the need for on-going direct counseling to help maintain enthusiasm and treatment adherence. Research conducted in women with diabetes from other cultures suggests those believing that their individual dietary choices play a role in disease development and the treatment were more compliant with the dietary recommendation.[13,14]
Within North Indian urban populations with type 2 diabetic, the present study aimed to: (1) Characterize aspects of current eating patterns, behaviors, and preferences; and (2) assess factors related to food decision-making including dominant beliefs and attitudes linked to food and health-related themes. Relationships between these factors and metabolic health were also examined in order to inform the effective nutrition and the life-style intervention strategies.
MATERIALS AND METHODS
The study was conducted in two large tertiary teaching hospitals in the National Capital region of New Delhi, India. The first, Indraprasthra Apollo Hospital is a 600 bed facility located in the southern district of New Delhi; the second, Medanta The Medicity is a 700 bed hospital located in Gurgaon, a growing business district 30 km southwest of New Delhi. Both hospitals cater to the population of North India and serve as national referral centers.
Between September 2010 and March 2011, upon presentation for an out-patient endocrine review, all males and females of Asian Indian origin, ≥25 to ≤65 years of age, with pre-existing type 2 diabetes (≥6 months), diagnosed according to standard World Health Organization criteria[15] were invited to take part in the study. Study participants were under the care of one of the authors (Ambrish Mithal). The Institutional Review Board of the sponsoring hospitals approved the study and all participants gave their informed consent.
The study involved the cross-sectional collection of a range of clinical, anthropometric and biochemical data, plus assessment of eating patterns, food-related knowledge and beliefs, and factors driving food choice.
Anthropometry and biochemistry
Respondents provided information on marital status; educational attainment; the number of persons living (and eating) within the household. Weight was recorded on electronic scales (Tanita Corporation, Japan) in kilograms to the nearest gram; height was recorded on a wall-mounted stadiometer in centimeters; waist circumference was taken at the umbilicus to the nearest millimeter. All readings were taken by a single, experienced observer. Metabolic health was assessed by a series of biochemical tests including fasting plasma glucose (mg/dL), glycosylated haemoglobin (HbA1c %), low density lipoprotein (LDL)-cholesterol (mg/dL), high density lipoprotein (HDL)-cholesterol (mg/dL) and triglycerides (mg/dL). Blood samples were taken during the routine care by experienced clinicians and analyzed according to standard procedures in hospital laboratories certified by the National Accreditation Board of Laboratories. Additional clinical information was recorded from the case notes after the out-patient appointment.
Food-related practices, beliefs, and knowledge
Information was collected on food-related practices, beliefs, and knowledge. Data were obtained by one of two clinic-based diabetes educators in a single semi-structured interview of approximately 30 min duration. The semi-structured interview consisted on a defined program of questions including multiple choice, 5-point rating scales, short answer, and dichotomous response. All diabetes educators received training to adhere to the interview outline. An initial draft was pilot-tested on ten individuals, which resulted in the restructuring and removal of a number of questions to reduce the survey length.
To obtain data on food purchasing habits within the home, respondents approximated how much of the family's income was spent on food (response range: ≤¼;¼ to ½; ≥½; don’t know); who was in charge of household food and meal choices and who purchased most household food. The consumption frequency of meals, snacks, fruits, and vegetables was determined by asking how many set meals; between-meal snacks; whole fruits; different types of vegetables subjects consumed during an average day. Individuals indicted if they were vegetarian.
Estimated use and intake of household fats
To investigate the quality and the quantity of habitually consumed fats and oils participants were presented with a list of 10 butters and oils: butter, desi ghee (clarified butter), vanaspati (hydrogenated vegetable oil), mustard seed oil, olive oil, sunflower oil, cotton seed oil, soyabean oil, canola oil, and blended vegetable oil. Subjects indicated which products were used in their home; the average size (in ml or liters) of the container(s) purchased; length of time each container lasted; and the number of people (including themselves) the container fed. If any usage details were unclear, the respondent was encouraged to contact the relevant individual for clarification. For analysis, all volumes were converted in ml and the average time a container lasted was converted into days (1 week = 7 days; 1 month = 30 days). To calculate the average daily household intake, the size of each container was divided by the number of people in the household that it fed. This figure was divided by the estimated number of days each container lasted and values were added to yield an estimate for the total daily household intake. At analysis, the top and bottom 2% of estimates were excluded.
Estimated consumption frequency of selected processed foods
To investigate intakes of high in fat processed foods, participants were asked how many times a week or month on average they consumed “fast-food” or “take-away food,” either inside or outside of the home. Participants also indicated how often they consumed 3 common fast-foods and 12 common snack items: Fried chips, burgers, commercial fried chicken, puri subzi (fried flat Indian bread with vegetables), namkeen (deep-fried snack), roasted channa (roasted chick peas), pakoras (deep-fried ball of chick pea flour mixed with vegetables), chholey bhature (chick pea curry with deep-fried Indian bread), chaat (spicy fried potato), samosas/kachauri (deep-fried pocket of wheat flour filled with vegetables), pav bhaji (white bun with a spicy vegetable gravy), plain sweet biscuits, chocolate, Indian sweets (burfi, rasgulla, jallebi) (milk-based Indian sweets), cakes/pastries. Consumption frequency options ranged from never; rarely; 1-3 times/month/1-7 times/week. A rating of “rarely” was considered to be an intake of 0.5 days/month. For analysis, all weekly values were multiplied by four in order to express consumption as days/month. A total estimate of processed snack intake/month was calculated by adding all monthly figures for each consumed food.
Food choice decisions and perceptions and knowledge
To assess the relative importance of factors involved in food choice decisions, subjects were asked to rate 13 possible factors on a 5-point scale from 1 (very much) to 5 (not at all), including: parents/upbringing; schooling/study; cultural background; job/working hours; dieting; food cost; food taste; perceived health value; convenience; what is served; habit; diabetes control; stress; other (extended response). Preference for fatty/savory food tastes was assessed from a list of eight commonly available foods (dal rice [a lentil curry and rice]; a sandwich; samosa; hot chips/French fries; fresh fruit; chat; home-cooked vegetable [vegetable curry]; pizza). Participants ranked their top three foods according to how much they enjoy the flavor.
In addition to indicating their consumption frequency of the various household fats/oils and fast foods snacks, participants also rated how healthy they perceived these foods to be. All ratings were based on a 5-point scale from 1 (very healthy) to 5 (very unhealthy).
Subjects were asked to specify, which statement defined simple carbohydrates (CHO): (a) Those digested quickly that are rapidly converted into sugar, or (b) those digested slowly, that are converted slowly into sugar.
Descriptive statistics were used to express values as mean ± SD for continuous demographic, anthropometric, clinical, biochemical, and dietary variables, median (interquartile range [IQR]) for categorical variables, and percentages. All continuous variables were normally distributed. Independent samples t-tests were used to compare differences between the continuous variables divided into respondents and non-respondents, by gender and groups split at the median value. Chi-square tested differences between categorical variables. Statistical Package for the Social Sciences (SPSS) version 12.0.1 was used for statistical analysis. A P value of less than 0.05 was considered as statistically significant.
RESULTS
Descriptive characteristics
Of 378 patients approached, 258 completed the study; a response rate of 68%. The final group included 101 individuals recruited from Indraprastha Apollo Hospital (39.1%) and 157 subjects from Medanta Medicity. There was no statistical differences between the grouped demographic, anthropometric or biochemical data of subjects recruited from either hospital (data not shown); therefore, group data were combined.
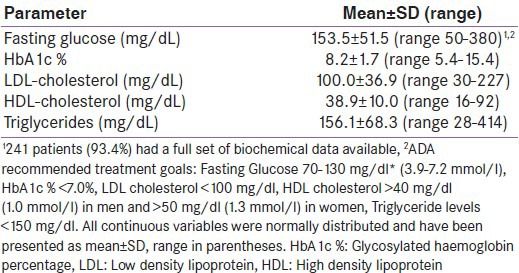
Group demographic and anthropometric descriptors are listed in Table 1. The study group included 185 males (71.7%) and 73 females, mostly older adults who, according to modified Asian Indian cut-offs,[16] were categorized as obese (body mass index [BMI] >25 kg/m2) with a high-risk waist circumference (men >90 cm, women: >80 cm).[16] Table 2 lists the group's biochemical indices. No differences in the plasma fasting glucose or HbA1c% were apparent between males and females; however, females recorded higher LDL-cholesterol (111.8 ± 38.4 vs. 95.5 ± 35.3, P = 0.003), HDL-cholesterol (43.7 ± 12.0 vs. 37.0 ± 8.6, P = 0.002), and triglyceride levels (161.6 ± 70.2 vs. 153.6 ± 67.7, P < 0.001).
Table 1.
Demographic and anthropometric characteristics of the sample of adults with diabetes attending an out-patient appointment (n=258)
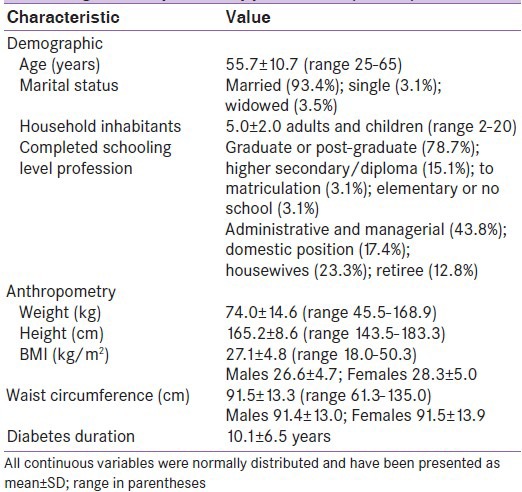
Table 2.
Biochemical parameters of the sample of adults with diabetes attending an out-patient appointment (n=258)
Eating patterns and food-related beliefs
Of those who responded (n = 188; 72.9%), 49.4% reported that ¼-½ and 44.7% reported ≤¼ of household income was used to purchase food. Within the household, the wife was most often responsible for food purchases (45%), then husband (22.1%) and cooks/servants (16.3%). Wives were most accountable for organizing household meals (57%); less so by cooks/servants (19.4%) and husbands (11.6%).
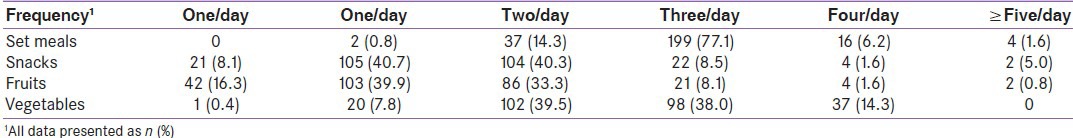
Within the group, 155 individuals (60.1%) were vegetarian. Compared to non-vegetarians, vegetarians had a smaller waist circumference (90.2 ± 13.8 cm vs. 93.5 ± 12.1; P = 0.05) but did not differ on any other measure (data not shown); therefore, all individuals were combined for the dietary analysis. Table 3 lists the frequency of the group's meal, snack, and fruit and vegetable consumption. Total daily eating episodes ranged from 1 (0.4%) to 8 (1.2%) with the majority reporting 4 (36.4%) or 5 (37.6%). The average participant consumed a combined total of 4.0 ± 2.0 (median ± IQR) types of fruits and vegetables/day. Compared to ≤4 serves/day of fruit/vegetables (n = 162; 62.8%), habitual consumption of ≥5 serves/day was associated with a smaller waist circumference (88.8 ± 13.3 vs. 93.0 ± 13.0; P = 0.014) and lower household fat usage (29.5 ± 12.0 vs. 38.4 ± 17.9; P < 0.001). Higher consumers rated “health-value” (P = 0.001) of food and “control of diabetes” (P < 0.001) as important factors in food choice, and were less likely to indicate “I eat what I am served” as influential (P = 0.004).
Table 3.
Number of reported daily meals, snacks, fruits, and vegetables consumed by the total group (n=258)
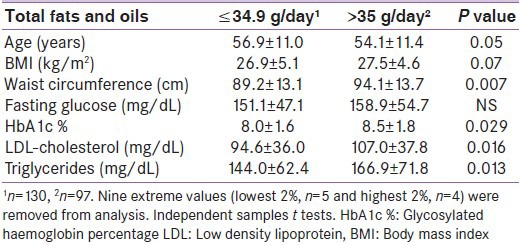
The number of different fats and oils used within the home ranged from 1 to 7 varieties and median ± IQR 3.0 ± 1.0 varieties per household. The most commonly used fats/oils were desi ghee (n = 184; 71.3%), butter (n = 166; 62.0%), mustard oil (n = 139; 53.9%), sunflower oil (n = 124; 48.1%), soyabean oil (n = 78; 30.2%), blended vegetable oils (n = 61; 23.6%), and olive oil (n = 52; 20.2%). Canola (n = 9; 3.5%), vanaspati (n = 8; 3.1%) and cotton seed oils (n = 1; 0.4%) were used infrequently. When extreme and improbable values were excluded, average household fat/oil usage (n = 229; 88.8%) was 35.0 ± 16.4 ml/day (range 10.3-95.7). Table 4 shows characteristics of lower and higher consumers. Ratings of the “health value” of each fat/oil varied; however, more favorable ratings were associated with a higher reported use of butter (P < 0.001); vanaspati (P = 0.005); olive oil (P < 0.001); soya bean oil (P = 0.001); canola (P = 0.05); and blended oil (P < 0.001).
Table 4.
Basic associations between clinical, biochemical, and behavioral parameters and the amount of fat and oil consumption within the home
Reported purchases of fast- or take-away food ranged from never to daily; median ± IQR 2.0 ± 4.2 days/month. Those consuming these foods >3 times/month (n = 125; 48.4%) were younger (53.2 ± 11.4 years vs. 57.9 ± 9.9 years; P = 0.001), with higher waist circumference (93.7 ± 12.8 cm vs. 89.3 ± 13.4 cm; P = 0.008) and triglycerides (168.4 ± 78.0 vs. 144.3 ± 55.8; P = 0.006). The most commonly consumed snack foods were roasted chana, sweet biscuits, namkeen, and Indian sweets (data not shown). As reported consumption increased and hence did the health rating of nine items. Those who believed between-meal snacks assisted their diabetic control (n = 154; 59.7%) ate more processed snacks (P < 0.001).
Factors involved in food choice decisions
From a list of 13 factors subjects rated on a 5-point scale from 1 (very much) to 5 (not at all) the extent to which each factor influenced their usual eating patterns. The most highly ranked group choices were the “health value” of food and “to control my diabetes” (both median ± IQR 2.0 ± 1.0), followed by “taste” and “out of habit” (both median ± IQR 2.0 ± 2.0).
Subjects ranked their top three foods according to flavor enjoyment. Those who preferred the taste of one or more high fat/salt foods (chat potato, samosa, fried chips or pizza) were younger, of higher BMI and waist circumference, with a higher LDL-cholesterol (107.8 ± 37.1 and triglycerides (data not shown) than the remainder.
CHO-related knowledge
Over one quarter of respondents (26.7%) were unsure of the definition of “simple CHO.” Of those who felt they knew, 98 (51.9%) individuals agreed that simple CHO are “those digested quickly that are rapidly converted into sugar.” A similar number; however, (91; 48.1%), designated simple CHO as “those digested slowly that are converted slowly into sugar.”
DISCUSSION
Investigation of particular eating patterns and food-related beliefs and associations between ranges of demographic, anthropometric, and biochemical characteristics in this older, well-educated grouping of Asian Indians living in urbanized regions of New Delhi yielded a number of useful findings.
While the use of household fats/oils and intakes of processed meals and snacks were common to some respondents, average daily intakes of fruits and vegetables were generally low. Intakes of at least three to five servings of non-starchy vegetables plus two to three portion-controlled servings of fruit are recommended.[17] Higher intakes of fruits and vegetables were linked with a lower average waist circumference and positively associated with food's perceived “health-value” and eating to maintain “control of diabetes.” Those reported to eat according to what they were served, thereby taking less personal food choice responsibility, consumed fewer fruits and vegetables. High ratings of “taste” as a food choice determinant were younger and showed a preference for processed, high fat foods.
This and other studies emphasize the need for personal accountability and active participation in diabetes self-management, the need for more dietary education with the inclusion of those responsible for meal planning,[18,19] and the benefits of recurring patient review.[20] Education should build dietary self-efficacy to change former habits and provide skills in forward planning and decision-making.[21] A study in urban South India also highlighted the role of family support and health-related values in facilitating compliance with recommended diabetes treatment regimes.[12]
Accurate knowledge about healthy food choice and patterns[21] and monitoring of carbohydrate types and amounts[9] are cornerstones of diabetes management. Despite a high level of education and socio-economic advantage, this study group displayed a generally low level of knowledge related to the healthy food choices and the basic concept of “simple carbohydrates” and dietary fiber. Erroneous beliefs regarding the “health-valuey” of foods also influenced consumption patterns. High health ratings of processed snack foods and the belief that snacking assisted control were associated with higher consumption frequency. An emphasis on small, frequent meals to manage the blood sugar and avoid hypoglycemia if on medication may have driven these beliefs and behaviors. A study based in Southern India also reported poor knowledge and diabetes about diabetes and management, more pronounced in females, and those less educated and in lower functioning jobs.[22]
Inside the home, use of butter and desi ghee, which can contain up to 50% trans fats,[6] was common and high fat snacks were especially common in younger participants. In India, addition of trans fats is currently unrestricted and commonly used in processed oils, biscuits, sweets, convenience and “street” foods.[23] High consumption of fats, especially trans fats, and saturates has been implicated in the etiology of insulin resistance and glucose intolerance, and poor diabetes management.[9] The ADA recommends intakes of saturated fat be ≤7% of total calories consumed, and trans fat should be minimized.[9]
This was a small, cross-sectional study with relatively more men; thus, the ability to generalize findings is limited. The dietary questions, although piloted were not tested for validity or reliability. The survey was reliant on memory; however, respondents asked household members for assistance. Although dietary interviews were semi-structured, terminology such as “meals” and “snacks” can vary in interpretation, and misreports and bias are possible,[24] and dietary estimates were based on novel calculations, as outlined. Strengths include the use of standardized anthropometric and biochemical measures, and a range of novel data in a north Indian population with type 2 diabetes.
India is not alone in its failure to provide optimal multidisciplinary care of individuals with diabetes.[25] Improved counseling skills and more resources allocated to the holistic dietary intervention could encourage beneficial food- and health-related beliefs to support sustained behavior change. Encouragement to reduce saturated fats and increase fruits/vegetables appears important. Future work should test cost-effective approaches to the dietary intervention in Asian Indian individuals with type 2 diabetes that include assessment of underlying beliefs and determinants of food choice, and strategies for change.
ACKNOWLEDGMENTS
The authors wish to thanks all of the individuals who participated in this study. The authors have no relevant conflict of interest to disclose.
Footnotes
Source of Support: Nil
Conflict of Interest: This research was undertaken within and as an extension of the routine care provided to outpatients. The first author provided her time and expertise without any form of funding or payment.
REFERENCES
- 1.Ramachandran A, Mary S, Yamuna A, Murugesan N, Snehalatha C. High prevalence of diabetes and cardiovascular risk factors associated with urbanization in India. Diabetes Care. 2008;31:893–8. doi: 10.2337/dc07-1207. [DOI] [PubMed] [Google Scholar]
- 2.5th ed. Brussels, Belgium: 2011. International Diabetes Federation. Diabetes Atlas. database on the Internet. [PubMed] [Google Scholar]
- 3.Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, et al. Diabetes in Asia: Epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129–40. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 4.McCarthy MI, Hitman GA, Shields DC, Morton NE, Snehalatha C, Mohan V, et al. Family studies of non-insulin-dependent diabetes mellitus in South Indians. Diabetologia. 1994;37:1221–30. doi: 10.1007/BF00399796. [DOI] [PubMed] [Google Scholar]
- 5.Ramachandran A. Epidemiology of diabetes in India – Three decades of research. J Assoc Physicians India. 2005;53:34–8. [PubMed] [Google Scholar]
- 6.Popkin BM, Horton S, Kim S, Mahal A, Shuigao J. Trends in diet, nutritional status, and diet-related noncommunicable diseases in China and India: The economic costs of the nutrition transition. Nutr Rev. 2001;59:379–90. doi: 10.1111/j.1753-4887.2001.tb06967.x. [DOI] [PubMed] [Google Scholar]
- 7.Odegaard AO, Pereira MA. Trans fatty acids, insulin resistance, and type 2 diabetes. Nutr Rev. 2006;64:364–72. doi: 10.1111/j.1753-4887.2006.tb00221.x. [DOI] [PubMed] [Google Scholar]
- 8.Shetty PS. Nutrition transition in India. Public Health Nutr. 2002;5:175–82. doi: 10.1079/PHN2001291. [DOI] [PubMed] [Google Scholar]
- 9.Americal Diabetes Association, Standards of medical care in diabetes–2011. Diabetes Care. 2011;34(Suppl 1):S11–61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raheja BS, Kapur A, Bhoraskar A, Sathe SR, Jorgensen LN, Moorthi SR, et al. DiabCare Asia – India Study: Diabetes care in India – Current status. J Assoc Physicians India. 2001;49:717–22. [PubMed] [Google Scholar]
- 11.Shobhana R, Begum R, Snehalatha C, Vijay V, Ramachandran A. Patients’ adherence to diabetes treatment. J Assoc Physicians India. 1999;47:1173–5. [PubMed] [Google Scholar]
- 12.Kapur K, Kapur A, Ramachandran S, Mohan V, Aravind SR, Badgandi M, et al. Barriers to changing dietary behavior. J Assoc Physicians India. 2008;56:27–32. [PubMed] [Google Scholar]
- 13.Hunt LM, Valenzuela MA, Pugh JA. Porque me tocó a mi? Mexican American diabetes patients’ causal stories and their relationship to treatment behaviors. Soc Sci Med. 1998;46:959–69. doi: 10.1016/s0277-9536(97)10014-4. [DOI] [PubMed] [Google Scholar]
- 14.Schoenberg NE, Amey CH, Coward RT. Stories of meaning: Lay perspectives on the origin and management of noninsulin dependent diabetes mellitus among older women in the United States. Soc Sci Med. 1998;47:2113–25. doi: 10.1016/s0277-9536(98)00277-9. [DOI] [PubMed] [Google Scholar]
- 15.Geneva: World Health Organization; 2006. World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: Report of a WHO/IDF consultation. [Google Scholar]
- 16.Misra A, Chowbey P, Makkar BM, Vikram NK, Wasir JS, Chadha D, et al. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Physicians India. 2009;57:163–70. [PubMed] [Google Scholar]
- 17.American Diabetes Association. [Accessed 2013 April 9]. Available from: http://www.diabetes.org/food-and-fitness/food/what-can-i-eat .
- 18.Gagliardino JJ, Aschner P, Baik SH, Chan J, Chantelot JM, Ilkova H, et al. Patients’ education, and its impact on care outcomes, resource consumption and working conditions: Data from the International Diabetes Management Practices Study (IDMPS) Diabetes Metab. 2011;38:128–34. doi: 10.1016/j.diabet.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Ellison GC, Rayman KM. Exemplars’ experience of self-managing type 2 diabetes. Diabetes Educ. 1998;24:325–30. doi: 10.1177/014572179802400307. [DOI] [PubMed] [Google Scholar]
- 20.Renders CM, Valk GD, Griffin S, Wagner EH, Eijk JT, Assendelft WJ. Interventions to improve the management of diabetes mellitus in primary care, outpatient and community settings. Diabetes Care. 2001;24:1821–33. doi: 10.2337/diacare.24.10.1821. [DOI] [PubMed] [Google Scholar]
- 21.Savoca M, Miller C. Food selection and eating patterns: Themes found among people with type 2 diabetes mellitus. J Nutr Educ. 2001;33:224–33. doi: 10.1016/s1499-4046(06)60035-3. [DOI] [PubMed] [Google Scholar]
- 22.Murugesan N, Snehalatha C, Shobhana R, Roglic G, Ramachandran A. Awareness about diabetes and its complications in the general and diabetic population in a city in southern India. Diabetes Res Clin Pract. 2007;77:433–7. doi: 10.1016/j.diabres.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Ghafoorunissa G. Role of trans fatty acids in health and challenges to their reduction in Indian foods. Asia Pac J Clin Nutr. 2008;17(Suppl 1):212–5. [PubMed] [Google Scholar]
- 24.Westerterp KR, Goris AH. Validity of the assessment of dietary intake: Problems of misreporting. Curr Opin Clin Nutr Metab Care. 2002;5:489–93. doi: 10.1097/00075197-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Narayan KM, Zhang P, Williams D, Engelgau M, Imperatore G, Kanaya A, et al. How should developing countries manage diabetes? CMAJ. 2006;175:733. doi: 10.1503/cmaj.060367. [DOI] [PMC free article] [PubMed] [Google Scholar]


